Abstract
Oligodendrogliomas are an important adult form of diffuse gliomas with a distinctive clinical and genetic profile. Histologically similar tumors occurring rarely in children are incompletely characterized. We studied 50 patients with oligodendrogliomas (median age at diagnosis 8 y, range 7mo to 20 y). Tumors resembling dysembryoplastic neuroepithelial tumors or pilocytic astrocytomas or those having a “mixed” histology were excluded. Tumors at first diagnosis were low grade (n=38) or anaplastic (n=12). Histologic features included uniform round cells with perinuclear halos (100%), secondary structures (predominantly perineuronal satellitosis) (90%), calcifications (46%), and microcysts (44%). Sequential surgical specimens were obtained in 8 low-grade oligodendroglioma patients, with only 1 progressing to anaplasia. Studies for 1p19q performed in 40 cases demonstrated intact 1p19q loci in 29 (73%), 1p19q codeletion in 10 (25%), and 1p deletion with intact 19q in 1 (2%). Except for 2 young patients (3 and 11 y of age), patients with 1p19q codeletion were older than 16 years at diagnosis. Mutant IDH1 (R132H) protein immunohistochemistry was positive in 4 (of 22) (18%) cases, 3 of which also had 1p19q codeletion, whereas 1p19q status was not available on the fourth case. There was a nonsignificant trend for worse overall survival in grade III tumors, but no significant association with age, extent of resection, or 1p19q status. In summary, oligodendrogliomas with classic histology occur in the pediatric population but lack 1p19q codeletion and IDH1 (R132H) mutations in most instances. They are predominantly low grade, recur/clinically progress in a subset, but demonstrate a relatively low frequency of histologic progression.
Keywords: oligodendroglioma, pediatric glioma, 1p19q, IDH1, brain tumor
Pediatric diffuse gliomas are clinically and pathologically poorly understood, mostly because of their rarity, histologic overlap with other entities, and the absence of a defining molecular feature. Nevertheless, there are tumors that fulfill all histologic criteria for oligodendroglioma in the pediatric population, and some even with 1p19q chromosomal arm codeletion typical of classical adult counterparts. Yet, the majority of the reported cases do not harbor this typical codeletion, and their demographic features are poorly delineated. In addition, some examples of these tumors arise in locations that are unusual for adult oligodendrogliomas, that is, brainstem, pineal region, cerebellum, or spinal cord.
Identification of prognostic factors in pediatric oligodendrogliomas has been difficult given the limited number of cases studied. In addition, earlier reports of pediatric oligodendroglioma may have been confounded by the inclusion of tumors currently placed in other categories such as dysembryoplastic neuroepithelial tumor (DNT). Thus, unlike the adult example, oligodendroglioma remains a poorly defined entity in children, partly because of the limited experience and small number of publications with more than a few patients.1–14
Numerous recent advances have been made in our molecular understanding of diffuse gliomas, particularly in adults. However, little is known about corresponding molecular alterations in pediatric oligodendrogliomas in particular. In adult infiltrating gliomas, whether oligodendroglial, astrocytic, or mixed, there is now a shift to a dichotomous division into 1p19q codeleted, ATRX/TP53 wild-type gliomas (oligodendrogliomas), and 1p19q intact tumors that histologically may be astrocytic or mixed (oligoastrocytoma).15 Many of the 1p19q intact adult tumors have abnormalities, that is, mutations in TP53 and ATRX and gain of chromosome 7, which are associated with neoplasms that are phenotypically more astrocytic.15–19 These latter changes are not typical of diffuse astrocytomas in children, however, and were not present in the series of Zhang et al.20
Regarding pediatric oligodendroglioma, little is known about molecular alterations characterizing these tumors. Using methylation-specific polymerase chain reaction, Suri et al13 found MGMT promoter methylation distributed in 10 oligodendroglioma patients from 4 to 25 years of age (in 5 of 7 patients 18 y of age or below and also in 5 of 7 patients 19 to 25 y of age). Interestingly, in a recent sequencing study, Zhang et al20 found FGFR1 TK duplications in 3 (of 5) pediatric oligodendrogliomas and 4 (of 8) oligoastrocytomas. FGFR1-TACC1 fusion, NAV1-NTRK2 fusion, FGFR1:p.N544K, and BRAF:p.G503 > EYSG were present in each of the 4 remaining oligoastrocytomas, and an MYB-MAML2 fusion and alterations typical of adult oligodendrogliomas (ie, CIC mutation, IDH1 mutation, and 1p19q codeletion, age 15 y) were present in each of the 2 remaining oligodendrogliomas.20 However, it remains to be seen how common and specific these changes are for pediatric oligodendrogliomas. They may not be specific, as mutations in FGFR and MYB are present in some pediatric diffuse astrocytomas as well.20,21
In the current study, we collected a large cohort of pediatric oligodendrogliomas to better define the histologic, molecular, and prognostic features of this rare entity. We have used diagnostic criteria established for adult tumors and made every attempt to exclude other entities and look-alikes.
MATERIALS AND METHODS
Patients
A pathology search algorithm was used to identify all children and adolescent patients (ages 0 to 20) with the diagnosis of oligodendroglioma from the Department of Pathology archives of Johns Hopkins Hospital and University of California San Francisco Medical Center. We chose this age cutoff as it is common for patients between 18 and 21 years of age to also be seen by pediatric practitioners, therefore reflecting the referral pattern observed among pediatric neurosurgeons/neuro-oncologists in our and similar practices, as well as cutoffs used in Children’s Oncology Group clinical trials. All available slides were reviewed by at least 2 neuropathologists in the study (P.C.B., W.M., F.J.R., T.T.). The inclusion criteria were: (1) intra-axial, supratentorial, or infratentorial location; (2) clinically symptomatic tumors; (3) radiologically diffuse/infiltrating appearance; (4) histologically consistent with adult type, infiltrative oligodendrogliomas of either World Health Organization (WHO) grade II or III. The exclusion criteria included intraspinal location, incidental tumors, tumors with unusual histologic features, and tumors diagnosed as anything other than oligodendroglioma after pathologic reviews (ie, DNT, clear cell ependymoma, pilocytic astrocytoma, and any glioneuronal tumor).
Appropriate IRB approvals were obtained in both institutions (CHR H41175-21977-01). Radiologic images were systematically reviewed in 18 patients by one of the authors (D.L.). Identification of DNTs was done along guidelines of the Children’s Cancer and Leukaemia group.22 Progression-free survival (PFS) was defined as the time of progression from initial surgery as abstracted from clinical and/or radiologic records.
Histopathology and Immunohistochemistry
Hematoxylin and eosin–stained slides and immunohistochemical slides were reviewed in all cases. Whenever available, additional sections obtained as unstained slides or blocks were used for special stains and immunohistochemistry. Immunohistochemical stains performed as part of the diagnostic workup were reviewed. Mutant IDH1 protein (H09; Dianova, Germany; 1:50) was systematically evaluated in a subset of cases.
1p19q Analysis
1p19q analysis was performed by various methods including polymerase chain reaction–based microsatellite analysis (n=18), fluorescence in situ hybridization (n=16), and single nucleotide polymorphism (SNP) array (n=9) by previously described methods.23–26
Statistical Evaluation
Frequencies and mean and median values were calculated for the variables as appropriate. Descriptive statistics and frequencies were generated for nominal and ordinal variables, respectively. Cumulative survival and median survival times with 95% confidence intervals were calculated. Survival analysis was performed using the Kaplan-Meier analysis for PFS (defined as the time between first surgery and first evidence of radiologic or clinical progression) and for overall survival (OS) (defined as the time between first surgery and death). Patients with tumors arising in unusual locations (ie, cerebellum, pineal gland, and brainstem, n=3) were excluded for survival analysis. All tests were 2 sided, with P values <0.05 considered statistically significant. Statistical analyses were performed using JMP version 10 software (SAS Institute Inc., Cary, NC).
RESULTS
Demographics and Clinical Characteristics
We identified 100 cases from both institutions that were originally diagnosed as oligodendroglioma among patients 20 years of age or under. Review of pathology material resulted in the exclusion of 50 cases because of either insufficient material or disagreement with the initial diagnosis. Eleven tumors were reclassified as DNT, 2 as ganglion cell tumors, 1 as ependymoma, and 9 as infiltrating astrocytomas. An additional 8 were neuroepithelial or glioneuronal neoplasms with unusual histologic features. For final analysis, there were 50 cases included as pediatric oligodendroglioma—36 male and 14 female. Sixteen were inhouse patients, and 34 were consultations referred to the Johns Hopkins Hospital. The median age at first surgical intervention was 7 years (range, 7 mo to 20 y). The predominant presenting symptom was a seizure disorder, either recent or remote (20 [of 33] cases), whereas the remainder presented with focal neurological deficits or symptoms attributable to mass effect.
Tumors occurred in the frontal (n = 22), temporal (n = 13), parietal (n = 7), occipital (n = 3), or frontoparietal lobes (n = 1) or in the brainstem (n = 1), cerebellum (n = 1), and pineal gland (n = 1). The exact intracranial location was not recorded in 1 patient. Postoperative treatment regimens included irradiation (n = 11), chemotherapy (n = 12), or observation only (n = 19). Subsequent treatment was not known for 17 patients. One patient had Noonan syndrome, and another had undergone central nervous system irradiation for leukemia 10 years earlier. Clinicopathologic features of patients are summarized in Table 1.
TABLE 1.
Case Summary of Pediatric Oligodendrogliomas
| Case | Sex | Age | Location | Extent of Resection | Path 1st Surgery | Treatment | Outcome | Follow-up Time (mo) |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 8 | L Frontal | STR | Low grade | Irradiation | AWD | 210 |
| 2 | M | 5 | L Temporal + thalamus | Biopsy | Low grade | Irradiation + chemotherapy | AWD | 10 |
| 3 | M | 12 | R Temporal | GTR | Low grade | Observation | Dead | 123 |
| 4 | F | 18 | R Frontal | GTR | Anaplastic | NA | NED | 166 |
| 5 | M | 8 | L Parietal lobe | STR | Anaplastic | Irradiation + chemotherapy | DOD | 25 |
| 6 | M | 7 | R Frontal | GTR | Anaplastic | Irradiation + chemotherapy | DOD | 18 |
| 7 | M | 2 | L Frontal | GTR | Low grade | Observation | AWD | 144 |
| 8 | F | 7 | Temporal | NA | Low grade | Observation | NED | 33 |
| 9 | M | 1 | Temporal | NA | Anaplastic | Irradiation + chemotherapy | NED | 212 |
| 10 | F | 2 | Parietal | NA | Low grade | Irradiation | AWD | 58 |
| 11 | F | 3 | Frontal | NA | Low grade | Observation | NED | 79 |
| 12 | M | 19 | Occipital | NA | Low grade | Observation | Alive | 204 |
| 13 | M | 5 | Frontal | NA | Low grade | Observation | Dead | 5 |
| 14 | M | 11 | Occipital | NA | Anaplastic | Irradiation + chemotherapy | AWD | 2 |
| 15 | M | 9 | R Temporal | NA | Low grade | Observation | Dead | 10 |
| 16 | M | 19 | Frontoparietal | NA | Low grade | NA | Alive | 217 |
| 17 | M | 3 | Frontal | NA | Low grade | NA | NED | 169 |
| 18 | M | 20 | Frontal | NA | Low grade | NA | AWD | 101 |
| 19 | M | 20 | R Frontal | GTR | Low grade | NA | DOD | 163 |
| 20 | M | 2 | Frontal | NA | Low grade | Observation | NED | 35 |
| 21 | F | 6 | Parietal | NA | Low grade | Observation | NED | 98 |
| 22 | F | 8 | L Temporal | GTR | Low grade | Observation | NED | 25 |
| 23 | F | 10 | R Parietal/pineal | GTR | Anaplastic | NA | Dead | 16 |
| 24 | M | 15 | L Temporal | GTR | Low grade | NA | alive | 50 |
| 25 | M | 6 | L Temporal | GTR | Low grade | Observation | NED | 52 |
| 26 | M | 19 | L Frontal | GTR | Low grade | NA | NED | 24 |
| 27 | M | 15 mo | R Frontal | STR | Low grade | Observation | AWD | 178 |
| 28 | M | 13 | L Frontal | Biopsy | Low grade | NA | LFU | NA |
| 29 | M | 16 | L Frontal | STR | Anaplastic | Irradiation + chemotherapy | AWD | 8 |
| 30 | F | 19 | R Temporal | NA | Low grade | NA | Alive | 15 |
| 31 | M | 21 mo | L Parietal | STR | Low grade | Observation | AWD | 3 |
| 32 | F | 11 | R Frontal | NA | Anaplastic | NA | AWD | 18 |
| 33 | M | 21 mo | L Frontal | STR | Low grade | NA | AWD | 9 |
| 34 | F | 17 | R Frontal | GTR | Anaplastic | Irradiation + chemotherapy | NED | 19 |
| 35 | F | 9 | NA | GTR | Low grade | NA | LFU | NA |
| 36 | M | 7 | L temporal lobe | STR | Low grade | Chemotherapy | AWD | 39 |
| 37 | M | 17 mo | Brainstem | Biopsy | Low grade | Chemotherapy | AWD | 32 |
| 38 | F | 26 mo | R Parietal | STR | Low grade | Observation | AWD | 80 |
| 39 | F | 18 | R Frontal | NA | Low grade | Chemotherapy | NED | 35 |
| 40 | M | 3 | cerebellum | STR | Anaplastic | Irradiation + chemotherapy | Dead | 115 |
| 41 | M | 16 | L Frontal | GTR | Low grade | Observation | NED | 21 |
| 42 | M | 20 | Pineal region | STR | Anaplastic | Irradiation + chemotherapy | DOD | 18 |
| 43 | M | 7 mo | L temporal lobe | NA | Low grade | Observation | LFU | NA |
| 44 | M | 4 | R medial Temporal | NA | Low grade | NA | LFU | NA |
| 45 | M | 14 | R frontal lobe | STR | Low grade | NA | AWD | 69 |
| 46 | M | 16 | R parietal lobe | STR | Low grade | Observation | AWD | 52 |
| 47 | M | 16 mo | R temporal lobe | Biopsy | Low grade | NA | DOD | 21 |
| 48 | M | 20 | R frontal lobe | NA | Low grade | NA | LFU | NA |
| 49 | M | 6 | L occipital lobe | GTR | Anaplastic | Observation | NED | 54 |
| 50 | M | 22 mo | L frontal lobe | Biopsy | Low grade | Observation | AWD | 7 |
AWD indicates alive with disease; DOD, dead of disease; LFU, lost to follow-up; NA, data not available.
Radiologic Findings
All patients had undergone brain magnetic resonance imaging (MRI), and 3 had undergone brain computed tomography preoperatively. Available radiologic studies were systematically reviewed by one of the authors (D.L.) in 18 patients (Fig. 1). In cases that had postoperative studies only (4/18), imaging features were extracted from the MRI reports.
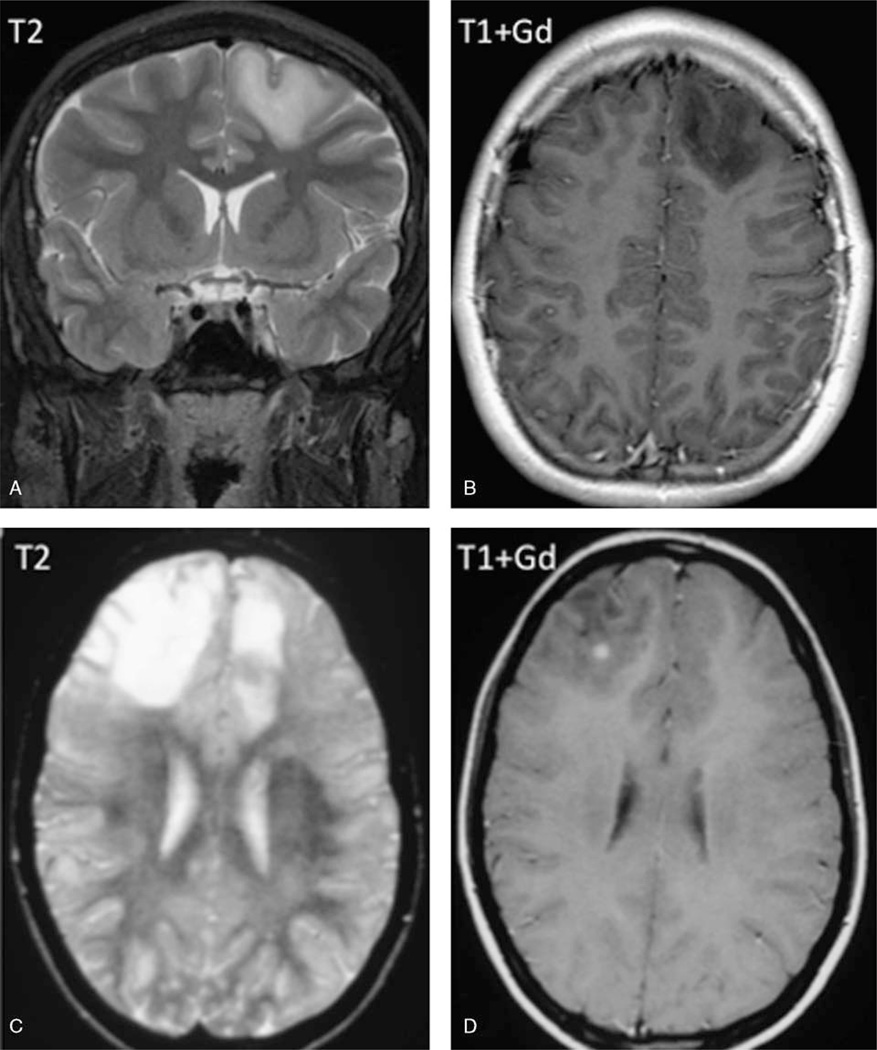
FIGURE 1.
Radiologic features of pediatric oligodendroglioma. Case 29. L frontal cortical-based, mildly expansile, T2 hyperintense, and T1 hypointense mass, also involving adjacent subcortical white matter, with minimal local mass effect and no enhancement. Histologically, this proved to be a low-grade oligodendroglioma (A and B). Case 34. MRI demonstrated T2 hyperintense, R frontal punctate-enhancing, and small L frontal nonenhancing infiltrative masses. Histologically, this tumor was an anaplastic oligodendroglioma (C and D).
Most tumors appeared infiltrative but circumscribed, some cystic or cyst-like with T1 hypointensity and T2/FLAIR hyperintensity (4 cases), and several showed small central cystic areas. Not infrequently there was evidence of remodeling of adjacent calvarium. In the majority of cases (13/18) the tumor was predominantly cortically based and showed both gray and white matter involvement with mild parenchymal expansion; in 2 cases only subcortical white matter was involved and in 1 only gray matter. Associated mass effect was most often minimal to mild, occasionally with mild adjacent vasogenic edema. In 3 cases calcification was present within the tumor (1 evident on computed tomography and 2 evident on MRI). Eight of 17 cases showed gadolinium contrast enhancement: 4/11 of grade II oligodendrogliomas showed enhancement, typically mild or minimal, compared with 4/5 grade III tumors showing enhancement, ranging from punctate, rim, to heterogenous, solid, and intense enhancement.
Pathologic Findings
Thirty-eight tumors (76%) were WHO grade II oligodendrogliomas (Fig. 2), and 12 (24%) were grade III on the basis of increased mitotic rate with or without the presence of microvascular proliferation (Fig. 3). Most anaplastic tumors had 7 or more mitoses per 10 high-power fields. Exceptions included 2 tumors with 4 mitoses per 10 high-power fields but demonstrating necrosis and/or microvascular proliferation.
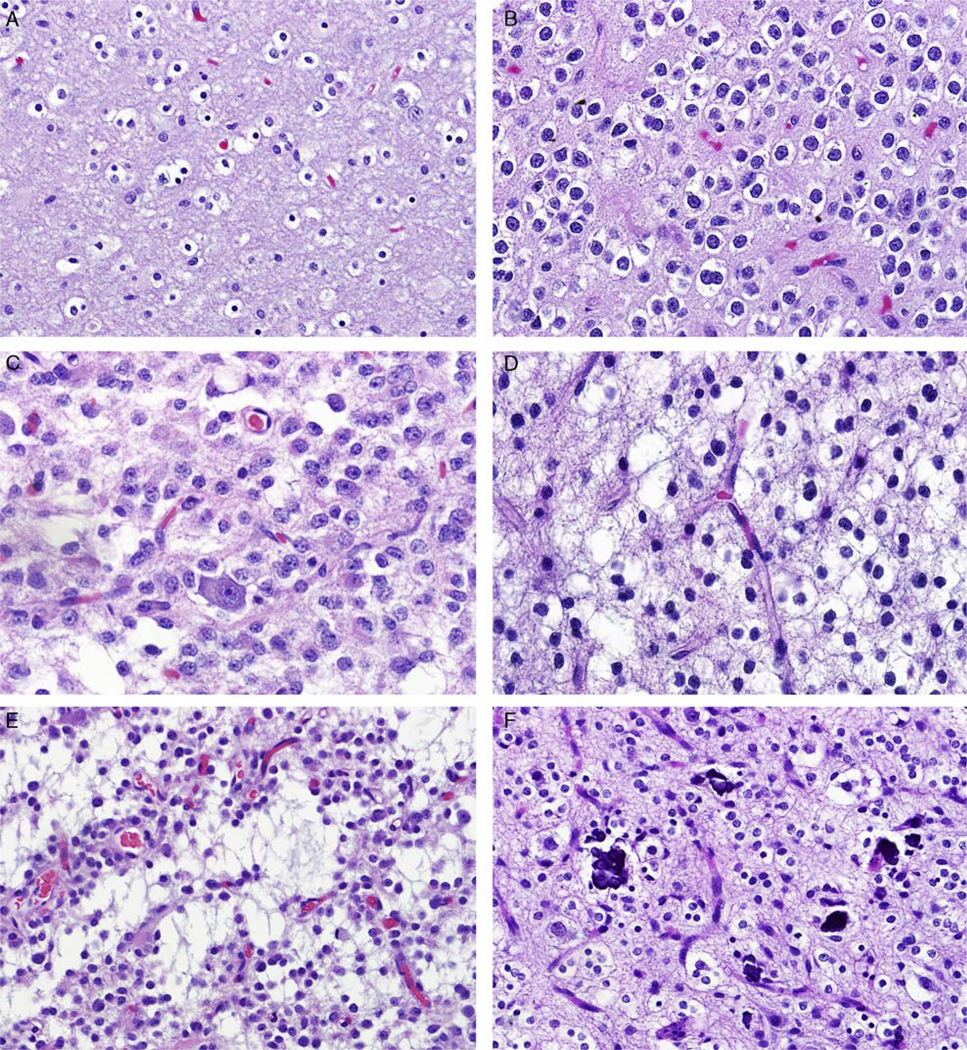
FIGURE 2.
Pediatric low-grade oligodendroglioma (WHO grade II). Most pediatric oligodendrogliomas are low grade and demonstrate features indistinguishable from their adult counterparts, particularly cells with round uniform nuclei with perinuclear halos (A), small nucleolus (B), perineuronal satellitosis (C), and delicate branching capillaries (D). Microcysts (E) and microcalcifications (F) were present in many cases as well.
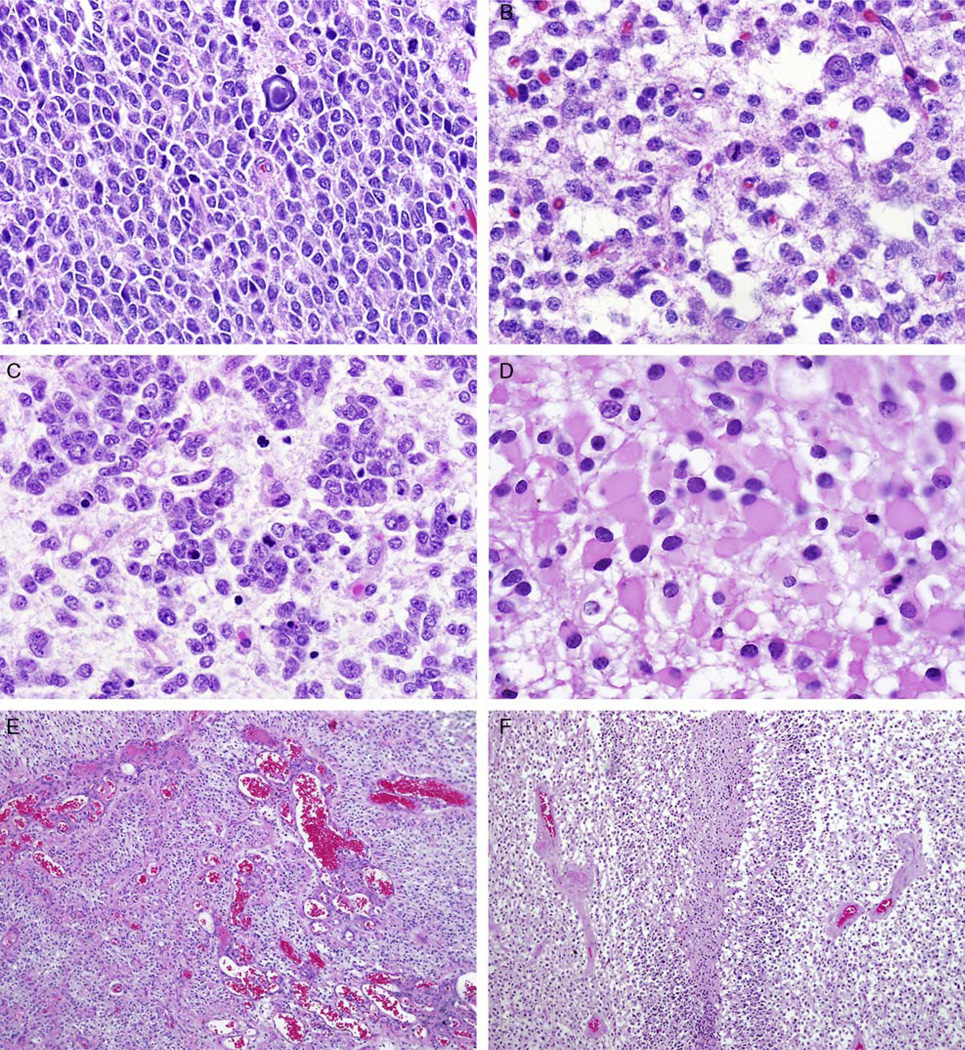
FIGURE 3.
Pediatric anaplastic oligodendroglioma (WHO grade III). A subset of pediatric oligodendrogliomas are anaplastic, particularly at first resection. These tumors are usually hypercellular (A) and contain obvious mitotic figures (B) and cellular areas with apoptotic bodies (C), minigemistocytes (D), and microvascular proliferation (E), and pseudopallisading necrosis may also be encountered (F).
All tumors had a uniform population of round monomorphous cells and often in a “chicken-wire” vascular background. All hemispheric tumors involved the cortex with satellitosis and subpial crowding. Cortical calcifications were present in 19 (of 41) (46%) cases and microcysts in 18 (of 41) (44%). Mitotic activity ranged from 0 to 4 mitoses per 10 high-power fields (median 0) in grade II tumors and 4 to 33 (median 12) in grade III tumors. These findings are summarized in Table 2.
TABLE 2.
Clinicopathologic and Molecular Features of Pediatric Oligodendrogliomas
| N (%) | ||
|---|---|---|
| Grade II Oligodendroglioma (N=38) | Grade III Oligodendroglioma (N=12) | |
| Round nuclei | 38 (100) | 12 (100) |
| Perinuclear halos | 38 (100) | 12 (100) |
| Microcysts | 14 (44) | 4 (40) |
| Microcalcifications | 15 (48) | 4 (40) |
| Secondary structures | 3 (91) | 6 (75) |
| Leptomeningeal extension | 3 (10) | 0 |
| Nodular architecture | 2 (6) | 1 (10) |
| Cortical involvement | 27 (90) | 6 (60) |
| Minigemistocytes | 3 (9) | 1 (10) |
| Gliofibrillary oligodendrocytes | 0 | 1 (10) |
| Microvascular changes | 1 (3) | 7 (70) |
| Necrosis | 1 (3) | 3 (30) |
| GFAP expression | ||
| Positive | 1 (17) | 0 |
| Focal | 2 (33) | 0 |
| S100 expression | ||
| Positive | 3 (100) | NA |
| Synaptophysin expression | ||
| Positive | 1 (13) | 1 (17) |
| Strong p53 immunostaining | 0 | 1 (14) |
| IDH1 (R132H) | ||
| Positive | 3 (19) | 1 (17) |
| 1p19q | ||
| Codeletion | 6 (21) | 4 (36) |
| 1p loss only | 0 | 1 (9) |
| 1p19q intact | 23 (79) | 6 (55) |
Cases Arising in Nonhemispheric Locations
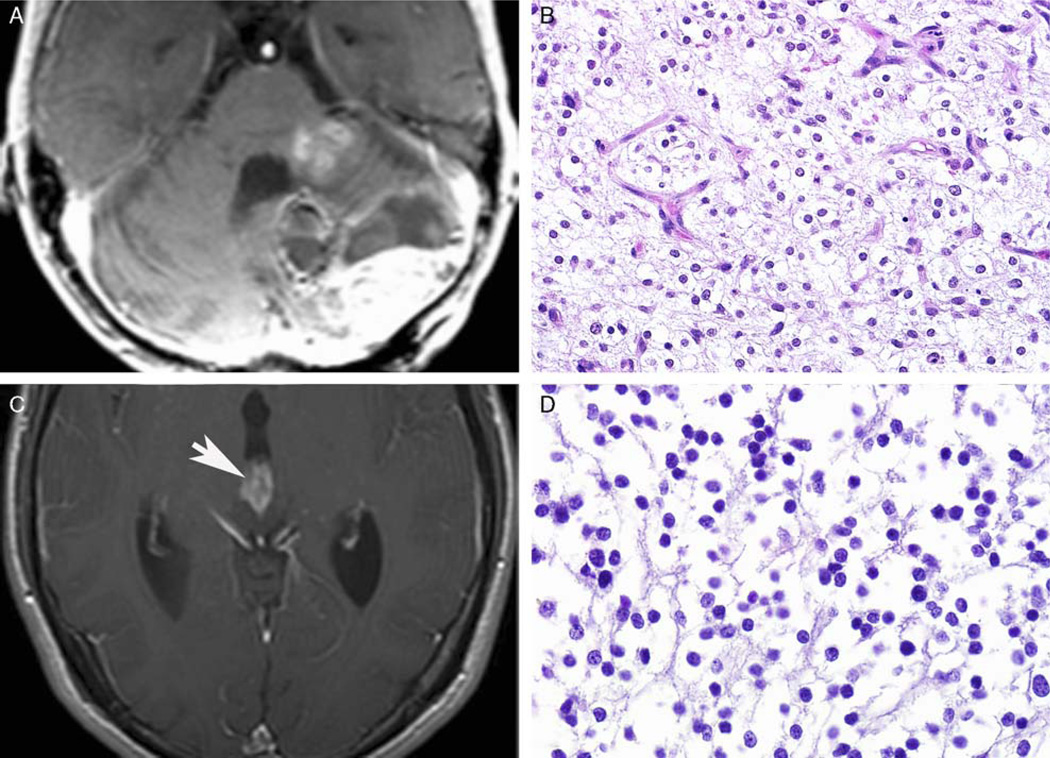
Three patients developed tumors in unusual locations, including a 17-month-old with a brainstem tumor, a 3-year-old with a cerebellar tumor, and a 20-year-old with a disseminated pineal region tumor (Fig. 4). The latter 2 patients died 115 and 18 months after surgery. Both the cerebellar and pineal region tumors were 1p19q codeleted.
FIGURE 4.
Pediatric oligodendrogliomas arising in unusual locations. Rare cases developed in unusual locations, including the cerebellum (A and B) and pineal gland (arrow) (C and D). Both of these tumors demonstrated 1p19q codeletion.
Cases With Sequential Pathologic Specimens
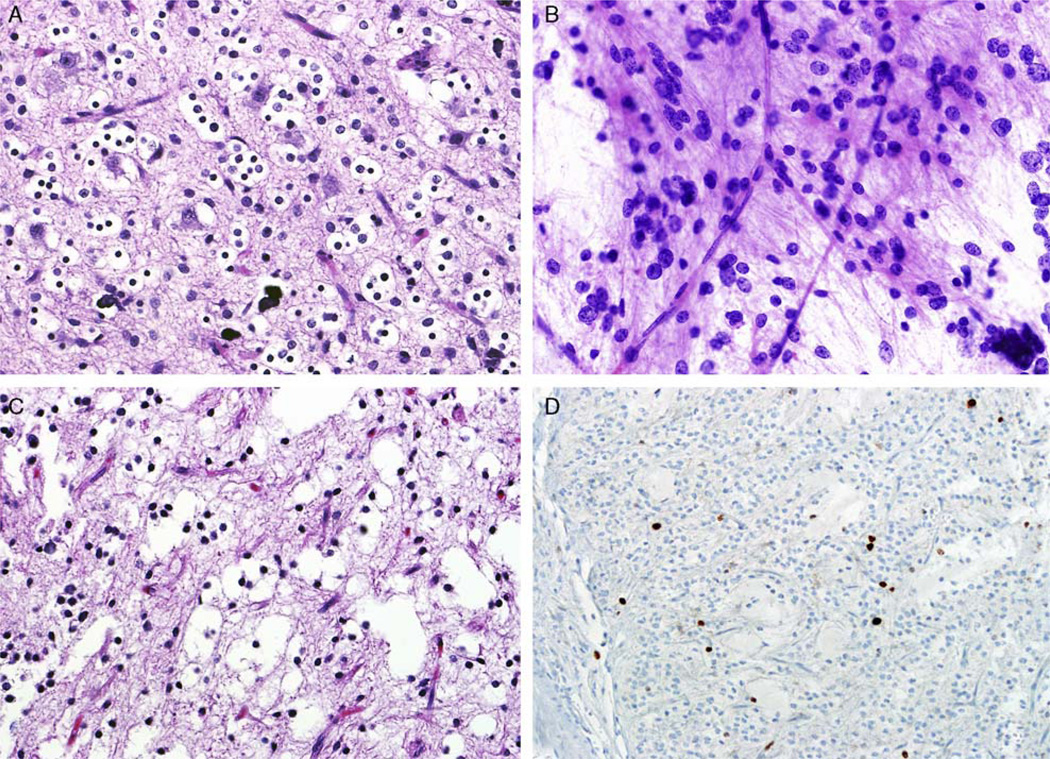
Eight patients had multiple surgical specimens; 4 patients with 2, 3 patients with 3, and 1 patient with 4 specimens (Fig. 5). Six (of 7) patients had grade II tumors, which remained low grade on follow-up surgeries. In the only patient with 4 specimens, subsequent material demonstrated anaplastic features.
FIGURE 5.
Pediatric oligodendroglioma with sequential biopsies. Histopathologic analysis of cases with sequential biopsies demonstrated persistent low-grade features in most of these tumors. For example, the tumor from this patient (case 27) showed classic low-grade oligodendroglial features at first resection (A), as well as 8 years (smear) (B) and 14 years (C) after. Ki67-labeling index in the most current resection remains low (D).
One patient with an anaplastic cerebellar oligodendroglioma underwent a second surgery as well as an autopsy. He developed myelodysplastic syndrome with evolution into acute myeloid leukemia several years after treatment (irradiation, cisplatin, BCNU, and thalidomide). Examination at autopsy demonstrated supratentorial spread of tumor into the right dorsal thalamus and right lateral ventricle as well as multiple infarcts.
Immunohistochemistry
Strong diffuse nuclear staining for p53 was present in only 1 (of 19) (5%) case (case 29). Immunohistochemical staining with GFAP was often weakly positive in tumor cells and was more pronounced in the surrounding reactive neuropil. Only 1 grade II oligodendroglioma showed strong GFAP immunoreactivity in tumor cells (case 50). Immunostaining for MIB1 was quite variable with much higher percentages of positive nuclei in grade III tumors. The staining ranged from <1% to 8% (median 4%) in 12 grade II tumors and 12% to 25% (median 23%) in 4 grade III tumors in which the staining could be performed. Key immunohistochemical features of cases are summarized in Table 2.
1p19q Status and Mutant IDH1 (R132H) Protein
Chromosome 1p19q status was analyzed in 40 tumors. There was no loss in either locus in 29 tumors, combined 1p19q loss in 10 (25%), and an isolated 1p loss in 1 tumor. The tumors with 1p19q codeletion were predominantly hemispheric (frontal lobe n = 7, temporal lobe n = 1, cerebellum n = 1, and pineal gland n = 1). Eight of the 10 tumors were in patients who were older than 15 years, which is the typical cutoff for the pediatric age group. The exceptions were one 11-year-old patient with a right frontal lobe tumor and a 3-year-old with a cerebellar tumor. Two of the cases with 1p19q codeletion and the single case with 1p loss analyzed by SNP array demonstrated the classic whole-arm deletion typical of oligodendroglial neoplasms.
Mutant IDH1 (R132H) protein was detected in 4 of 22 tested cases (18%). Mutated IDH1 (R132H) protein was present in 3 (of 6) cases tested in the 1p19q codeleted group (cases 4, 30, and 39). The 1p19q status was not available in the fourth case with mutant IDH1 (R132H).
Clinical Follow-up
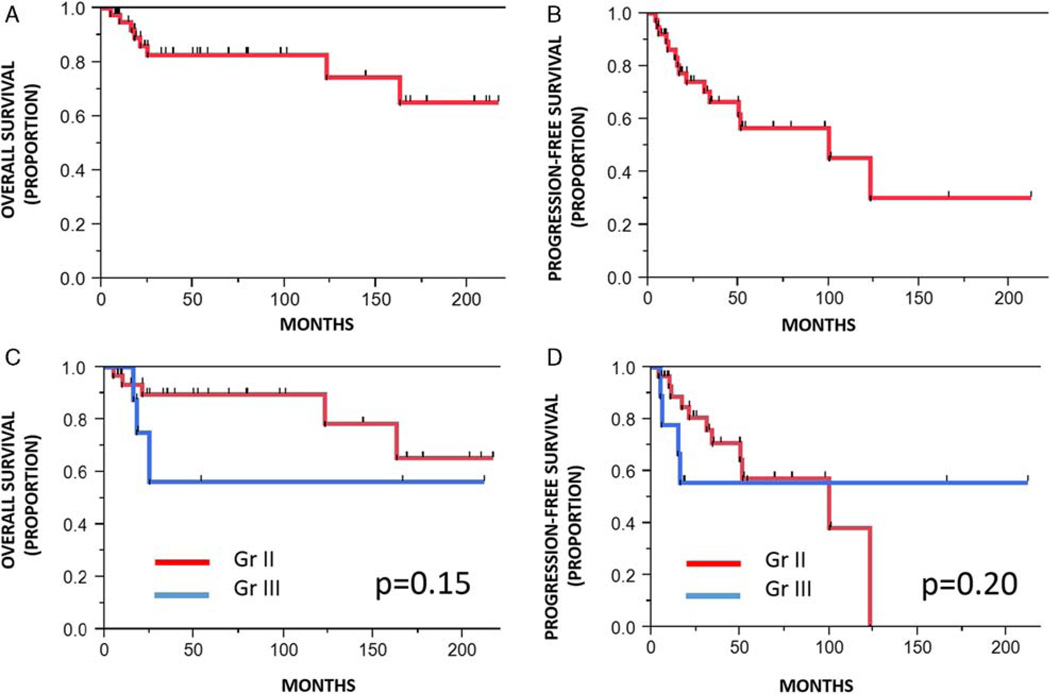
Postoperative follow-up information was available in 45 patients and ranged from 2 to 217 months (median 39 mo). Information on >1 year follow-up was available in 37 patients. At the end of the follow-up period of 38 grade II patients, 10 were alive with no evidence of disease, 14 were alive with disease, 4 alive with unclear status, 4 were dead (5 to 123 mo after surgery, median 15.5 mo), and 6 were lost to follow-up. Among 12 grade III patients, 4 were alive with no evidence of disease, 2 were alive with disease, 5 were dead (16 to 115 mo after surgery, median 18 mo), and 1 patient was lost to follow-up. There was a nonsignificant trend for worse OS in patients with grade III versus grade II tumors (P = 0.15, Wilcoxon) but no trends or significant difference for recurrence-free survival (P = 0.20) (Fig. 6). Although it was not possible to perform formal survival analyses by MIB1-labeling index given the relatively limited follow-up for the group with MIB1 data, 4 (of 5) patients who died during follow-up had an MIB1-labeling index of 12% or more. Conversely, 4 (of 8) tumors that progressed clinically or recurred had MIB1-labeling indices of 8 or less, and 3 had 2% or less. There was no significant difference in OS or PFS by age, extent of resection, or 1p19q status (P > 0.05).
FIGURE 6.
OS and PFS in pediatric oligodendroglioma. Survival analysis in pediatric oligodendroglioma patients demonstrated good OS (A) but decreased PFS (B) in the group as a whole. There was a nonsignificant trend for worse OS in anaplastic tumors compared with low-grade tumors (C), whereas PFS remained similar in both groups (D).
Treatment and Outcome in Institutional Cases
Eleven patients received their ongoing care at Johns Hopkins Hospital, and a more detailed analysis of their treatment and outcome was performed. Treatment for the patients with grade II tumors consisted of resection (gross total resection [GTR], n = 4; subtotal resection [STR], n = 2). One child with an STR went on to receive irradiation and remains alive with persistent imaging abnormalities. All but 1 child with a GTR and a grade II tumor had recurrence of disease.
Grade III tumor treatment included resection (GTR, n = 1; STR, n = 4), of whom 1 child went on to receive irradiation, and 5 went on to receive irradiation/chemotherapy. Of the 5 who received adjuvant chemotherapy, all regimens included alkylator-based therapy (eg, CCNU, procarbazine, BCNU, cyclophosphamide, temozolomide). Two patients received PCV (procarbazine, lomustine, vincristine) therapy, which has been shown to be an effective regimen to increase both OS and PFS in adult anaplastic oligodendroglioma.27 Neither of these patients had 1p19q codeletion, and both progressed early in therapy. Two patients with grade III tumors in this series had 1p19q codeletion, but despite treatment with alkylating agents, both patients recurred early in their disease course and are deceased. With the exception of 1 child who is early in follow-up, all other children with grade III tumors are deceased. Because of the small patient size and the variability of treatment regimens, we cannot determine the efficacy of adjuvant treatment in our study. Although there was a suggestion that patients with grade II tumors fared better than those with grade III tumors, recurrence or progression was common in both groups.
DISCUSSION
From both the present series and previously reported cases, pediatric oligodendrogliomas emerge as a histologic entity without a unifying molecular signature. Our findings support the suggestion that there may be >1 molecular pathway.6,10
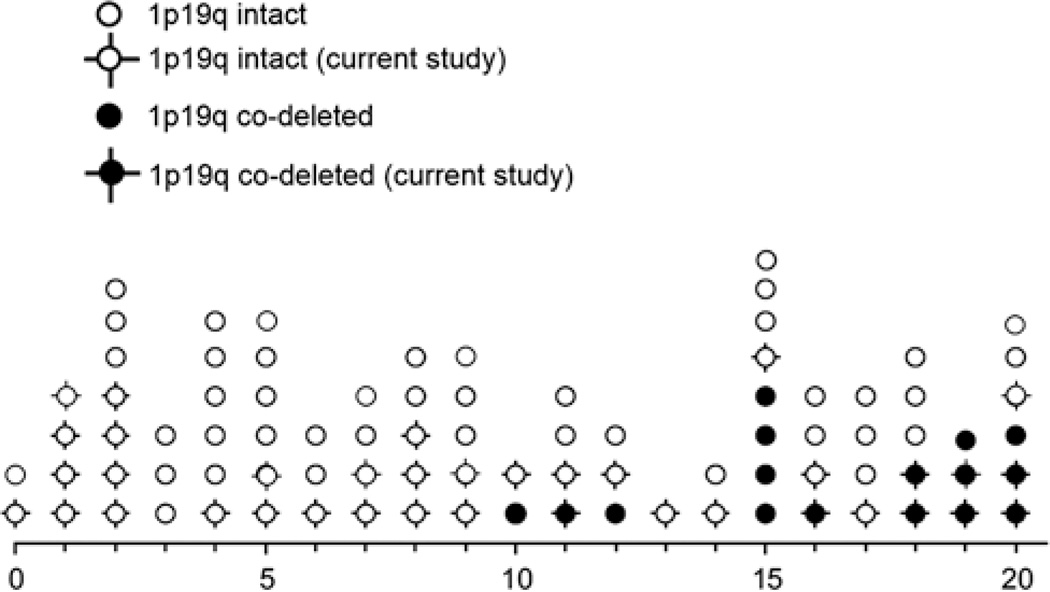
One group includes tumors without the typical 1p19q codeletion, yet a classical histologic appearance of oligodendroglioma. These tumors were predominantly in the true pediatric age group, that is, 0 to 15 years: they may be referred to as the “childhood-type” of pediatric oligodendroglioma. In contrast, codeletions were present in older children and adolescents, with 2 exceptions that were 3 (cerebellar) and 11 (cerebral hemispheric) years old at presentation, and therefore this could represent the “adult-type” of pediatric oligodendroglioma. The distribution in our series is thus similar to the findings of Raghavan and colleagues who found no codeletions in 15 patients 0 to 9 years of age but in 3 patients 10 to 18 years of age. Similarly, Suri et al13 reported no codeletions in 7 patients 18 years of age or under but in 4 of 7 patients between 19 and 25 years of age. Zhang et al20 in a recent sequencing study of pediatric low-grade gliomas found 1 case, age 15, with a codeletion and a mutation in CIC, a molecular abnormality present in most codeleted adult oligodendrogliomas.15 Other series, in contrast, found no codeletions, even in older children.6,7,28 Considering all the reported cases to date, it is fair to suggest that tumors with the classical histologic appearance of oligodendroglioma in the pediatric population (ie, 0 to 15 y) do not typically harbor 1p19q codeletions (Fig. 7). Whether oligodendrogliomas of this “childhood” type can present initially in adulthood is not clear. Codeletions in adult oligodendrogliomas develop through a translocation (1;19)q10;p10).29,30 Whether this mechanism is operational in children remains to be determined, but SNP array analysis of 2 of our codeleted cases revealed whole-arm deletions as would be expected with such a translocation.
FIGURE 7.
Age distribution and frequency of 1p19q codeletion in pediatric oligodendroglioma. Pediatric oligodendroglioma 1p19q codeleted status by age in the literature and the current series. Pediatric oligodendrogliomas in our series arising in unusual locations (ie, cerebellum, pineal gland, brainstem, n = 3) are excluded.
Little is known about the IDH1/2 status of pediatric oligodendrogliomas and its relation to 1p19q codeletion, other than the fact that mutations seem to be uncommon in most pediatric low-grade gliomas. Immunohistochemical positivity against the most frequent IDH1 mutant protein (R132H) is present in approximately 80% to 90% of adult oligodendrogliomas in the literature but in only 8% in our series. As might be predicted, all 4 IDH1 R132H-positive tumors were from older patients (ages 16 to 19 y), and 3 of these also showed 1p19q codeletions. It thus appears that IDH1 mutations may be associated with 1p19q codeletions as they are in adults and are more common in tumors in older children, as has been reported in malignant gliomas.31
The relation of outcome to grade is also unclear, but we found a trend to shorter OS in the grade III tumors when the 50 tumors at all sites were considered, as well as when the 3 tumors arising in unusual sites were excluded. Wang et al14 noted that all cases with anaplasia (n = 4) or positive cerebrospinal fluid cytology had a poor outcome. Three (of 10) anaplastic tumors arising in typical locations in our series were also ultimately lethal, and an additional one recurred. An interesting observation by Raghavan10 was that 2 grade III codeleted tumors with polysomies were resistant to treatment, as has been noted in adult oligodendrogliomas.32 Rizk et al12 noted that children with oligodendrogliomas presenting with consequences of mass effect had anaplastic tumors and poor OS, whereas those presenting with seizures had “benign,” presumably grade II, tumors with excellent outcomes.
Although there is a significant difference in outcome between 1p19q codeleted and non-codeleted oligodendrogliomas in adults, the more favorable outlook associated with the 1p19q codeletion has been stated not to be present in children.33 This could be true; however, a publication making this assertion included a broad range of high-grade pediatric gliomas, not just oligodendrogliomas,33 and its applicability to the latter tumors is therefore at issue. In our study and review of the literature, the non-codeleted oligodendrogliomas in children may be equally or even less aggressive than the deleted types. Clinical and histologic contexts thus need to be considered in interpreting the significance of genetic features. For example, glioblastomas in adults with 1p19q codeletion are not associated with improved survival.34,35 Diffuse astrocytomas in children are considerably more benign and less prone to anaplastic transformation than their adult counterparts.36 This same issue arises in adult, non-codeleted oligodendrogliomas, the percentage of which decreases as diagnostic stringency and percentage of assenting reviewers increase.37
Current models in brain tumor biology suggest that tumor location and relation to molecular features, patient age, and biological behavior may shed some light on cytogenesis. Specifically, the relation between codeletion and IDH1/2 status might also explain, or at least correlate with, the preference for the frontal lobe. If the tumors of the pineal region, brainstem, and cerebellum in our series were excluded, there were 14 hemispheric cases of age 15 years or greater, with 7 being codeleted. There was also one 11-year-old with a codeletion. If these are combined, 10 of the resulting 15 (67%) cases were located in a frontal lobe, a figure in accord with distribution of adult codeleted oligodendrogliomas.38 Of the remaining 26 cases less than 15 years of age and not codeleted, only 10 (38%) were frontal. Adult oligodendrogliomas with 1p loss are also more likely to be frontal.38,39 In adults, lower-grade IDH-mutant diffuse astrocytomas are more common in the frontal lobe than tumors with wild-type IDH,40 and IDH-mutant, secondary glioblastomas favor the frontal lobe as well.41
Although usually hemispheric, pediatric oligodendrogliomas occasionally arise elsewhere, including cerebellum/posterior fossa,5,6,8,9,14 brainstem,6,9,42,43 thalamus/ basal ganglia,1,9,14 and spinal cord.14 We had 1 case each in the pineal region, cerebellum, and brainstem. The latter 2 were anaplastic. The molecular features of these unusually situated oligodendrogliomas have been variable. Some are codeleted (our cases 40 and 42 and those in Furtado et al5 and Hewer et al42), and some are not (our case 37 and those in Furtado et al5 and Kreiger et al6). Of our codeleted grade III examples, the single pineal region and cerebellar tumors were certainly aggressive and ultimately lethal. Other series also noted aggressive behavior with grade III cerebellar lesions.8
Any study of pediatric oligodendrogliomas must confront the issue of DNT, a lesion whose relationship to oligodendroglioma remains to be defined. It certainly can be difficult to distinguish the 2, especially in small fragmented specimens. Although classified as glioneuronal, DNT’s alleged neuronal qualities have been difficult to substantiate, especially with regard to the small, infiltrating oligodendroglioma-like cells. Synaptophysin staining is variable, at best, and is often reported as only focal.44 The most consistent immunopositivity is for OLIG1 or OLIG2, the latter a frequently used glial marker.45,46 It has been suggested that DNTs are grade I oligodendrogliomas,46 a designation not recognized by the WHO classification. A related interpretation is that grade I oligodendroglioma might lie within the DNT spectrum.47 The neoplastic versus reactive nature of the large “floating” neurons is an issue as well, although they seem likely to be trapped preexisting cortical ganglion cells.46 The complexities and uncertainties of the oligodendroglioma versus DNT issue are apparent in a series that included 14 DNTs, of which 2 were felt to have both classic cortical DNT and underlying grade II oligodendroglioma.48 Both components were 1p19q codeleted in both cases. DNTs with 1p19q codeletion are reported by others as well.44 Whatever the relationship between pediatric oligodendroglioma and DNT, we were mindful of histologic similarities and made use of radiologic, pathologic, and clinical features to minimize the chance of including DNTs. Given definitional issues and ambiguities, however, it is difficult to be assured that we were successful.
Another lesion of uncertain relation to oligodendroglioma is an oligodendroglioma-like disseminated leptomeningeal tumor that is often associated with a spinal intramedullary component.49–53 Also OLIG2 positive, it has in some cases clear neuronal differentiation, although this in itself does not necessarily exclude oligodendroglioma as the parent neoplasm. A frequent isolated 1p deletion appears to individualize the disseminated tumor; however, some such leptomeningeal lesions have the 1p19q codeletion typical of adult oligodendroglioma.51–54 Mutant IDH1/2 immunostaining has been negative.52,53 We did not include this type of tumor in the present study.
In conclusion, there appear to be 2 types of pediatric oligodendrogliomas each needing further study. It remains to be established for the “adult” types whether the genes CIC and FUBP1 are mutated and that the translocation that maintains the codeleted state is present, although there seems no reason why this should not be the case. The molecular basis of the “childhood” type is less clear, and awaits further molecular characterization.
Acknowledgments
Source of Funding: Funded in part by the Pilocytic Pilomyxoid Research Fund, including Lauren’s First and Goal (P.C.B., F.J.R.), and the Childhood Brain Tumor Foundation (F.J.R.), as well as the Bruce T Halle Family Foundation, Barrow Neurological Foundation, and NIH CA 13525 (B.F.).
Footnotes
Conflicts of Interest: The authors have disclosed that they have no significant relationships with, or financial interest in, any commercial companies pertaining to this article.
REFERENCES
- 1.Bowers DC, Mulne AF, Weprin B, et al. Prognostic factors in children and adolescents with low-grade oligodendrogliomas. Pediatr Neurosurg. 2002;37:57–63. doi: 10.1159/000065106. [DOI] [PubMed] [Google Scholar]
- 2.Bruggers C, White K, Zhou H, et al. Extracranial relapse of an anaplastic oligodendroglioma in an adolescent: case report and review of the literature. J Pediatr Hematol Oncol. 2007;29:319–322. doi: 10.1097/MPH.0b013e318054756e. [DOI] [PubMed] [Google Scholar]
- 3.Dohrmann GJ, Farwell JR, Flannery JT. Oligodendrogliomas in children. Surg Neurol. 1978;10:21–25. [PubMed] [Google Scholar]
- 4.Favier J, Pizzolato GP, Berney J. Oligodendroglial tumors in childhood. Childs Nerv Syst. 1985;1:33–38. doi: 10.1007/BF00706728. [DOI] [PubMed] [Google Scholar]
- 5.Furtado SV, Venkatesh PK, Ghosal N, et al. Clinical and radiological features of pediatric cerebellar anaplastic oligodendrogliomas. Indian J Pediatr. 2011;78:880–883. doi: 10.1007/s12098-010-0318-4. [DOI] [PubMed] [Google Scholar]
- 6.Kreiger PA, Okada Y, Simon S, et al. Losses of chromosomes 1p and 19q are rare in pediatric oligodendrogliomas. Acta Neuropathol. 2005;109:387–392. doi: 10.1007/s00401-004-0976-2. [DOI] [PubMed] [Google Scholar]
- 7.Myal Y, Del Bigio MR, Rhodes RH. Age-related differences in 1p and 19q deletions in oligodendrogliomas. BMC Clin Pathol. 2003;3:6. doi: 10.1186/1472-6890-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Packer RJ, Sutton LN, Rorke LB, et al. Oligodendroglioma of the posterior fossa in childhood. Cancer. 1985;56:195–199. doi: 10.1002/1097-0142(19850701)56:1<195::aid-cncr2820560133>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 9.Peters O, Gnekow AK, Rating D, et al. Impact of location on outcome in children with low-grade oligodendroglioma. Pediatr Blood Cancer. 2004;43:250–256. doi: 10.1002/pbc.20111. [DOI] [PubMed] [Google Scholar]
- 10.Raghavan R, Balani J, Perry A, et al. Pediatric oligodendrogliomas: a study of molecular alterations on 1p and 19q using fluorescence in situ hybridization. J Neuropathol Exp Neurol. 2003;62:530–537. doi: 10.1093/jnen/62.5.530. [DOI] [PubMed] [Google Scholar]
- 11.Razack N, Baumgartner J, Bruner J. Pediatric oligodendrogliomas. Pediatr Neurosurg. 1998;28:121–129. doi: 10.1159/000028635. [DOI] [PubMed] [Google Scholar]
- 12.Rizk T,Mottolese C, Bouffet E, et al. Cerebral oligodendrogliomas in children: an analysis of 15 cases. Childs Nerv Syst. 1996;12:527–529. doi: 10.1007/BF00261605. [DOI] [PubMed] [Google Scholar]
- 13.Suri V, Jha P, Agarwal S, et al. Molecular profile of oligodendrogliomas in young patients. Neuro-oncol. 2011;13:1099–1106. doi: 10.1093/neuonc/nor146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang KC, Chi JG, Cho BK. Oligodendroglioma in childhood. J Korean Med Sci. 1993;8:110–116. doi: 10.3346/jkms.1993.8.2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiao Y, Killela PJ, Reitman ZJ, et al. Frequent ATRX CIC, FUBP1 and IDH1 mutations refine the classification of malignant gliomas. Oncotarget. 2012;3:709–722. doi: 10.18632/oncotarget.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Camelo-Piragua S, Jansen M, Ganguly A, et al. A sensitive and specific diagnostic panel to distinguish diffuse astrocytoma from astrocytosis: chromosome 7 gain with mutant isocitrate dehydrogenase 1 and p53. J Neuropathol Exp Neurol. 2011;70:110–115. doi: 10.1097/NEN.0b013e31820565f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kannan K, Inagaki A, Silber J, et al. Whole-exome sequencing identifies ATRX mutation as a key molecular determinant in lower-grade glioma. Oncotarget. 2012;3:1194–1203. doi: 10.18632/oncotarget.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu XY, Gerges N, Korshunov A, et al. Frequent ATRX mutations and loss of expression in adult diffuse astrocytic tumors carrying IDH1/IDH2 and TP53 mutations. Acta Neuropathol. 2012;124:615–625. doi: 10.1007/s00401-012-1031-3. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen DN, Heaphy CM, de Wilde RF, et al. Molecular and morphologic correlates of the alternative lengthening of telomeres phenotype in high-grade astrocytomas. Brain Pathol. 2013;23:237–243. doi: 10.1111/j.1750-3639.2012.00630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang J, Wu G, Miller CP, et al. Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nat Genet. 2013;45:602–612. doi: 10.1038/ng.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramkissoon LA, Horowitz PM, Craig JM, et al. Genomic analysis of diffuse pediatric low-grade gliomas identifies recurrent oncogenic truncating rearrangements in the transcription factor MYBL1. Proc Natl Acad Sci USA. 2013;110:8188–8193. doi: 10.1073/pnas.1300252110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Brien DF, Farrell M, Delanty N, et al. The Children’s Cancer and Leukaemia Group guidelines for the diagnosis and management of dysembryoplastic neuroepithelial tumours. Br J Neurosurg. 2007;21:539–549. doi: 10.1080/02688690701594817. [DOI] [PubMed] [Google Scholar]
- 23.Harada S, Henderson LB, Eshleman JR, et al. Genomic changes in gliomas detected using single nucleotide polymorphism array in formalin-fixed, paraffin-embedded tissue: superior results compared with microsatellite analysis. J Mol Diagn. 2011;13:541–548. doi: 10.1016/j.jmoldx.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hatanpaa KJ, Burger PC, Eshleman JR, et al. Molecular diagnosis of oligodendroglioma in paraffin sections. Lab Invest. 2003;83:419–428. doi: 10.1097/01.lab.0000059948.67795.ef. [DOI] [PubMed] [Google Scholar]
- 25.Jenkins RB, Curran W, Scott CB, et al. Pilot evaluation of 1p and 19q deletions in anaplastic oligodendrogliomas collected by a national cooperative cancer treatment group. Am J Clin Oncol. 2001;24:506–508. doi: 10.1097/00000421-200110000-00018. [DOI] [PubMed] [Google Scholar]
- 26.Nigro JM, Takahashi MA, Ginzinger DG, et al. Detection of 1p and 19q loss in oligodendroglioma by quantitative microsatellite analysis, a real-time quantitative polymerase chain reaction assay. Am J Pathol. 2001;158:1253–1262. doi: 10.1016/S0002-9440(10)64076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van den Bent MJ, Brandes AA, Taphoorn MJ, et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol. 2013;31:344–350. doi: 10.1200/JCO.2012.43.2229. [DOI] [PubMed] [Google Scholar]
- 28.Padovani L, Colin C, Fernandez C, et al. Search for distinctive markers in DNT and cortical grade II glioma in children: same clinicopathological and molecular entities? Curr Top Med Chem. 2012;12:1683–1692. doi: 10.2174/156802612803531450. [DOI] [PubMed] [Google Scholar]
- 29.Griffin CA, Burger P, Morsberger L, et al. Identification of deR1;19)(q10;p10) in five oligodendrogliomas suggests mechanism of concurrent 1p and 19q loss. J Neuropathol Exp Neurol. 2006;65:988–994. doi: 10.1097/01.jnen.0000235122.98052.8f. [DOI] [PubMed] [Google Scholar]
- 30.Jenkins RB, Blair H, Ballman KV, et al. A t(1;19)(q10;p10) mediates the combined deletions of 1p and 19q and predicts a better prognosis of patients with oligodendroglioma. Cancer Res. 2006;66:9852–9861. doi: 10.1158/0008-5472.CAN-06-1796. [DOI] [PubMed] [Google Scholar]
- 31.Pollack IF, Hamilton RL, Sobol RW, et al. IDH1 mutations are common in malignant gliomas arising in adolescents: a report from the Children’s Oncology Group. Childs Nerv Syst. 2011;27:87–94. doi: 10.1007/s00381-010-1264-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Snuderl M, Eichler AF, Ligon KL, et al. Polysomy for chromosomes 1 and 19 predicts earlier recurrence in anaplastic oligodendrogliomas with concurrent 1p/19q loss. Clin Cancer Res. 2009;15:6430–6437. doi: 10.1158/1078-0432.CCR-09-0867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pollack IF, Finkelstein SD, Burnham J, et al. Association between chromosome 1p and 19q loss and outcome in pediatric malignant gliomas: results from the CCG-945 cohort. Pediatr Neurosurg. 2003;39:114–121. doi: 10.1159/000071647. [DOI] [PubMed] [Google Scholar]
- 34.Boots-Sprenger SH, Sijben A, Rijntjes J, et al. Significance of complete 1p/19q co-deletion, IDH1 mutation and MGMT promoter methylation in gliomas: use with caution. Mod Pathol. 2013;26:922–929. doi: 10.1038/modpathol.2012.166. [DOI] [PubMed] [Google Scholar]
- 35.Clark KH, Villano JL, Nikiforova MN, et al. 1p/19q testing has no significance in the workup of glioblastomas. Neuropathol Appl Neurobiol. 2013;39:706–717. doi: 10.1111/nan.12031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pollack IF, Claassen D, al-Shboul Q, et al. Low-grade gliomas of the cerebral hemispheres in children: an analysis of 71 cases. J Neurosurg. 1995;82:536–547. doi: 10.3171/jns.1995.82.4.0536. [DOI] [PubMed] [Google Scholar]
- 37.McDonald JM, See SJ, Tremont IW, et al. The prognostic impact of histology and 1p/19q status in anaplastic oligodendroglial tumors. Cancer. 2005;104:1468–1477. doi: 10.1002/cncr.21338. [DOI] [PubMed] [Google Scholar]
- 38.Zlatescu MC, TehraniYazdi A, Sasaki H, et al. Tumor location and growth pattern correlate with genetic signature in oligodendroglial neoplasms. Cancer Res. 2001;61:6713–6715. [PubMed] [Google Scholar]
- 39.Laigle-Donadey F, Martin-Duverneuil N, Lejeune J, et al. Correlations between molecular profile and radiologic pattern in oligodendroglial tumors. Neurology. 2004;63:2360–2362. doi: 10.1212/01.wnl.0000148642.26985.68. [DOI] [PubMed] [Google Scholar]
- 40.Gorovets D, Kannan K, Shen R, et al. IDH mutation and neuroglial developmental features define clinically distinct subclasses of lower grade diffuse astrocytic glioma. Clin Cancer Res. 2012;18:2490–2501. doi: 10.1158/1078-0432.CCR-11-2977. [DOI] [PubMed] [Google Scholar]
- 41.Lai A, Kharbanda S, Pope WB, et al. Evidence for sequenced molecular evolution of IDH1 mutant glioblastoma from a distinct cell of origin. J Clin Oncol. 2011;29:4482–4490. doi: 10.1200/JCO.2010.33.8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hewer E, Beck J, Vassella E, et al. Anaplastic oligodendroglioma arising from the brain stem and featuring 1p/19q co-deletion. Neuropathology. 2013;34:32–38. doi: 10.1111/neup.12043. [DOI] [PubMed] [Google Scholar]
- 43.Mittelbronn M, Wolff M, Bultmann E, et al. Disseminating anaplastic brainstem oligodendroglioma associated with allelic loss in the tumor suppressor candidate region D19S246 of chromosome 19 mimicking an inflammatory central nervous system disease in a 9-year-old boy. Hum Pathol. 2005;36:854–857. doi: 10.1016/j.humpath.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 44.Thom M, Toma A, An S, et al. One hundred and one dysembryoplastic neuroepithelial tumors: an adult epilepsy series with immunohistochemical, molecular genetic, and clinical correlations and a review of the literature. J Neuropathol Exp Neurol. 2011;70:859–878. doi: 10.1097/NEN.0b013e3182302475. [DOI] [PubMed] [Google Scholar]
- 45.Azzarelli B, Miravalle L, Vidal R. Immunolocalization of the oligodendrocyte transcription factor 1 (Olig1) in brain tumors. J Neuropathol Exp Neurol. 2004;63:170–179. doi: 10.1093/jnen/63.2.170. [DOI] [PubMed] [Google Scholar]
- 46.Komori T, Arai N. Dysembryoplastic neuroepithelial tumor, a pure glial tumor? Immunohistochemical and morphometric studies. Neuropathology. 2013;33:459–468. doi: 10.1111/neup.12033. [DOI] [PubMed] [Google Scholar]
- 47.Takahashi H, Kakita A, Tomikawa M, et al. Oligodendroglioma (WHO grade I) in a young epilepsy patient: a specific entity lying within the spectrum of dysembryoplastic neuroepithelial tumor? Neuropathology. 2013;33:645–651. doi: 10.1111/neup.12026. [DOI] [PubMed] [Google Scholar]
- 48.Gonzales M, Dale S, Susman M, et al. Dysembryoplastic neuroepithelial tumor (DNT)-like oligodendrogliomas or Dnts evolving into oligodendrogliomas: two illustrative cases. Neuropathology. 2007;27:324–330. doi: 10.1111/j.1440-1789.2007.00783.x. [DOI] [PubMed] [Google Scholar]
- 49.Agamanolis DP, Katsetos CD, Klonk CJ, et al. An unusual form of superficially disseminated glioma in children: report of 3 cases. J Child Neurol. 2012;27:727–733. doi: 10.1177/0883073811426500. [DOI] [PubMed] [Google Scholar]
- 50.Gardiman MP, Fassan M, Nozza P, et al. Diffuse leptomeningeal glioneuronal tumours: clinico-pathological follow-up. Pathologica. 2012;104:428–431. [PubMed] [Google Scholar]
- 51.Rhiew RB, Manjila S, Lozen A, et al. Leptomeningeal dissemination of a pediatric neoplasm with 1p19q deletion showing mixed immunohistochemical features of an oligodendroglioma and neurocytoma. Acta Neurochir (Wien) 2010;152:1425–1429. doi: 10.1007/s00701-010-0674-x. [DOI] [PubMed] [Google Scholar]
- 52.Rodriguez FJ, Perry A, Rosenblum MK, et al. Disseminated oligodendroglial-like leptomeningeal tumor of childhood: a distinctive clinicopathologic entity. Acta Neuropathol. 2012;124:627–641. doi: 10.1007/s00401-012-1037-x. [DOI] [PubMed] [Google Scholar]
- 53.Schniederjan MJ, Alghamdi S, Castellano-Sanchez A, et al. Diffuse leptomeningeal neuroepithelial tumor: 9 pediatric cases with chromosome 1p/19q deletion status and IDH1 (R132H) immunohistochemistry. Am J Surg Pathol. 2013;37:763–771. doi: 10.1097/PAS.0b013e31827bf4cc. [DOI] [PubMed] [Google Scholar]
- 54.Rossi S, Rodriguez FJ, Mota RA, et al. Primary leptomeningeal oligodendroglioma with documented progression to anaplasia and t(1;19)(q10;p10) in a child. Acta Neuropathol. 2009;118:575–577. doi: 10.1007/s00401-009-0565-5. [DOI] [PubMed] [Google Scholar]