Abstract
Background
Intraductal papillary mucinous neoplasms (IPMNs) have malignant potential, and can progress from low- to high-grade dysplasia to invasive adenocarcinoma. The management of patients with IPMNs is dependent on their risk of malignant progression, with surgical resection recommended for patients with branch duct-IPMN (BD-IPMN) who develop high-risk features.
There is increasing evidence that liver transplant patients are at increased risk of extra-hepatic malignancy. However there are few data regarding the risk of progression of BD-IPMNs in liver transplant recipients. The aim of this study was to determine if liver transplant recipients with BD-IPMNs are at higher risk of developing high-risk features than patients with BD-IPMNs who did not receive a transplant.
Methods
Consecutive patients who underwent a liver transplant with BD-IPMNs were included. Patients with BD-IPMNs with no history of immunosuppression were used as controls. Progression of the BD-IPMNs was defined as development of a high-risk feature (jaundice, dilated main pancreatic duct, mural nodule, cytology suspicious or diagnostic for malignancy, cyst diameter ≥3cm).
Results
Twenty three liver transplant patients with BD-IPMN were compared with 274 control patients. The median length of follow-up was 53.7 and 24 months in liver transplant and control groups respectively. Four (17.4%) liver transplant patients and 45 (16.4%) controls developed high-risk features (p=0.99). In multivariate analysis, progression of BD-IPMNs was associated with age at diagnosis but not with liver transplantation.
Conclusion
There was no statistically significant difference in the risk of developing high-risk features between the liver transplant and control groups.
Keywords: Pancreatic cyst, Pancreatic cancer, IPMN, intraductal papillary mucinous neoplasm, liver transplant, OLT, immunosuppression
Introduction
Pancreatic cysts are very common, with incidental cysts detected in 3% to 13% of patients who undergo computed tomography (CT) or magnetic resonance imaging (MRI)(1, 2). Intraductal papillary mucinous neoplasms (IPMNs) are among the most common types of neoplastic pancreatic cysts, accounting for almost 50% of resected pancreatic cysts (3). IPMNs have malignant potential, and can progress from low-, or intermediate-grade dysplasia, to high-grade dysplasia to invasive adenocarcinoma in a manner similar to the adenoma-carcinoma sequence in colonic polyps. This risk of malignant progression is dependent on the type of IPMN; main-duct and mixed-IPMNs, which both affect the main pancreatic duct (Fig. 1), are associated with the greatest risk (45% to 62%) of high-grade dysplasia or invasive adenocarcinoma (4). In contrast, branch-duct IPMNs (BD-IPMN), which are characterized by enlarged, cyst-like side branch ducts with a non-dilated main pancreatic duct (Fig. 1), are associated with a much lower risk of malignant transformation, with high-grade dysplasia or invasive adenocarcinoma found in 17% to 24% of surgically resected branch-duct IPMNs(4). The management of patients with IPMNs is dependent on their risk of malignant progression, with surgical resection recommended for patients with main-, or mixed-duct IPMNs. In contrast, surgery is only recommended for patients with BD-IPMN who develop high-risk features (Table 1) which suggest the cyst is at risk of malignant transformation(4).
Figure 1.
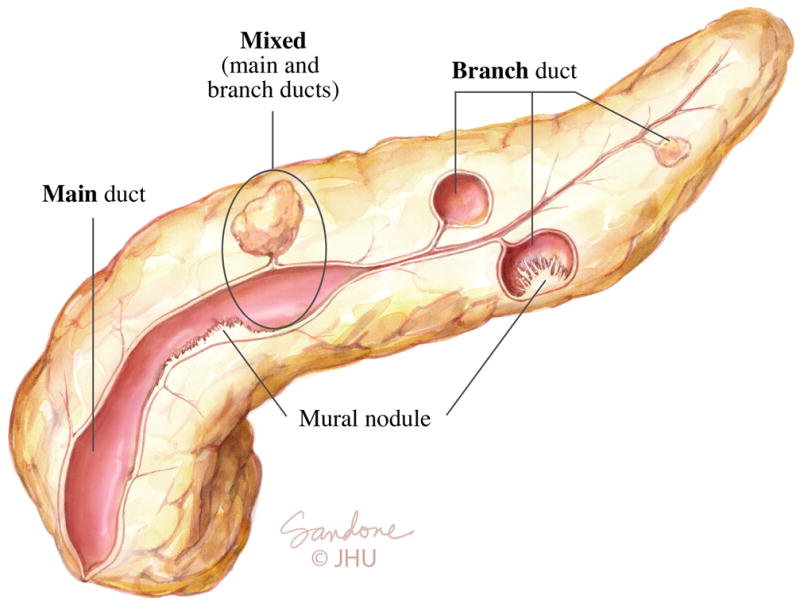
Intraductal papillary mucinous neoplasms (IPMNs)
There are three types of IPMNs based on the involvement, or not of the main pancreatic duct. Main duct IPMN is associated with a marked dilation of a portion, or all, of the main pancreatic duct. In branch-duct IPMN, the main pancreatic duct is of normal caliber, however pancreatic cysts which connect, or communicate, with the main pancreatic duct are present. Mixed-type IPMN have both a dilated main pancreatic duct and pancreatic cysts.
Table 1.
Features associated with increased risk of malignant transformation in BD-IPMN(4)
| Jaundice secondary to the pancreatic cyst Mural nodule or solid component within the cyst Cytology suspicious or diagnostic for malignancy Cyst diameter ≥3cm Main pancreatic duct involvement (pancreatic duct diameter ≥5mm) |
There is increasing evidence that liver transplant patients are at increased risk of extra-hepatic malignancy, with their overall risk of de novo solid organ malignancy of 2.0%, 6.7% and 13.6% at one, five and 10 years post transplantation(5). However there are few data regarding the risk of progression of BD-IPMNs in liver transplant recipients. The aim of this study was to determine if liver transplant recipients with BD-IPMNs are at higher risk of developing high-risk features than patients with BD-IPMNs who did not receive a transplant.
Methods
The study was approved by the Institutional Review Board (IRB) for Human Research and complied with Health Insurance Portability and Accountability Act regulations.
Patients
Consecutive adult patients who had undergone a liver transplant in a single, tertiary care center, between January 2000 and May 2010 were identified from a liver transplant database. Patients with a BD-IPMN identified on imaging either prior to, or after liver transplant, were included in the study. Patients with BD-IPMNs who did not receive a transplant were identified from a prospective multidisciplinary pancreatic cyst clinic database, and were used as controls. A detailed clinical history is recorded on all patients seen in the multidisciplinary pancreatic cyst clinic, which includes a comprehensive family history, the presence of any gene mutation associated with an increased pancreatic cancer risk, and whether they have ever been on immunosuppressant medications. Patients were excluded from either group if they had any of the following: 1) high-risk cyst features at the time of diagnosis of their IPMN (Table 1), 2) pancreatic resection for IPMN within six months of diagnosis, 3) less than six months of follow-up, or 4) fewer than two sequential imaging studies. In addition, patients were excluded from the control group if they had 1) a transplant, 2) had taken immunosuppressant medication, 3) had a strong family history of pancreatic cancer (defined as ≥ two family members), 4) or had a germline genetic mutation known to predispose to pancreatic cancer(6).
Medical records were reviewed and relevant information documented. The indication for liver transplant and immunosuppression history were documented. Surveillance was undertaken with a combination of CT, MRI and endoscopic ultrasound, following the International Consensus Criteria Guidelines (4). Sequential imaging was reviewed in both groups. The cyst size, number, location, and the presence of high-risk features were documented. The length of follow-up was defined as the time between the first and last imaging test. Pathological diagnosis was documented in patients who underwent surgical resection. Patients were deemed to have progressed if they developed any high-risk features or underwent surgical resection (Table 1).
Statistical Analyses
Stata version 11 (StataCorp, College Station, TX, USA) was used for statistical analysis. Variables were presented as number (%) or median (interquartile range, IQR). Statistical difference was assessed using the Fisher Exact test (categorical variables) or Mann Whitney U test (continuous variables). Cumulative progression rates were compared using Kaplan-Meier estimates and log rank test. Multivariable Cox proportional hazard regression model was constructed to assess the variables associated with BD-IPMN progression. Variables with a p value <0.2 in univariate analysis were included in multivariable analysis. The best fit model was constructed based on likelihood ratio test. A sensitivity analysis was undertaken to compare age-matched control patients with LT group to adjust for the age difference between two groups. A p value < 0.5 was considered statistically significant.
Results
General demographics
A total of 376 patients underwent a liver transplant, 31 (8.2%) of whom had BD-IPMNs. No liver transplant patients were identified with main- or mixed-duct IPMN. Eight patients were excluded due to less than six months follow up (n=7) or fewer than two sequential imaging studies (n=1). A total of 23 liver transplant patients with BD-IPMNs (10 (43.5%) female; median age 56.8 (interquartile range (IQR) 50.1–61.9) years) were included in the analysis. Of the 551 patients with BD-IPMN seen at the multidisciplinary pancreatic cyst clinic, 274 patients (184 (67.2%) female; median age 63.7 (IQR 55.2–72.4) years) fulfilled the inclusion and exclusion criteria and formed the control group. The general demographics of both groups are presented in Table 2. The control group contained more females, was older, had less abdominal pain and less weight loss than the liver transplant group. Over 60% of the control group never smoked, compared with 44% of the liver transplant group, while 21.7% of the liver transplant group were current smokers in contrast to only 4.7% of the control group (p=0.008). The median length of follow-up was longer in the liver transplant at 53.7 (IQR 16.9–71.8) months, compared with the control group with 24 (IQR 13.8–38.7) months. Eleven (47.8%) liver transplant and 28 (10.2%) of the controls had more than five years follow up (p<0001).
Table 2.
General characteristics of the patients
| Characteristic | Liver Transplant (n=23) |
Controls (n=274) |
P value |
|---|---|---|---|
| Female, n (%) | 10 (43.5) | 184 (67.2) | 0.03 |
| Race, n (%) | |||
| African American | 5 (21.7) | 18 (6.6) | 0.05 |
| White | 17 (73.9) | 238 (86.9) | |
| Other | 1 (4.4) | 18 (6.6) | |
| Median age at diagnosis, years (IQR) | 56.8 (50.1–61.9) | 63.7 (55.2–72.4) | 0.006 |
| Symptoms | 17 (73.9) | 97 (35.4) | 0.001 |
| Abdominal Pain, n (%) | |||
| Acute pancreatitis, n (%) | 3 (13.0) | 16 (5.8) | 0.17 |
| Jaundice*, n (%) | 0 | 0 | – |
| Weight loss, n (%) | 11(47.8) | 36 (13.1) | <0.001 |
| Diabetes mellitus, n (%) | 5 (21.7) | 53 (19.3) | 0.78 |
| Median length of follow up, months (IQR) | 53.7 (16.9–71.8) | 24 (13.8–38.7) | 0.002 |
| Smoking History, n (%) | |||
| Never smoked | 10 (43.5) | 176 (64.2) | 0.008 |
| Current smoker | 5 (21.7) | 13 (4.7) | |
| Ex-smoker | 8 (34.8) | 85 (31.0) | |
| Cyst location | |||
| Head or uncinate | 2 (8.7) | 49 (17.9) | 0.28 |
| Neck | – | 13 (4.7) | |
| Body | 3 (13.0) | 34 (12.4) | |
| Tail | 4 (17.4) | 16 (5.8) | |
| Multifocal | 14 (60.9) | 162 (59.1) |
IQR: Interquartile range;
secondary to IPMN
Liver Transplant indications and immunosuppression
The indication for liver transplant were decompensated liver disease secondary to hepatitis B virus (n=3), hepatitis C virus (n=6), alcoholic liver disease (n=3), primary biliary cirrhosis (n=3), primary sclerosing cholangitis (n=3), nonalcoholic steatohepatitis (n=1), autoimmune cirrhosis (n=1) and cryptogenic cirrhosis (n=3). The median length of follow-up after transplantation was 19.3 (IQR 11.7–94.0) months. All liver transplant patients received prednisone after liver transplant which was tapered in a standard fashion over one year. Twenty (87%) liver transplant patients initially received calcineurin inhibitors (tacrolimus or cyclosporine), of whom six changed to an mTOR inhibitor (sirolimus). Three patients (13%) received sirolimus alone. Thirteen (56.5%) patients also received mycophenolate mofetil as additional immunosuppression.
Pre- and post- liver transplant imaging
Pre and post- liver transplant imaging was available in 16 of the 23 liver transplant patients. All 16 patients had BD-IPMNs identified prior to transplant. Median pancreatic cyst size prior to liver transplant was 1.1cm (IQR, 0.9–1.7) which increased to 1.35cm (IQR 1.2–2.4, p value=0.01) following transplantation. Two patients developed high-risk features (main-duct involvement (n=1), solid component (n=1)) during follow-up but this was not statistically significant compared to pre-operative imaging.
Risk of progression in liver transplant recipients versus controls
A comparison of the progression of the BD-IPMNs in liver transplant versus control patients is presented in Table 3. Four (17.4%) liver transplant patients developed high-risk features compared to 45 (16.4%) controls, which was not statistically significant (p=0.99). The cumulative rate of progression did not differ significantly between two groups (log-rank test p value=0.18). In univariate Cox proportional hazard regression analysis age at diagnosis of IPMN was associated with an increased risk, while a history of a liver transplant or female gender were associated with a decreased risk, of developing of high-risk features (Table 4). In multivariate analysis, age at diagnosis was the only variable associated with the development of high-risk features and history of liver transplant was no longer significant. To ensure that the difference in age between the controls and the liver transplant group did not affect the results, an analysis was performed between liver transplant and age-matched controls, and did not find any association between a history of liver transplant and the development of high-risk features (hazard ratio=0.78 and p value=0.58 in multivariate analysis).
Table 3.
Progression of BD-IPMN during follow-up in liver transplant versus non-immunosuppressed controls
| Characteristic | Liver Transplant (n=23) | Controls (n=274) | ||
|---|---|---|---|---|
|
| ||||
| First imaging | Last imaging | First imaging | Last imaging | |
|
|
||||
| Median cyst size, cm (IQR) | 0.9 (0.6–1.5) | 1.3 (1–1.9) | 1.4 (0.9–2) | 1.7 (1.2–2.4) |
| Size >3cm, n (%) | 0 | 1 (4.4) | 0 | 19 (6.7) |
| Main pancreatic duct dilation*, n (%) | 0 | 2 (8.7) | 0 | 12 (4.38) |
| Solid component, n (%) | 0 | 1 (4.4) | 0 | 14 (5.1) |
| Markedly atypical cytology, n (%) | 0 | 0 | 0 | 3 (1.1) |
| Pancreatic resection, n(%) | 0 | 0 | 0 | 9 (3.3) |
|
| ||||
| Total numbers progressed€, n(%) | 4 (17.4) | 45 (16.4) | ||
defined as ≥5mm,
Patients with one or more high risk features
IQR: Interquartile range
Table 4.
Cox proportional hazard regression analysis of characteristics associated with the development of high-risk features
| Characteristics | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
|
| ||||
| Hazard ratio (CI 95%) | P value | Hazard ratio (CI 95%) | P value | |
|
|
||||
| Liver transplant | 0.5 (0.17–1.4) | 0.19 | 0.6 (0.2–1.7) | 0.34 |
| Age at diagnosis of BD-IPMN | 1.03 (1–1.07) | 0.013 | 1.03 (1–1.06) | 0.04 |
| Female gender | 0.63 (0.35–1.13) | 0.12 | 0.65 (0.36–1.2) | 0.15 |
| Race | ||||
| African American | Reference | Reference | – | – |
| White | 0.7 (0.27–1.78) | 0.45 | – | – |
| Other | 0.38 (0.07–2) | 0.26 | – | – |
| Weight loss | 1.15 (0.53–2.47) | 0.72 | – | – |
| Abdominal pain | 1.1 (0.61–2) | 0.73 | – | – |
| Diabetes | 0.98 (0.49–1.95) | 0.9 | – | – |
| Acute pancreatitis | 1.27 (0.52–3.05) | 0.6 | – | – |
| Smoking history | ||||
| Never smoker | Reference | Reference | – | – |
| Current smoker | 1.3 (0.45–3.75) | 0.63 | – | – |
| Ex-smoker | 1.41 (0.76–2.63) | 0.28 | – | – |
Outcomes of patients with high-risk features
Pancreatic resection was performed in 9 control patients, and no liver transplant patient. The indication for resection was: cyst size ≥3 cm (n=3), mural nodule or solid component (n=2), main duct involvement (n=1), markedly atypical cytology (n=2) and one patient had two indications for resection (mural nodule or solid component and cyst size ≥3 cm). Surgical pathology revealed high-grade dysplasia in two (22.2%) patients, intermediate-grade dysplasia in three (33.3%), and low-grade dysplasia in four (44.4%).
Four liver transplant, and 36 controls, developed high-risk features, but did not undergo surgical resection. The reason for not proceeding with surgery were high surgical risk (n=11) or patient refused surgery (n=4). In the remaining 25 patients the high-risk features was cyst size ≥3cm (n=11), main pancreatic ductal dilation ≥0.5cm (n=9), or a small (<5mm) mural nodule seen on endoscopic ultrasound but with no concerning features seen on any other imaging modality (n=5). To date, none of these patients have developed evidence of invasive pancreatic cancer, with a median follow-up of 32.9 (IQR 14–72.2) months for the LT group, and 7.2 (IQR 0–21.2) months in the control group.
Discussion
Patients who receive a liver transplant are at increased risk of developing malignancies, with an overall incidence of 3% to 15% (7). Several risk factors have been identified in these patients including increasing age, smoking, alcoholic liver disease, and primary sclerosing cholangitis (5). In addition to these factors, there is increasing evidence linking immunosuppressant medication, particularly calcineurin inhibitors, azathioprine, and anti-lymphocyte agents, with an increased risk of malignancy (8). Although high-level evidence is lacking, several groups recommend that liver transplant patients undergo careful surveillance for cancer (7).
BD-IPMNs have clear malignant potential, with high-grade and invasive adenocarcinoma found in 15% to 24% of patients who undergo surgical resection (4). Several factors, such as a strong family history of pancreatic cancer (9, 10), and certain genetic mutations (11), are known to increase the risk of malignant transformation of IPMNs, and closer surveillance is recommended for these patients compared with patients with sporadic IPMN (6). There are very little data published examining whether patients with BD-IPMN who have undergone a liver transplant are at increased risk of progression to malignancy compared to an average risk patient with a BD-IPMN (12).
The prevalence of BD-IPMNs in this study in liver transplant recipients was just over 8%. The true prevalence of BD-IPMNs is unclear, with a wide variation reported in the literature. One reason for this is the imaging modality used, as MRI is superior to CT for identifying small pancreatic cysts (13). However even in studies which use MRI alone, the prevalence varies greatly from 3% (14) to 32% (15).
As the number of patients who undergo surgical resection is relatively low, we used the development of features for which surgery is indicated (Table 1) as a marker of progression of the BD-IPMN. There was no statistically significant difference in the risk of developing high-risk features between the liver transplant and control groups, with just over 17% of liver transplant patients and 16% of the control group progressing, a finding similar to other studies (12). Several factors are known to be associated with an increased risk of malignancy in liver transplant recipients including immunosuppression, smoking and age (7, 16). On multivariate analysis, each year increment in age increased the hazard of development of high-risk features by 3% in patients with BD-IPMN.
This study highlights the limitations of the current criteria for identifying patients who require surgical resection. Surgical resection is recommended for BD-IPMNs which are associated with jaundice, a definite mural nodule or solid component, cytology suspicious or diagnostic for malignancy, while surgery should be considered in patients with a main pancreatic duct of ≥5mm or cyst diameter of ≥3cm (4). Pancreatic resection is associated with not insignificant risks, with morbidity reported in more than a third of patients, and a mortality of between 0.5% to 5% for a pancreaticoduodenectomy even in high-volume centers (3, 17, 18). In this study, just over 20% of patients who underwent surgical resection were found to have high-grade dysplasia, with no cases of invasive adenocarcinoma, findings which are very similar to large surgical series (19). Forty patients developed high-risk features were followed with surveillance, rather than surgery, following a multidisciplinary review. To date, none of these patients have developed evidence of invasive pancreatic cancer. The majority of these patients had a cyst which was just over 3cm in size, a main pancreatic duct which fulfilled the criteria (≥0.5cm) for main duct IPMN but had no other concerning features, or a small mural nodule seen on endoscopic ultrasound but not on other imaging modalities. There is significant debate in the literature about whether cyst size alone is a predictor of malignancy, while the optimum size for surgical resection in main duct IPMN is also debated (4). Imaging is also imperfect, and it can be difficult to differentiate a mural nodule from mucin within a cyst (20). This study highlights that in these complex cases, a review by a multidisciplinary cyst clinic is helpful, and has been shown in prior studies to alter the management of 30% of patients (21). New tools, such as molecular markers, are urgently required to help identify those patients with high-grade dysplasia or invasive adenocarcinoma who would benefit from surgical resection (22).
This study has limitations that should be considered when interpreting the results. It is a single center, retrospective study. The sample size, particularly of liver transplant recipients, is small, and thus the risk of malignant transformation may have been underestimated (Type II error). Although the median length of follow-up in the liver transplant group was almost five years, it is possible that with larger sample size and longer follow up, changes would be detected between the two groups. Studies have suggested that calcineurin inhibitors, azathioprine and antilymphocyte agents are associated with an increased risk of malignancy, while sirolimus and everolimus may be protective (8). In addition, the length of time on immunosuppressant medications may increase the risk of malignant transformation. There were insufficient number of patients in this study to allow us to assess these questions, and further multi-center, long-term studies are required.
The findings from this study add to our growing knowledge of the risk of progression of BD-IPMNs among patients who receive a liver transplant. The data suggest that liver transplant patients with BD-IPMNs do not have a statistically significant increased risk of progression compared to sporadic immunocompetent patients with BD-IPMNs. This supports the current practice that the presence of BD-IPMN without suspicious features should not preclude a liver transplant and that following liver transplant, patients with BD-IPMNs should undergo careful surveillance in a similar manner to sporadic IPMNs.
Acknowledgments
Support:
Supported by Cancer Center grant P30 CA006973, NIH SPORE grant CA62924, the Lustgarten Foundation for Pancreatic Cancer Research, Full Moon Full Circle, and the Michael Rolfe Foundation. Dr Law is the Volker Dolch Fellow in Gastroenterology at Johns Hopkins Hospital.
Footnotes
Conflict Statement:
Mouen A. Khashab is a consultant for Boston Scientific and Olympus America and has received research support from Cook Medical. Anne Marie Lennon is a consultant for Boston Scientific. Vikesh K. Singh is a consultant for Abbvie, Santarus, D-Pharm, Enteromedics, Novo Nordisk and Boston Scientific.
Author contributions:
Drs. Victor, Law, Ostovaneh, Rezaee, Girotra, Ahn, DalMolin, Wu performed data acquisition, were involved in editing and final approval of the manuscript.
Dr. Zaheer reviewed the cross-sectional imaging, edited and approved the final manuscript.
Dr. Singh, Dr. Cameron, Dr. Khashab, Dr. Ahuja, Dr. Makary, Dr. Weiss, Dr. Hirose, Dr. Goggins, Dr. Hruban and Dr. Wolfgang were involved in data interpretation, editing and final approval of the manuscript.
Dr. Ostovaneh and Ms. Jeh performed the statistical analysis, edited and approved the final manuscript.
Dr. Lennon and Dr. Gurakar developed the concept, wrote and edited the manuscript.
References
- 1.Laffan TA, Horton KM, Klein AP, Berlanstein B, Siegelman SS, Kawamoto S, et al. Prevalence of unsuspected pancreatic cysts on MDCT. AJR Am J Roentgenol. 2008;191:802–807. doi: 10.2214/AJR.07.3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee KS, Sekhar A, Rofsky NM, Pedrosa I. Prevalence of incidental pancreatic cysts in the adult population on MR imaging. Am J Gastroenterol. 2010;105:2079–2084. doi: 10.1038/ajg.2010.122. [DOI] [PubMed] [Google Scholar]
- 3.Valsangkar NP, Morales-Oyarvide V, Thayer SP, Ferrone CR, Wargo JA, Warshaw AL, et al. 851 resected cystic tumors of the pancreas: a 33-year experience at the Massachusetts General Hospital. Surgery. 2012;152:S4–12. doi: 10.1016/j.surg.2012.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanaka M, Fernandez-Del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183–197. doi: 10.1016/j.pan.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Watt KD, Pedersen RA, Kremers WK, Heimbach JK, Sanchez W, Gores GJ. Long-term probability of and mortality from de novo malignancy after liver transplantation. Gastroenterology. 2009;137:2010–2017. doi: 10.1053/j.gastro.2009.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Canto MI, Harinck F, Hruban RH, Offerhaus GJ, Poley JW, Kamel I, et al. International Cancer of the Pancreas Screening (CAPS) Consortium summit on the management of patients with increased risk for familial pancreatic cancer. Gut. 2012;62:339–347. doi: 10.1136/gutjnl-2012-303108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chandok N, Watt KD. Burden of de novo malignancy in the liver transplant recipient. Liver Transpl. 2012;18:1277–1289. doi: 10.1002/lt.23531. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez-Peralvarez M, De la Mata M, Burroughs AK. Liver transplantation: immunosuppression and oncology. Curr Opin Organ Transplant. 2014;19:253–260. doi: 10.1097/MOT.0000000000000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klein AP, Brune KA, Petersen GM, Goggins M, Tersmette AC, Offerhaus GJ, et al. Prospective risk of pancreatic cancer in familial pancreatic cancer kindreds. Cancer Res. 2004;64:2634–2638. doi: 10.1158/0008-5472.can-03-3823. [DOI] [PubMed] [Google Scholar]
- 10.Shi C, Klein AP, Goggins M, Maitra A, Canto M, Ali S, et al. Increased Prevalence of Precursor Lesions in Familial Pancreatic Cancer Patients. Clin Cancer Res. 2009;15:7737–7743. doi: 10.1158/1078-0432.CCR-09-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shin EJ, Canto MI. Pancreatic cancer screening. Gastroenterol Clin North Am. 2012;41:143–157. doi: 10.1016/j.gtc.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gill KR, Pelaez-Luna M, Keaveny A, Woodward TA, Wallace MB, Chari ST, et al. Branch duct intraductal papillary mucinous neoplasm of the pancreas in solid organ transplant recipients. Am J Gastroenterol. 2009;104:1256–1261. doi: 10.1038/ajg.2009.62. [DOI] [PubMed] [Google Scholar]
- 13.Canto MI, Hruban RH, Fishman EK, Kamel IR, Schulick R, Zhang Z, et al. Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology. 2012;142:796–804. doi: 10.1053/j.gastro.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Jong K, Nio CY, Hermans JJ, Dijkgraaf MG, Gouma DJ, van Eijck CH, et al. High prevalence of pancreatic cysts detected by screening magnetic resonance imaging examinations. Clin Gastroenterol Hepatol. 2010;8:806–811. doi: 10.1016/j.cgh.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 15.Girometti R, Intini SG, Cereser L, Bazzocchi M, Como G, Del Pin M, et al. Incidental pancreatic cysts: a frequent finding in liver-transplanted patients as assessed by 3D T2-weighted turbo spin echo magnetic resonance cholangiopancreatography. JOP. 2009;10:507–514. [PubMed] [Google Scholar]
- 16.Rodriguez-Peralvarez M, Manousou P, Lerut J, De la Mata M, Burroughs AK. How much immunosuppression is needed after liver transplantation? Clin Transplant. 2014;28:6–7. doi: 10.1111/ctr.12242. [DOI] [PubMed] [Google Scholar]
- 17.de Wilde RF, Besselink MG, van der Tweel I, de Hingh IH, van Eijck CH, Dejong CH, et al. Impact of nationwide centralization of pancreaticoduodenectomy on hospital mortality. Br J Surg. 2012;99:404–410. doi: 10.1002/bjs.8664. [DOI] [PubMed] [Google Scholar]
- 18.Winter JM, Cameron JL, Campbell KA, Arnold MA, Chang DC, Coleman J, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: A single-institution experience. J Gastrointest Surg. 2006;10:1199–1210. doi: 10.1016/j.gassur.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 19.Sahora K, Mino-Kenudson M, Brugge W, Thayer SP, Ferrone CR, Sahani D, et al. Branch duct intraductal papillary mucinous neoplasms: does cyst size change the tip of the scale? A critical analysis of the revised international consensus guidelines in a large single-institutional series. Ann Surg. 2013;258:466–475. doi: 10.1097/SLA.0b013e3182a18f48. [DOI] [PubMed] [Google Scholar]
- 20.Zhong N, Zhang L, Takahashi N, Shalmiyev V, Canto MI, Deutsch JC, et al. Histologic and imaging features of mural nodules in mucinous pancreatic cysts. Clin Gastroenterol Hepatol. 2012;10:192–198. doi: 10.1016/j.cgh.2011.09.029. [DOI] [PubMed] [Google Scholar]
- 21.Lennon AM, Manos LL, Hruban RH, Ali SZ, Fishman EK, Kamel IR, et al. Role of a Multidisciplinary Clinic in the Management of Patients with Pancreatic Cysts: A Single-Center Cohort Study. Ann Surg Oncol. 2014 doi: 10.1245/s10434-014-3739-x. dx.doi.org/10.1245/s10434–014-3739-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lennon AM, Wolfgang CL, Canto MI, Klein AP, Herman JM, Goggins M, et al. The Early Detection of Pancreatic Cancer: What Will It Take to Diagnose and Treat Curable Pancreatic Neoplasia? Cancer Res. 2014;74:3381–3389. doi: 10.1158/0008-5472.CAN-14-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]


