Abstract
Purpose
Long-term survival rates for patients with resected pancreatic ductal adenocarcinoma (PDAC) have stagnated at 20% for more than a decade, demonstrating the need to develop novel adjuvant therapies. Gemcitabine-erlotinib therapy has demonstrated a survival benefit for patients with metastatic PDAC. Here we report the first phase 2 study of erlotinib in combination with adjuvant chemoradiation and chemotherapy for resected PDAC.
Methods and Materials
Forty-eight patients with resected PDAC received adjuvant erlotinib (100 mg daily) and capecitabine (800 mg/m2 twice daily Monday-Friday) concurrently with intensity modulated radiation therapy (IMRT), 50.4 Gy over 28 fractions followed by 4 cycles of gemcitabine (1000 mg/m2 on days 1, 8, and 15 every 28 days) and erlotinib (100 mg daily). The primary endpoint was recurrence-free survival (RFS).
Results
The median follow-up time was 18.2 months (interquartile range, 13.8–27.1). Lymph nodes were positive in 85% of patients, and margins were positive in 17%. The median RFS was 15.6 months (95% confidence interval [CI], 13.4–17.9), and the median overall survival (OS) was 24.4 months (95% CI, 18.9–29.7). Multivariate analysis with adjustment for known prognostic factors showed that tumor diameter >3 cm was predictive for inferior RFS (hazard ratio, 4.01; P = .001) and OS (HR, 4.98; P = .02), and the development of dermatitis was associated with improved RFS (HR, 0.27; P = .009). During CRT and post-CRT chemotherapy, the rates of grade 3/4 toxicity were 31%/2% and 35%/8%, respectively.
Conclusion
Erlotinib can be safely administered with adjuvant IMRT-based CRT and chemotherapy. The efficacy of this regimen appears comparable to that of existing adjuvant regimens. Radiation Therapy Oncology Group 0848 will ultimately determine whether erlotinib produces a survival benefit in patients with resected pancreatic cancer.
Introduction
Pancreatic cancer remains the fourth leading cause of cancer death in the United States (1). Surgical resection is potentially curative; however, ~70% of surgical patients experience recurrence in the first 2 years and succumb to their disease (2), and 5-year overall survival remains poor at 20% (3). Adjuvant therapy improves outcomes, but the optimal postoperative treatment remains controversial. Although several studies have shown a survival benefit for adjuvant chemoradiation therapy (CRT) (3), others have shown comparable outcomes for chemotherapy alone (4). Some have suggested that upfront chemoradiation after surgery may delay high-dose systemic therapy and result in worse survival. Adding novel targeted agents to concurrent 5-fluorouracil-based CRT may help address this issue, if such agents can inhibit metastasis, enhance local control, or both.
Amplification of the epidermal growth factor receptor (EGFR) gene and overexpression of the EGFR surface protein have been described in up to 60% of pancreatic tumors (5), making EGFR an attractive therapeutic target. A randomized phase 3 trial demonstrated superior survival in patients with metastatic pancreatic cancer treated with gemcitabine and erlotinib versus gemcitabine alone (6), leading to U. S. Food and Drug Administration approval of erlotinib for the treatment of pancreatic ductal adenocarcinoma (PDAC). Preclinical data indicate that 1 mechanism of neoplastic cell resistance to radiation therapy is through paracrine activation of EGFR by transforming growth factor alpha (TGF-α), which is released after radiation exposure. EGFR blocks the antiapoptotic effects of TGF-α shedding and restores the apoptotic response of tumor cells to radiation (7).
These preclinical findings, along with the positive results of the phase 3 trial of gemcitabine and erlotinib in metastatic disease, provide a strong rationale to test the adjuvant combination of erlotinib, chemoradiation, and chemotherapy. We previously reported phase 1 results demonstrating that concurrent erlotinib (100 mg daily) with capecitabine and intensity modulated radiation therapy (IMRT) as adjuvant therapy for resected PDAC was feasible and safe (8). Here we report the results of a phase 2 trial evaluating the safety and efficacy of erlotinib combined with adjuvant CRT and chemotherapy for resected PDAC.
Methods and Materials
Enrollment and eligibility
Patients with histologically confirmed stage I/II PDAC who underwent surgical resection at our institution without prior chemotherapy or radiation therapy were enrolled in this study. Eligibility criteria also included age 18 years or older; Eastern Cooperative Oncology Group performance status 0–1, and adequate bone marrow/liver/kidney function. Exclusion criteria included metastasis, other malignancies diagnosed within 5 years, previous chemotherapy for pancreatic cancer, previous abdominal radiation therapy, and incomplete postoperative healing. The study protocol was approved by the institutional review board, and all patients provided written informed consent before study enrollment.
Treatment intervention and toxicity assessment
Beginning 4 to 12 weeks after surgery, eligible patients received adjuvant erlotinib (100 mg daily), capecitabine (800 mg/m2 twice daily, Monday-Friday), and IMRT. Eight patients received this regimen as part of a phase 1 trial (8) and are included in this report despite receiving a higher dose of erlotinib (150 mg/day) and 7 days of capecitabine instead of 5. The total radiation dose was 50.4 Gy in 28 fractions (1.8 Gy/fraction). All patients received 45 Gy to an initial planning target volume (PTV1) that included the pancreatic tumor bed and adjacent lymph nodes. An additional 5.4 Gy was given to a boost volume (PTV2) including the tumor bed plus 1 to 1.5 cm. Dose-limiting structures included the liver (50% <30 Gy), kidney (66% of 1 kidney <18 Gy), and spinal cord (maximum dose 45 Gy). Radiation doses to the bowel and stomach were limited to the extent possible. The PTV was covered by the 95% isodose line, and any hot spots greater than 10% of the prescribed dose were avoided. Four to 8 weeks after chemoradiation, patients received 4 cycles of gemcitabine (1000 mg/m2 intravenously on days 1, 8, and 15 every 28 days) plus erlotinib (100 mg oral daily) or until disease progression or toxicity occurred. Toxicity was assessed weekly during chemoradiation and during every cycle of adjuvant chemotherapy by use of the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.
Endpoints and follow-up
The primary endpoint was recurrence-free survival (RFS), defined as the time between surgical resection and death or first radiographic evidence of disease recurrence. Overall survival (OS) was a secondary endpoint, defined as the time between surgical resection and death. Patients who did not experience recurrence or die were censored at the date of last follow-up. After adjuvant therapy, patients were followed up with surveillance computed tomographic scan, physical examination, and laboratory tests every 3 months for 2 years, then every 6 months for the next 3 years.
Quality of life
Quality of life (QOL) was assessed before CRT was started or during the first week of its administration (baseline [BL]), between completion of CRT and starting maintenance chemotherapy (time 1 [t1]), and within 3 months after completion of maintenance chemotherapy (time 2 [t2]). Assessments included 2 questionnaires: the EORTC QLQ-C30 (version 3.0) and the disease-specific QOL module for pancreatic cancer, QLQ-PAN26.
Statistical analysis
Demographic, baseline, toxicity, and QOL data were summarized by use of descriptive statistics. Efficacy analysis was performed in a modified intention-to-treat fashion. The Kaplan-Meier method was used to estimate time-to-event curves and survival rates. The expected median RFS for standard 5-fluorouracil-based CRT and gemcitabine maintenance therapy without erlotinib is 12.0 months. To detect an 8-month improvement in RFS with 89% power and 2-sided type I error of 5%, 40 patients were needed. Patient characteristics associated with RFS and OS were identified in univariate Cox regression analysis by use of a value of P≤.05 and selected as covariates to construct multivariate proportional hazards models for RFS and OS. Established prognostic factors for patients with resected pancreatic cancer, including Eastern Cooperative Oncology Group performance status, margin status, nodal status, age, sex, and a term indicating whether the patient received a higher dose of erlotinib, were added as covariates to these models to estimate the hazard ratio (HR) for recurrence and death attributable to each covariate. Differences in QOL scores between time points were assessed for significance using paired t tests. All P values reported are 2-sided, and the a priori level of significance was set at P≤.05. Analyses were performed with R, version 2.15.1.
Results
Patient characteristics
Fifty patients enrolled in the study from March 2006 to January 2012. Two patients, 1 with an open drain site and the other with clinical deterioration, were removed from the trial before receiving study treatment and were not included in the analyses. Table 1 summarizes the demographic and baseline disease characteristics.
Table 1.
Patient demographics and baseline disease characteristics (n = 48)
| Characteristic | Value |
|---|---|
| Median age at diagnosis, y (range) | 62 (46–82) |
| Age ≥65 | 16 (33%) |
| Age <65 | 32 (67%) |
| Sex, n (%) | |
| F | 28 (58%) |
| M | 20 (42%) |
| Resection type, n (%) | |
| Pancreaticoduodenectomy | 36 (75%) |
| Distal pancreatectomy | 10 (21%) |
| Total pancreatectomy | 2 (4%) |
| Location of tumor, n (%) | |
| Head | 38 (79%) |
| Body/tail | 10 (21%) |
| Stage, n (%) | |
| I | 4 (8%) |
| II | 44 (92%) |
| No. with nodal involvement (%) | 41 (85%) |
| No. with positive margins, (%) | 8 (17%) |
| Differentiation, n (%) | |
| Well | 2 (4%) |
| Moderate | 30 (62%) |
| Poor | 16 (33%) |
| ECOG performance status, n (%) | |
| 0 | 34 (71%) |
| 1 | 14 (29%) |
Abbreviation ECOG = Eastern Cooperative Oncology Group.
Efficacy
The median follow-up time was 18.2 months (interquartile range, 13.8–27.1). At the time of analysis, 31 patients (64%) had experienced recurrence and 29 (60%) had died. No patients experienced progression during CRT. Six patients (13%) experienced progression before starting post-CRT chemotherapy, 4 (8%) experienced progression during maintenance chemotherapy, and 21 (44%) experienced progression after completing the study protocol. Eighteen patients (37%) experienced recurrence, 9 (19%) had local recurrence, and 4 (8%) had synchronous local/distant recurrence. The median plasma CA19-9 before CRT was 32.3 U/mL. CRT plus erlotinib resulted in CA19-9 reduction or stabilization in 25 of 44 patients (57%) for whom CA19-9 laboratory values were available.
Recurrence-free survival
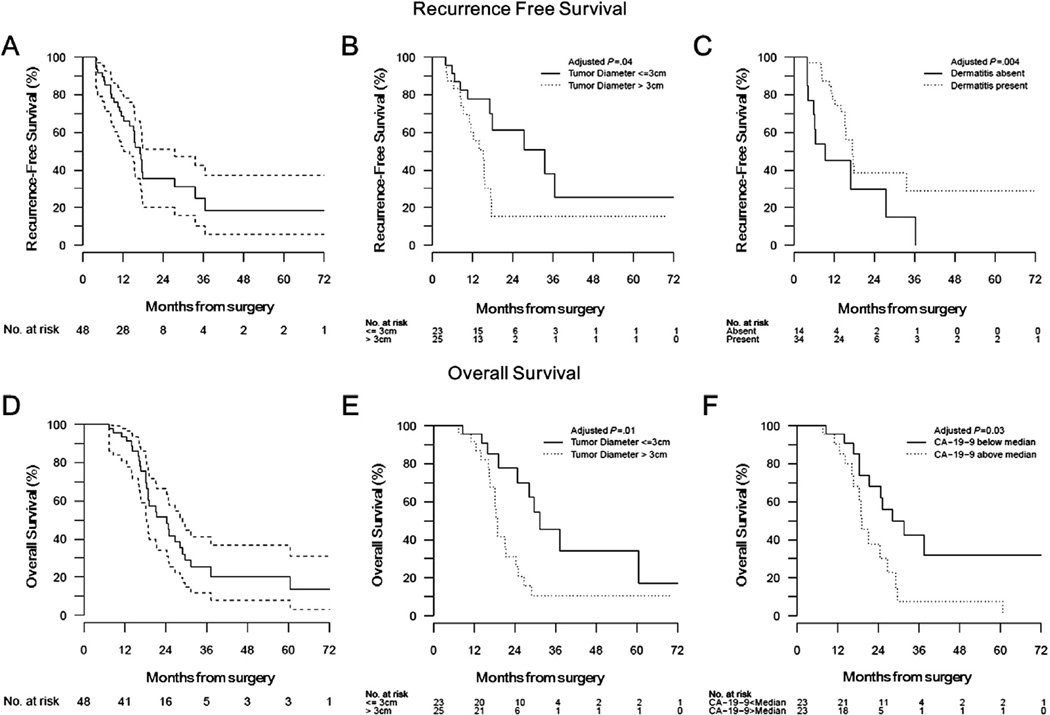
The median RFS was 15.6 months (95% CI, 13.4–17.9); the 1-year and 2-year RFS rates were 65.1% (95% CI, 50.9–79.3) and 30.5% (95% CI, 15.5–45.5), respectively (Table 2). The median local RFS was 21.1 months (95% CI, 17.5–29.1); the 1-year and 2-year local RFS rates were 86.9% (95% CI, 76.9–96.9) and 44.4% (95% CI, 27.6–61.2), respectively (Fig. 1A).
Table 2.
Recurrence-free survival (RFS) for 48 patients enrolled on study
| Variable | N | Median (mo) | 1-year RFS | 2-year RFS | 5-year RFS | HR | 95% CI | P |
|---|---|---|---|---|---|---|---|---|
| All subjects | 48 | 15.62 (13.35, 17.88) | 65 (53, 81) | 31 (19, 49) | 16 (7, 39) | |||
| Age <65 | 32 | 17.03 (11.51, 72.8+) | 65 (50, 84) | 33 (19, 58) | 17 (5, 51) | 1 | - | |
| Age 65+ | 16 | 15.35 (9.3, 72.8+) | 66 (46, 95) | 25 (9, 65) | 16 (5, 58) | 1.17 | (0.56, 2.44) | .67 |
| Tumor diameter ≤3 cm | 23 | 17.88 (16.31, 72.8+) | 74 (58, 94) | 50 (31, 79) | 21 (7, 65) | 1 | - | |
| Tumor diameter >3 cm | 25 | 13.97 (9.3, 17.49) | 57 (40, 81) | 14 (5, 41) | 14 (5, 41) | 2.03 | (0.99, 4.16) | .05 |
| Isolated recurrence pattern | 27 | 11.51 (8.35, 15.62) | 48 (33, 71) | 11 (4, 32) | 0 (NA, NA) | 1 | - | |
| Synchronous recurrence pattern | 4 | 12.92 (4.08, 36.4+) | 50 (19, 100) | 0 (NA, NA) | 0 (NA, NA) | 0.97 | (0.33, 2.83) | .96 |
| Negative margin status | 40 | 15.62 (13.97, 72.8+) | 68 (55, 85) | 35 (22, 57) | 23 (10, 50) | 1 | - | |
| Positive margin status | 8 | 11.88 (8.35, 72.8+) | 50 (25, 100) | 12 (2, 78) | 0 (NA, NA) | 1.88 | (0.84, 4.22) | .13 |
| Male | 20 | 15.62 (13.97, 72.8+) | 74 (56, 96) | 29 (13, 64) | 11 (2, 58) | 1 | - | |
| Female | 28 | 16.31 (8.45, 72.8+) | 60 (44, 81) | 32 (17, 58) | 21 (8, 58) | 0.97 | (0.48, 1.97) | .94 |
| Uninvolved lymph nodes | 7 | 17.88 (13.97, 72.8+) | 86 (63, 100) | 36 (12, 100) | 0 (NA, NA) | 1 | - | |
| Involved lymph nodes | 41 | 15.35 (11.51, 27.39) | 61 (48, 79) | 30 (18, 51) | 19 (8, 44) | 1.28 | (0.49, 3.34) | .61 |
| Grade <3 | 32 | 17.03 (13.97, 72.8+) | 74 (60, 91) | 32 (18, 57) | 0 (NA, NA) | 1 | - | |
| Grade 3+ | 16 | 13.38 (9.3, 72.8+) | 50 (31, 82) | 29 (13, 64) | 21 (8, 57) | 1.05 | (0.51, 2.18) | .9 |
| Tumor in body/tail | 10 | 13.35 (8.45, 72.8+) | 56 (31, 100) | 22 (7, 75) | 0 (NA, NA) | 1 | - | |
| Tumor in head | 38 | 17.03 (13.97, 33.57) | 68 (54, 85) | 33 (19, 55) | 20 (9, 48) | 0.69 | (0.31, 1.55) | .37 |
| ECOG 0 | 34 | 15.48 (11.51, 27.39) | 63 (48, 82) | 26 (14, 51) | 9 (2, 49) | 1 | - | |
| ECOG 1 | 14 | 17.03 (12.26, 72.8+) | 71 (50, 100) | 39 (20, 77) | 26 (9, 75) | 0.71 | (0.32, 1.55) | .39 |
| Baseline CA19-9 above median | 23 | 15.48 (11.51, 72.8+) | 66 (49, 90) | 15 (4, 51) | 0 (NA, NA) | 1 | - | |
| Baseline CA19-9 below median | 23 | 17.88 (13.35, 72.8+) | 70 (53, 91) | 45 (28, 72) | 29 (13, 64) | 0.57 | (0.27, 1.21) | .14 |
| No dermatitis | 14 | 9.3 (5.95, 72.8+) | 45 (24, 83) | 22 (7, 71) | 0 (NA, NA) | 1 | - | |
| Dermatitis | 34 | 16.31 (15.25, 72.8+) | 73 (59, 90) | 34 (20, 57) | 25 (12, 54) | 0.43 | (0.21, 0.9) | .02 |
Abbreviations CI = confidence interval; ECOG = Eastern Cooperative Oncology Group; HR = hazard ratio.
Median RFS is shown in months with a 95% CI. Values for RFS probability at 1, 2, and 5 years are shown with 95% CIs. All HRs and P values are derived from univariate models.
Fig. 1.
Kaplan-Meier curves showing (A) recurrence-free survival (RFS) (solid line) with 95% confidence intervals (dashed lines); (B) RFS stratified by the absence (solid line) or presence (dotted line) of dermatitis during erlotinib therapy; (C) RFS stratified by tumor diameter less than (solid line) or greater than (dotted line) 3 cm; (D) overall survival (OS) (solid line) with 95% confidence intervals (dashed lines); (E) OS stratified by tumor diameter less than (solid line) or greater than (dotted line) 3 cm; (F) OS stratified by baseline CA19-9 less than (solid line) or greater than (dotted line) the median value for the cohort (32.3 U/mL). P values shown are derived from univariate analyses; multivariate results are discussed in the text.
RFS was significantly associated with tumor diameter larger than or less than or equal to 3 cm (median, 14.0 months vs 17.9 months, HR 2.03, 95% CI 0.99–4.16, P = .05) and the presence or absence of dermatitis (16.3 months vs 9.3 months, HR 0.43, 95% CI 0.21–0.90, P = .02) in univariate Cox regression models (Table 2, Fig. 1B, C). These 2 variables remained significant independent predictors for RFS (P = .002 and <.001, respectively) in multivariate analysis (Table E1, available online at www.redjournal.org). RFS was not significantly associated with histologic grade, tumor location, lymph node, or margin status at resection (Table 2).
Overall survival
The median OS was 24.4 months (95% CI, 18.9–29.7); the 1- and 2-year OS rates were 93.4% (95% CI, 86.0–100) and 51.4% (95% CI, 34.6–68.2), respectively (Fig. 1D). OS was significantly associated with tumor diameter larger than versus less than or equal to 3 cm (18.9 months vs 31.5 months, HR 2.69, 95% CI 1.22–5.96, P = .01) and baseline CA19-9 below versus above the median (28.2 months vs 19.0 months, HR 0.37, 95% CI 0.17–0.83, P = .02) in univariate Cox regression models (Table 3). When both were included in the multivariate Cox proportional hazards model, only tumor diameter above versus less than or equal to 3 cm remained a significant independent predictor of OS (P = .03) (Table E2, available online at www.redjournal.org).
Table 3.
Overall survival (OS) for 48 patients enrolled on study
| Variable | N | Median (mo) | 1-year OS | 2-year OS | 5-year OS | HR | 95% CI | P |
|---|---|---|---|---|---|---|---|---|
| All subjects | 48 | 24.39 (18.87, 29.65) | 93 (86, 100) | 51 (37, 71) | 20 (10, 42) | |||
| Age <65 | 32 | 24.69 (19, 37.38) | 94 (85, 100) | 57 (40, 81) | 19 (8, 49) | 1 | - | |
| Age 65+ | 16 | 21.11 (18.28, 72.8+) | 93 (82, 100) | 42 (22, 82) | 25 (10, 67) | 0.91 | (0.41, 2.01) | .81 |
| Tumor diameter ≤3 cm | 23 | 31.46 (28.18, 72.8+) | 96 (88, 100) | 78 (60, 100) | 34 (15, 78) | 1 | - | |
| Tumor diameter >3 cm | 25 | 18.87 (18.21, 25.08) | 91 (81, 100) | 31 (16, 60) | 10 (3, 38) | 2.69 | (1.22, 5.96) | .01 |
| Isolated recurrence pattern | 40 | 26.73 (18.38, 72.8+) | 92 (84, 100) | 60 (45, 81) | 29 (16, 55) | 1 | - | |
| Synchronous recurrence pattern | 8 | 19.13 (18.87, 72.8+) | 100 (100, 100) | 25 (8, 83) | 0 (NA, NA) | 1.90 | (0.82, 4.39) | .13 |
| Negative margin status | 20 | 19 (18.21, 72.8+) | 100 (100, 100) | 39 (21, 72) | 10 (2, 53) | 1 | - | |
| Positive margin status | 28 | 25.08 (19.27, 72.8+) | 89 (77, 100) | 62 (44, 88) | 31 (15, 63) | 0.65 | (0.31, 1.38) | .26 |
| Male | 7 | 29.65 (24.69, 72.8+) | 100 (100, 100) | 83 (58, 100) | 28 (5, 100) | 1 | - | |
| Female | 41 | 21.11 (18.38, 31.46) | 92 (84, 100) | 46 (31, 68) | 18 (8, 43) | 1.26 | (0.44, 3.66) | .67 |
| Uninvolved lymph nodes | 32 | 25.08 (19, 72.8+) | 97 (90, 100) | 54 (36, 81) | 15 (3, 61) | 1 | - | |
| Involved lymph nodes | 16 | 19.27 (18.28, 72.8+) | 88 (73, 100) | 48 (28, 81) | 20 (7, 56) | 1.09 | (0.51, 2.33) | .82 |
| Grade <3 | 10 | 21.34 (18.87, 72.8+) | 89 (71, 100) | 38 (16, 92) | 25 (8, 84) | 1 | - | |
| Grade 3+ | 38 | 24.69 (18.38, 37.38) | 95 (88, 100) | 56 (40, 78) | 19 (8, 46) | 0.88 | (0.37, 2.07) | .76 |
| Tumor in body/tail | 34 | 21.34 (19, 29.06) | 97 (91, 100) | 48 (31, 74) | 16 (6, 44) | 1 | - | |
| Tumor in head | 14 | 24.69 (16.21, 72.8+) | 85 (67, 100) | 54 (33, 89) | 26 (9, 74) | 0.79 | (0.35, 1.78) | .57 |
| ECOG 0 | 23 | 19 (16.57, 29.65) | 90 (79, 100) | 38 (19, 73) | 8 (1, 49) | 1 | - | |
| ECOG 1 | 23 | 28.18 (21.34, 72.8+) | 96 (88, 100) | 68 (50, 93) | 32 (14, 71) | 0.37 | (0.17, 0.83) | .02 |
| Baseline CA19-9 above median | 14 | 18.28 (16.57, 72.8+) | 85 (67, 100) | 42 (20, 88) | 11 (2, 67) | 1 | - | |
| Baseline CA19-9 below median | 34 | 24.69 (19.27, 72.8+) | 97 (91, 100) | 55 (38, 78) | 24 (10, 54) | 0.53 | (0.24, 1.16) | .11 |
Abbreviations CI = confidence interval; ECOG = Eastern Cooperative Oncology Group; HR = hazard ratio.
Median survival is shown in months with a 95% CI. Values for OS probability at 1, 2, and 5 years are shown with 95% CIs. All HRs and P values are from univariate models.
Tolerability and safety
During CRT, 24 patients (50%) experienced grade 2 toxicity, 15 (31%) grade 3, and 1 (2%) grade 4. The most common toxicities were nausea, anorexia, weight loss, fatigue, and dermatitis (Table 4). Seven patients (15%) required a treatment break during CRT. Eight patients (17%) stopped CRT early, 2 because of abdominal pain, 1 fistula, 1 rash, 1 diarrhea, 1 neutropenia, 1 intractable nausea, and 1 ischemic bowel. Nine patients (19%) were unable to receive any maintenance chemotherapy after CRT, 2 because of metastatic disease, 2 because of patient preference, 1 because of neutropenia, and 4 because of gastrointestinal complications, including bowel ischemia, enterocutaneous fistula, gastritis, and intractable vomiting.
Table 4.
Toxicity during chemoradiation plus erlotinib and during adjuvant gemcitabine plus erlotinib, both broken down by type and severity
| Category | Total (%) | Grade 1 (%) | Grade 2 (%) | Grade 3 (%) | Grade 4 (%) |
|---|---|---|---|---|---|
| Chemoradiation plus erlotinib | |||||
| Elevated AST/elevated ALT | 12 (25%) | 8 (17%) | 1 (2%) | 3 (6%) | 0 (0%) |
| Elevated ALP | 7 (15%) | 5 (10%) | 1 (2%) | 1 (2%) | 0 (0%) |
| Hyperbilirubinemia | 4 (8%) | 2 (4%) | 1 (2%) | 1 (2%) | 0 (0%) |
| Abdominal pain | 3 (6%) | 1 (2%) | 2 (4%) | 0 (0%) | 0 (0%) |
| Diarrhea | 19 (40%) | 13 (27%) | 5 (10%) | 1 (2%) | 0 (0%) |
| Vomiting | 8 (17%) | 3 (6%) | 5 (10%) | 0 (0%) | 0 (0%) |
| Nausea | 31 (65%) | 15 (31%) | 15 (31%) | 1 (2%) | 0 (0%) |
| Dermatitis | 28 (58%) | 16 (33%) | 10 (21%) | 2 (4%) | 0 (0%) |
| Anorexia | 30 (62%) | 17 (35%) | 12 (25%) | 1 (2%) | 0 (0%) |
| Fatigue | 29 (60%) | 17 (35%) | 12 (25%) | 0 (0%) | 0 (0%) |
| Weight loss | 29 (60%) | 16 (33%) | 12 (25%) | 1 (2%) | 0 (0%) |
| Neutropenia | 10 (21%) | 4 (8%) | 0 (0%) | 5 (7%) | 1 (2%) |
| Adjuvant gemcitabine plus erlotinib | |||||
| Nonhematologic | |||||
| Elevated AST/elevated ALT | 16 (41%) | 13 (33%) | 2 (5%) | 1 (3%) | 0 (0%) |
| Elevated ALP | 20 (51%) | 13 (33%) | 6 (15%) | 1 (3%) | 0 (0%) |
| Hyperbilirubinemia | 1 (3%) | 1 (3%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Diarrhea | 19 (49%) | 14 (36%) | 3 (8%) | 2 (5%) | 0 (0%) |
| Vomiting | 3 (8%) | 2 (5%) | 0 (0%) | 1 (3%) | 0 (0%) |
| Nausea | 15 (38%) | 14 (36%) | 0 (0%) | 1 (3%) | 0 (0%) |
| Dermatitis | 17 (44%) | 11 (28%) | 5 (13%) | 1 (3%) | 0 (0%) |
| Anorexia | 8 (21%) | 5 (13%) | 3 (8%) | 0 (0%) | 0 (0%) |
| Fatigue | 28 (72%) | 23 (59%) | 3 (8%) | 2 (5%) | 0 (0%) |
| Alopecia | 2 (5%) | 0 (0%) | 2 (5%) | 0 (0%) | 0 (0%) |
| Neuropathy (sensory) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Weight loss | 9 (23%) | 9 (23%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Hematologic | |||||
| Neutropenia | 9 (23%) | 1 (3%) | 1 (3%) | 5 (13%) | 2 (5%) |
| Anemia | 25 (64%) | 9 (23%) | 10 (26%) | 6 (15%) | 0 (0%) |
| Thrombocytopenia | 12 (31%) | 9 (23%) | 2 (5%) | 0 (0%) | 1 (3%) |
Abbreviations ALP = alkaline phosphatase; ALT = alanine aminotransferase; AST = aspartate aminotransferase.
Of the 39 patients who received maintenance chemotherapy after CRT, gemcitabine dose reduction was required in 10 (26%), and reduction in gemcitabine and erlotinib was required in 3 (8%). On average, patients completed 3.4 (SD, 1.0) of the planned 4 cycles of full-dose gemcitabine/erlotinib. Regarding the highest grade of toxicity experienced, 15 patients (38%) experienced grade 2 toxicity, 14 (36%) grade 3, and 3 (8%) grade 4. The most common toxicities were fatigue, anemia, elevated alkaline phosphatase, diarrhea, and dermatitis (Table 4).
Overall, when toxicities from the CRTand chemotherapy phases of the trial are considered collectively, 26 of 48 patients (54%) experienced grade 3 or higher toxicity, with anemia, neutropenia, and elevated aspartate aminotransferase and alanine aminotransferase being the most commonly encountered toxicities. Sixteen (33%) patients required hospital admission for a serious adverse event, including fever (8%), gastrointestinal bleeding (6%), altered mental status (4%), syncope (4%), small bowel obstruction (4%), anemia (4%), abdominal pain (4%), enteritis (4%), pneumonia (4%), and intractable nausea/vomiting (2%). Thirteen patients had their first hospitalization during treatment, 2 during routine follow-up, and 1 after being taken off protocol.
Quality of life
Despite receiving aggressive treatment, the mean global QOL scores for the 33 patients with available QOL data remained stable throughout both phases of treatment (fluctuated by less than 5.0 points between BL, t1, and t2; all P>>.05). Similarly, there were no significant changes in 4 of the 5 functional QOL scales (role, cognition, emotional, social), although physical function score declined slightly (by 6.2 points) from t1 to t2 (P = .01). Symptoms of pain, fatigue, nausea/vomiting, dyspnea, insomnia, and constipation did not change significantly from BL. (Tables E3 and E4, available online at www.redjournal.org).
Discussion
This phase 2 study is the first prospective trial examining the efficacy of adding erlotinib to adjuvant CRT and chemotherapy for resected PDAC. The results suggest acceptable safety and efficacy in comparison with other adjuvant regimens (Table 5); however, our study did not meet its primary endpoint for median RFS (15.6 months actual vs 20.0 months targeted).
Table 5.
Survival outcomes in selected adjuvant studies of resected pancreatic cancer
| Study | Regimen | Sample size |
Positive margins (%) |
Nodal involvement (%) |
Local recurrence (%)* |
RFS (mo) |
Median (mo) |
|---|---|---|---|---|---|---|---|
| GITSG, 1985 | 5-FU+XRT → 5-FU | 21 | 0 | 28 | 33 | 11 | 20 |
| EORTC, 1999 | 5-FU+XRT | 104 | 19 | 47 | 36 | 17.4† | 17.1 |
| ESPAC-1, 2001 | 5-FU+XRT → 5-FU + LV | 75 | - | - | - | - | 19.9 |
| CONKO-001, 2007 | Gem | 179 | 19 | 71 | 34 | 13.4‡ | 22.1‡ |
| ROTG 97-04, 2008 | Gem; 5-FU/XRT → gem | 221 | 35 | 68 | 23 | - | 20.6 |
| ESPAC-3, 2010 | Gem | 537 | 35 | 73 | - | 14.3 | 23.6 |
| Bao et al, 2011 (9) | Erlotinib+FDR gem | 25 | 0 | 64 | 59 | 14§ | ~24§, ‖ |
| This study | Erlotinib+capecitabine+IMRT → erlotinib+gem | 48 | 17 | 85 | 27 | 15.6 | 24.4 |
Abbreviations 5-FU = 5-fluorouracil; CONKO = Charité Onkologie; ESPAC = European Study Group for Pancreatic Cancer; EORTC = European Organization for Research and Treatment of Cancer; FDR = fixed dose rate; Gem = gemcitabine; GITSG = Gastro-Intestinal Study Group; IMRT = intensity modulated radiation therapy; LV = leukovorin; RFS = recurrence-free survival; ROTG = Radiation Therapy Oncology Group; XRT = radiation therapy. Unless otherwise indicated, event times for RFS and median survival were measured from the date of surgical resection.
Including synchronous local and distant recurrences.
Including periampullary cancer.
Event time from randomization.
Event time from the start of adjuvant therapy.
Median survival was not reached in this study, but the 2-year overall survival was 53%, suggesting a median survival of approximately 24 months
Bao et al (9) performed a phase 2 study of adjuvant gemcitabine and erlotinib (without radiation) for margin-negative resected PDAC that provides a useful comparison for the current study. In our study, median RFS and OS were similar (Table 5), but local recurrence rates were markedly lower (27% vs 59%), even though our cohort had higher rates of positive margins (17% vs 0%) and lymph node involvement (85% vs 64%) (Table 5). The median survival for margin-negative patients in our series was slightly more favorable (26.7 months). Despite the addition of chemoradiation, the toxicity rates for our regimen were similar to those reported by Bao et al (9) (35% grade 3, 4% grade 4).
Cetuximab, another EGFR inhibitor, has also demonstrated good local tumor control in unresectable PDAC, suggesting a potential radiosensitizing effect of EGFR inhibitors (10). Local disease recurrence has been associated with symptoms of pain, bowel obstruction, portal hypertension, biliary obstruction, and decreased quality of life. Therefore, chemoradiation with erlotinib either before (R1) or after maintenance chemotherapy may benefit patients with resected PDAC.
Tumor size at resection has been previously identified as a prognostic factor for survival (11). Other factors influencing survival include lymph node involvement, margin status, and histologic grade (12, 13). In our study, tumor diameter was associated with RFS and OS, whereas margin status, nodal involvement, and histologic grade were not. This suggests that more aggressive regimens may be needed for larger tumors such as higher doses of radiation therapy and multi-agent chemotherapy.
The development of a rash during treatment with tyrosine kinase inhibitors or anti-EGFR antibodies is associated with superior outcomes in colorectal (14) and metastatic pancreatic cancer (15). Dermatitis during erlotinib therapy was an independent predictor for RFS in our study. In fact, the only patients who were recurrence free beyond 36 months (n = 5) were those in whom dermatitis developed during treatment. In future studies, it is possible that dermatitis could be used to distinguish patients who should continue erlotinib therapy from those who could derive greater benefit from an alternative regimen.
Given the modest survival benefit observed for erlotinib plus gemcitabine versus gemcitabine alone (6.24 vs 5.91 months, respectively) in patients with advanced pancreatic cancer (6), it is perhaps not surprising that our study did not meet its intended primary endpoint of 20.0 months RFS. The most straightforward explanation may be that erlotinib is only modestly effective against pancreatic adenocarcinoma. Still, there are several possible factors that may have additionally contributed to the reason why a larger benefit was not observed as a result of adding erlotinib to standard adjuvant therapy. As shown in Table 5, our study population contained a remarkably high proportion of patients with lymph node involvement. The observation that lymph node involvement was not a significant prognostic factor for survival in our analysis could be explained by the fact that we had an overwhelming proportion of patients with positive lymph nodes (85%), leaving only a small group of patients with negative nodes for comparison. Moreover, a substantial portion of patients with resectable disease already harbor occult micrometastatic disease at the time of diagnosis (16); therefore, there may be an inherent limitation in the efficacy of an adjuvant sequencing approach that delays full-dose systemic therapy. A phase 3 trial (RTOG-0848) is currently investigating whether adding erlotinib to standard adjuvant gemcitabine chemotherapy improves survival and whether the addition of chemoradiation improves survival. By moving full-dose chemotherapy earlier in the treatment sequence, this regimen may more effectively prevent systemic spread of disease and improve outcomes.
In other malignancies, high levels of EGFR protein expression and high EGFR gene copy number are strongly associated with objective responses to EGFR tyrosine kinase inhibitors and increased survival (17). Overexpression of the EGFR protein and amplification of the EGFR gene have been documented in up to 60% of pancreatic tumor specimens (5), rendering EGFR a logical target. However, given that the majority (>90%) of pancreatic tumors harbor somatic activating mutations of the KRAS oncogene (18), inhibition of EGFR in the face of downstream constitutive mutational activation of KRAS may have a diminished effect on oncogenic cell signaling. In fact, in multiple advanced lung cancer trials, tumors positive for KRAS mutation were associated with resistance to EGFR tyrosine kinase inhibitors (19). Direct DNA sequencing revealed KRAS mutations in 92% of resected specimens in the study by Bao et al (9) of adjuvant gemcitabine with erlotinib, and a trend toward improved RFS was observed among patients with higher levels of EGFR expression on immunohistochemistry. The optimal approach for targeted inhibition of EGFR in pancreatic cancer, therefore, may be to carefully select patients with wild-type KRAS and overexpression of EGFR.
Ben-Josef et al (21) recently studied concurrent IMRT and gemcitabine in patients with unresectable PDAC and reported promising results (20). However, to our knowledge, this is the first prospective study using IMRT as adjuvant treatment for resectable PDAC. A phase 1 trial of the EGFR inhibitor gefitinib with capecitabine and 3-dimensional conformal radiation therapy for resectable and locally advanced PDAC produced significant doselimiting diarrhea that precluded determination of a recommended phase 2 gefitinib dose (21). The fact that 83% of our patients were able to complete CRT with erlotinib is encouraging evidence that the use of IMRT rather than 3-dimensional conformal radiation therapy may improve treatment tolerability, although further investigation is required. The overall rate of severe (grade 3– 4) toxicity observed in this study (54%) compares favorably with that observed for the chemoradiation plus gemcitabine arm in RTOG 97-04 (79%) (22), suggesting that erlotinib can be combined with adjuvant IMRT-based CRT and chemotherapy with acceptable safety compared with more conventional regimens.
Conclusions
Erlotinib can be safely combined with IMRT-based CRT and chemotherapy. This regimen appears promising compared with existing regimens for resected PDAC. Patients with dermatitis in response to erlotinib therapy smaller appear to especially benefit from this adjuvant regimen. The RTOG 0848 trial will further elucidate whether the addition of erlotinib to gemcitabine confers a survival benefit in patients receiving chemotherapy alone or with adjuvant chemoradiation.
Supplementary Material
Summary.
The optimal adjuvant treatment for pancreatic cancer remains controversial. Here we report the results of a single-institution phase 2 trial investigating the efficacy and safety of erlotinib in combination with adjuvant chemoradiation and chemotherapy for resectable pancreatic cancer. Our results show that erlotinib can be safely combined with adjuvant chemoradiation and chemotherapy for resected pancreatic cancer. Patients who develop dermatitis from erlotinib had improved RFS.
Acknowledgment
Supported by Genentech, Inc. We would like to thank the Claudio X. Gonzalez family foundation and Flannery Family for their generous contributions to help support this study.
Footnotes
Conflict of interest: none.
Supplementary material for this article can be found at www.redjournal.org.
References
- 1.Jemal A, Simard EP, Xu J, et al. Selected cancers with increasing mortality rates by educational attainment in 26 states in the United States, 1993–2007. Cancer Causes Control. 2013;24:559–565. doi: 10.1007/s10552-012-9993-y. [DOI] [PubMed] [Google Scholar]
- 2.Yeo TP, Hruban RH, Leach SD, et al. Pancreatic cancer. Curr Probl Cancer. 2002;26:176–275. doi: 10.1067/mcn.2002.129579. [DOI] [PubMed] [Google Scholar]
- 3.Herman JM, Swartz MJ, Hsu CC, et al. Analysis of fluorouracil-based adjuvant chemotherapy and radiation after pancreaticoduodenectomy for ductal adenocarcinoma of the pancreas: Results of a large, prospectively collected database at the Johns Hopkins Hospital. J Clin Oncol. 2008;26:3503–3510. doi: 10.1200/JCO.2007.15.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: A randomized controlled trial. JAMA. 2007;297:267–277. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- 5.Fjällskog M-LH, Lejonklou MH, Oberg KE, et al. Expression of molecular targets for tyrosine kinase receptor antagonists in malignant endocrine pancreatic tumors. Clin Cancer Res. 2003;9:1469–1473. [PubMed] [Google Scholar]
- 6.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: A phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 7.Bianco C, Tortora G, Bianco R, et al. Enhancement of antitumor activity of ionizing radiation by combined treatment with the selective epidermal growth factor receptor-tyrosine kinase inhibitor ZD1839 (Iressa) Clin Cancer Res. 2002;8:3250–3258. [PubMed] [Google Scholar]
- 8.Ma WW, Herman JM, Jimeno A, et al. A tolerability and pharmacokinetic study of adjuvant erlotinib and capecitabine with concurrent radiation in resected pancreatic cancer. Transl Oncol. 2010;3:373–379. doi: 10.1593/tlo.10196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bao PQ, Ramanathan RK, Krasinkas A, et al. Phase II study of gemcitabine and erlotinib as adjuvant therapy for patients with resected pancreatic cancer. Ann Surg Oncol. 2011;18:1122–1129. doi: 10.1245/s10434-010-1401-9. [DOI] [PubMed] [Google Scholar]
- 10.Crane CH, Varadhachary GR, Yordy JS, et al. Phase II trial of cetuximab, gemcitabine, and oxaliplatin followed by chemoradiation with cetuximab for locally advanced (T4) pancreatic adenocarcinoma: Correlation of Smad4(Dpc4) immunostaining with pattern of disease progression. J Clin Oncol. 2011;29:3037–3043. doi: 10.1200/JCO.2010.33.8038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.You DD, Lee HG, Heo JS, et al. Prognostic factors and adjuvant chemoradiation therapy after pancreaticoduodenectomy for pancreatic adenocarcinoma. J Gastrointest Surg. 2009;13:1699–1706. doi: 10.1007/s11605-009-0969-5. [DOI] [PubMed] [Google Scholar]
- 12.Kato K, Yamada S, Sugimoto H, et al. Prognostic factors for survival after extended pancreatectomy for pancreatic head cancer: Influence of resection margin status on survival. Pancreas. 2009;38:605–612. doi: 10.1097/MPA.0b013e3181a4891d. [DOI] [PubMed] [Google Scholar]
- 13.Corsini MM, Miller RC, Haddock MG, et al. Adjuvant radiotherapy and chemotherapy for pancreatic carcinoma: The Mayo Clinic experience (1975–2005) J Clin Oncol. 2008;26:3511–3516. doi: 10.1200/JCO.2007.15.8782. [DOI] [PubMed] [Google Scholar]
- 14.Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 15.Wacker B, Nagrani T, Weinberg J, et al. Correlation between development of rash and efficacy in patients treated with the epidermal growth factor receptor tyrosine kinase inhibitor erlotinib in two large phase III studies. Clin Cancer Res. 2007;13:3913–3921. doi: 10.1158/1078-0432.CCR-06-2610. [DOI] [PubMed] [Google Scholar]
- 16.Haeno H, Gonen M, David MB, et al. Computational modeling of pancreatic cancer reveals kinetics of metastasis suggesting optimum treatment strategies. Cell. 2012;148:362–375. doi: 10.1016/j.cell.2011.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsao M-S, Sakurada A, Cutz JC, et al. Erlotinib in lung cancer: Molecular and clinical predictors of outcome. N Engl J Med. 2005;353:133–144. doi: 10.1056/NEJMoa050736. [DOI] [PubMed] [Google Scholar]
- 18.Jones S, Zhang X, Parsons DW, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Massarelli E, Varella-Garcia M, Tang X, et al. KRAS mutation is an important predictor of resistance to therapy with epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. Clin Cancer Res. 2007;13:2890–2896. doi: 10.1158/1078-0432.CCR-06-3043. [DOI] [PubMed] [Google Scholar]
- 20.Ben Josef E, Schipper M, Francis IR, et al. A phase I/II trial of intensity modulated radiation (IMRT) dose escalation with concurrent fixed-dose rate gemcitabine (FDR-G) in patients with unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 2012;84(5):1166–1171. doi: 10.1016/j.ijrobp.2012.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Czito BG, Willett CG, Bendell JC, et al. Increased toxicity with gefitinib, capecitabine, and radiation therapy in pancreatic and rectal cancer: Phase I trial results. J Clin Oncol. 2006;24:656–662. doi: 10.1200/JCO.2005.04.1749. [DOI] [PubMed] [Google Scholar]
- 22.Regine WF, Winter KA, Abrams RA, et al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: A randomized controlled trial. JAMA. 2008;299:1019–1026. doi: 10.1001/jama.299.9.1019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.