Abstract
Object
The aim of this study was to demonstrate that paclitaxel could function as a radiosensitizer for malignant glioma in vitro and in vivo.
Methods
The radiosensitizing effect of paclitaxel was tested in vitro using the human U373MG and rat 9L glioma cell lines. Cell cycle arrest in response to paclitaxel exposure was quantified by flow cytometry. Cells were subsequently irradiated, and toxicity was measured using the clonogenic assay. In vivo studies were performed in Fischer 344 rats implanted with intracranial 9L gliosarcoma. Rats were treated with control polymer implants, paclitaxel controlled-release polymers, radiotherapy, or a combination of the 2 treatments. The study end point was survival.
Results
Flow cytometry demonstrated G2-M arrest in both U373MG and 9L cells following 6–12 hours of paclitaxel exposure. The order in which the combination treatment was administered was significant. Exposure to radiation treatment (XRT) during the 6–12 hours after paclitaxel treatment resulted in a synergistic reduction in colony formation. This effect was greater than the effect from either treatment alone and was also greater than the effect of radiation exposure followed by paclitaxel. Rats bearing 9L gliosarcoma tumors treated with paclitaxel polymer administration followed by single-fraction radiotherapy demonstrated a synergistic improvement in survival compared with any other treatment, including radiotherapy followed by paclitaxel treatment. Median survival for control animals was 13 days; for those treated with paclitaxel alone, 21 days; for those treated with XRT alone, 21 days; for those treated with XRT followed by paclitaxel, 45 days; and for those treated with paclitaxel followed by XRT, more than 150 days (p < 0.0001).
Conclusions
These results indicate that paclitaxel is an effective radiosensitizer for malignant gliomas because it renders glioma cells more sensitive to ionizing radiation by causing G2-M arrest, and induces a synergistic response to chemoradiotherapy.
Keywords: brain tumor, glioma, controlled-release polymer, radiotherapy, oncology
Resection followed by external beam radiation therapy and chemotherapy have produced modest advances in overall survival for patients with malignant gliomas.10 The effectiveness of resection for local tumor control is limited by the extent of eloquent cortex infiltrated by neoplasm and by the presence of transformed glia in the periphery of the tumor mass. As a result, nearly all recurrences arise within centimeters of the original resection cavity.6
A persistent challenge in the development of malignant glioma chemotherapy treatment modalities is the restricted permeability of the blood-brain barrier (BBB) to systemic chemotherapy. To overcome this obstacle, local delivery methods—such as convection-enhanced delivery, 16 osmotic disruptions,2,11 and controlled release polymers4,23,32,37— have been developed to bypass the BBB. These local delivery methods, used at the time of surgery, circumvent the BBB and allow for local, sustained, and tightly regulated drug delivery to the CNS with reproducible release kinetics.14,36
Paclitaxel is an antineoplastic agent with established cytotoxicity against diverse tumors and clinical efficacy in the treatment of ovarian, breast, and lung cancers.8 Despite paclitaxel’s potent efficacy against glioma cells7,29 and promising results following intracranial delivery in a rodent model of malignant glioma,35 the results of clinical trials of systemically administered paclitaxel for the treatment of brain tumors have been discouraging.13,19,24 This failure was attributed to the inadequate bioavailability of paclitaxel in the CNS at its maximum tolerated systemic dose13,15 due to its poor BBB penetration, as well as a dose-limiting toxicity when administered intravenously.12
Radiotherapy is an important treatment adjunct for malignant gliomas. There is a need for optimizing radiotherapy regimens in combination with novel compounds that would result in a synergistic effect. Such a strategy could improve the therapeutic index, achieve effectiveness at lower overall doses, and reduce attendant toxicity to surrounding tissues. Because paclitaxel arrests cells in the G2-M phase of the cell cycle1,17 through microtubule stabilization, it can function as a putative radiosensitizer.9,19
In this study, we demonstrate that not only is paclitaxel effective as a single agent against glioma in the form of a controlled-release polymer, but it also functions as a radiosensitizer for glioma cell lines in vitro. This sensitivity correlates with paclitaxel’s effect of G2-M cell cycle arrest. More importantly, we report that paclitaxel incorporated into a novel polyphosphoester (PPE): polilactofate microsphere formulation acts synergistically with radiation treatment (XRT) to prolong survival in a rodent model of malignant glioma20 when it is implanted as a pretreatment prior to the XRT regimen.
Methods
Cell Lines
Human (U373) and rat (9L) glioma cells were maintained in minimal essential medium (Invitrogen) supplemented with 10% fetal bovine serum, L-glutamine, penicillin, and streptomycin at 37°C in a 5% CO2 environment. Cells were passaged every 3–4 days. All chemicals were obtained from Sigma-Aldrich unless otherwise stated.
Flow Cytometry
Flow cytometry was performed on asynchronous U373 and 9L glioma cells. Cells were plated in 100 mm plates, and incubated for 24 hours. Cells were then treated either with paclitaxel (Taxol) dissolved in 0.01% dimethyl sulfoxide (DMSO) or with an equal volume of 0.01% DMSO as control treatment. Cells were incubated with paclitaxel for 6–36 hours, harvested, and fixed in 70% ethanol at 4°C. After addition of DNase-free RNase A and propidium iodide, flow cytometry (FACScan; Becton, Dickinson) was used to measure the DNA content per cell. Cell cycle distribution was quantified using CellQuest software (Becton, Dickinson) to determine the fraction of cells in the G1, G2-M, and S phases.
Cytotoxicity Assay
We characterized tumor cell survival and proliferation using the clonogenic assay.7 U373 and 9L cells were plated in monolayer, incubated for 24 hours, and either treated with various concentrations of paclitaxel or exposed to a single fraction of XRT at different doses (Gammacell 40 irradiator; Best Theratronics). Five days posttreatment, colony formation was assessed as a marker for cell survival and proliferation. This was used to establish separate paclitaxel and XRT dose-response curves for glioma cell viability.
Next, the interaction between paclitaxel and XRT was examined to elucidate the effect of treatment order and identify potential synergistic or additive effects. Cells were plated as above, incubated for 24 hours, and given an initial treatment of paclitaxel (U373, 1 nM; 9L, 50 nM) or XRT (1 Gy). In the paclitaxel pretreatment group (P/ XRT), cells were irradiated with a 1-Gy dose of XRT at a specific time point 6–36 hours after paclitaxel exposure. In the radiation pretreatment group (XRT/P), the treatment order was reversed such that 6–36 hours after radiation pretreatment, cells were treated with paclitaxel. Colony formation was determined 5 days after pretreatment.
Polymer Preparation
Paclitaxel and PPE microspheres were generous gifts from Guilford Pharmaceuticals. Paclitaxel was incorporated into a biodegradable PPE polymer in the form of microspheres at 10% (w/w) loading as previously described. 20 The paclitaxel microspheres were then combined with polyethylene glycol-1000 at a 1:1 ratio (w/w). After thorough mixing, 10-mg aliquots of the suspension were pressed into 4 × 1 mm discs for intracranial implantation. To make the control implants, an analogous procedure was followed while omitting paclitaxel. In a previous publication we showed that an almost-constant release of paclitaxel was measured for 90 days in solution. 20 In vivo biodistribution of these microspheres showed intact paclitaxel 5–7 mm from the implant site 30 days after implantation, with histological changes, including apoptotic cells, evident in brains 12 weeks after microsphere implantation.20
Intracranial Efficacy Study
Under a protocol approved by the Johns Hopkins University Institutional Animal Care and Use Committee, 65 female Fischer 344 rats (Charles River) weighing 150–175 g were obtained and housed in standard animal facilities. On Day 0, all animals were anesthetized with an intra-peritoneal injection (3 ml/kg) of a solution of ketamine (25 mg/ml), xylazine (2.5 mg/ml), and 14.25% ethanol in normal saline. The animals underwent direct surgical implantation of a 1-mm3 fragment obtained from a solid 9L gliosarcoma that had been propagated in a rat flank.20 The animals were then divided into 5 experimental groups (n = 13 each): 1) treatment with blank microspheres (control); 2) paclitaxel-only; 3) XRT-only; 4) XRT followed by paclitaxel (XRT/P); and 5) paclitaxel followed by XRT (P/XRT) groups.
On Day 5, the animals were re-anesthetized. The control group underwent implantation of blank micro-sphere discs and received no further treatment. The paclitaxel-only and P/XRT groups underwent surgical implantation of paclitaxel microspheres next to the tumor. The remaining animals—those in the XRT-only and XRT/P groups—underwent a 20-Gy external beam single-dose radiation treatment delivered by a 137Cs laboratory irradiator (Mark I Irradiator, Model 68; J. L. Shepard). The animals undergoing radiotherapy were anesthetized, restrained at a fixed distance from the radiation source, and shielded with a square primary collimator (7 × 7 cm) and a circular secondary collimator (1 cm diameter) that were centered over the tumor implantation bur hole.
On Day 10, the order of treatment for the P/XRT and XRT/P groups was reversed such that the animals in the P/XRT group underwent XRT as above, and the XRT/P animals underwent surgical implantation of paclitaxel microspheres. The animals were observed for signs of neurological or systemic toxicity, and their survival was recorded.
Statistical Analysis
Survival was plotted on a Kaplan-Meier survival curve. Results were then analyzed for statistical significance using a nonparametric Kruskal-Wallis analysis of variance test and individual groups were compared by the Mann-Whitney rank-sum test.
Results
Flow Cytometry
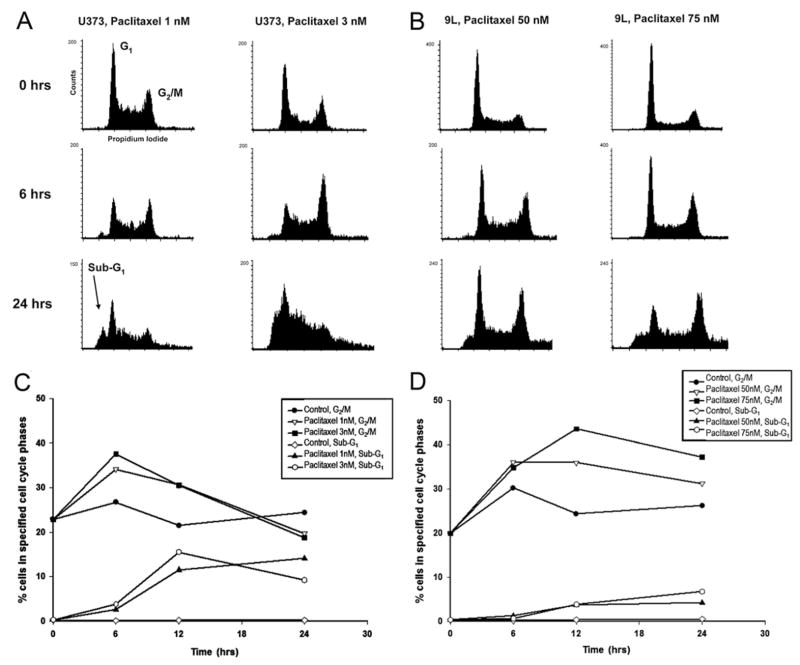
Flow cytometry revealed a dose- and time-dependent alteration in the cell cycle phase distribution of glioma cells (Fig. 1A and B). Treatment with high-dose paclitaxel for as little as 6–12 hours resulted in arrest of cells in the G2-M phase (41% increase compared with control for U373; 79% relative increase for 9L) (Fig. 1C and D). In the 9L cell line, G2-M arrest became evident after longer paclitaxel exposures than for the U373 cell line. Longer duration of paclitaxel exposure in U373 cells resulted in a gradual increase in the number of aneuploid and hypo-diploid (sub-G1) cells and deterioration of the DNA histogram (Fig. 1A and B). The sub-G1 population in the U373 line was observed after 6-hour exposure to low-dose paclitaxel. There was a marked increase in this population at 24 hours for both U373 and 9L cells (Fig. 1C and D).
Fig. 1.
Effect of paclitaxel on the cell cycle distribution of glioma cells. Flow cytometric analysis of asynchronous cultures of U373 (A) and 9L (B) cells following continuous paclitaxel exposure (U373, 1 and 3 nM; 9L, 50 and 75 nM) for the indicated durations. Cell frequency is plotted against DNA content; for each sample, 15,000 events were analyzed. Paclitaxel induced progression of cells to G2-M and appearance of sub-G1 cells in U373 (C) and 9L (D) cell cultures. DNA content was quantified by gating events with DNA content of 4N (C) or < 2N (D). The percentage of cells in the specified phases of the cell cycle was plotted as a function of time. The experiments were done a minimum of 3 times and standard errors of the mean (SEMs) were below 10%.
In Vitro Efficacy of Paclitaxel and XRT
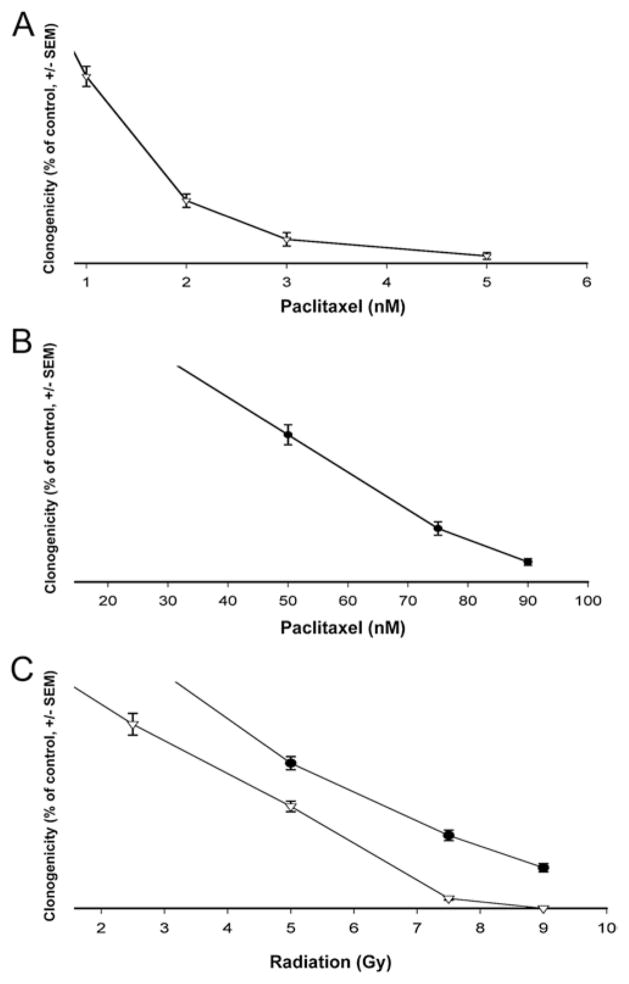
We employed a clonogenic assay to measure the global effect of radiation and paclitaxel on cellular viability. U373 and 9L cell lines were sensitive to paclitaxel and XRT. The 9L cells exhibited greater resistance to both treatments (Fig. 2). Paclitaxel’s IC50 (half maximal inhibitory concentration) was 37-fold greater for 9L than for U373 cells (IC50: 1.2 nM for U373, 44.6 nM for 9L) (Fig. 2A and B). The LD50 (median lethal dose) following single-fraction XRT was 2.7 Gy for U373 and 4.3 Gy for 9L cells (Fig. 2C).
Fig. 2.
Malignant glioma cell survival in response to paclitaxel and XRT. U373 (▽) and 9L (●) cells were plated in monolayer and exposed to paclitaxel or XRT on Day 1; clonogenicity was evaluated on Day 5 and expressed as mean relative to control (± SEM). The results represent a minimum of 2 independent experiments. A and B: Graphs showing changes in colony formation of U373 cells (A) and 9L cells (B) in response to paclitaxel exposure. C: Graph showing the single-fraction XRT dose-response curve for U373 and 9L cells.
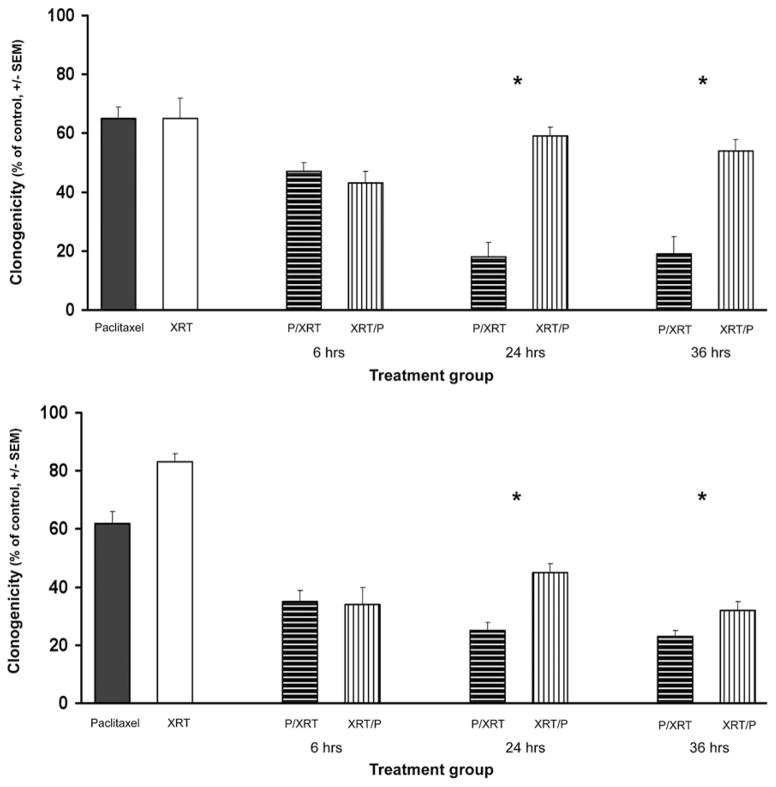
When U373 and 9L cells were treated with a combination of paclitaxel and XRT, there was significance in the order of treatment; an additive relationship was seen when XRT preceded paclitaxel exposure, whereas a synergistic effect was seen when paclitaxel exposure preceded XRT (Fig. 3). For instance, at the 24-hours time point, paclitaxel plus XRT had additive efficacy because this combination produced further reduction in colony formation than either treatment alone (U373: 9.2% decrease for XRT/P 24-hours group vs XRT or paclitaxel [p < 0.001, Mann-Whitney rank-sum test]; 9L: 27.4% decrease for XRT/P 24-hours group vs XRT (p < 0.001), or 45.8% decrease vs paclitaxel (p < 0.001)] (Fig. 3). However, synergistic interaction between paclitaxel and XRT was observed at the 24-hours time point, when paclitaxel was administered before XRT (U373: 72.3% decrease for P/XRT 24-hours group vs paclitaxel or XRT (p < 0.001); 9L: 59.7% decrease for P/XRT 24-hours group vs paclitaxel (p < 0.001), or 69.9% decrease vs XRT [p < 0.001]) (Fig. 3). Moreover, the difference in colony formation between the P/XRT and XRT/P groups was statistically significant (U373: 17.5% vs 59.1% [p < 0.001]; 9L: 25.5% vs 44.8% [p < 0.001]). Similar results were obtained for the 36-hours time point, but not for 6-hour exposure, indicating that cell accumulation in G2-M is necessary in order for the radiosensitizing effect of paclitaxel to occur (Fig. 3).
Fig. 3.
Synergistic effect of paclitaxel and XRT against malignant glioma cell lines. U373 (upper) and 9L (lower) cells were plated in monolayer and treated with paclitaxel (U373, 1 nM; 9L, 50 nM) or radiation (1 Gy). After the specified period of time (6, 24, 36 hours), cells originally exposed to paclitaxel were irradiated (P/XRT) and cells initially treated with radiation were exposed to paclitaxel (XRT/P). Colony formation was assessed on Day 5 for each group and plotted relative to control (mean ± SEM). The results represent a minimum of 2 independent experiments. * Statistically significant reduction in survival in P/XRT group compared to XRT/P group.
Intracranial Efficacy Study
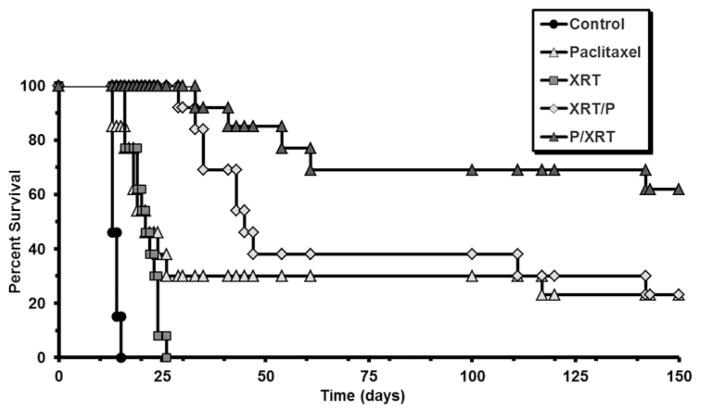
In rats bearing intracranial 9L gliosarcoma, individual treatments of 20-Gy single-fraction XRT or intracranially implanted paclitaxel microspheres significantly extended survival (median survival 13 days for control group, 21 days for paclitaxel-only or XRT-only groups, p < 0.0001) (Fig. 4). In animals receiving combination treatment, both administration of XRT before implantation of paclitaxel microspheres (XRT/P group) and pre-treatment with paclitaxel prior to XRT (P/XRT group) extended survival compared with control or either treatment alone (median survival 45 days for the XRT/P group and > 150 days for P/XRT group [p < 0.0001 vs control, p < 0.033 vs paclitaxel only, or p < 0.0001 vs XRT only]). Comparison of the dual-treatment protocols, however, revealed a synergistic improvement in survival when paclitaxel microspheres were implanted prior to XRT (median survival > 150 days for P/XRT vs 45 days for XRT/P, p < 0.036)]. In long-term survivors, there were no adverse events in relation to weight gain, grooming behavior, or motor deficits.
Fig. 4.
Kaplan-Meier curves demonstrating survival following treatment with intracranial delivery of paclitaxel and/or XRT. On Day 0, animals underwent intracranial implantation of 9L tumor. On Day 5, control animals underwent implantation of blank polymer in the tumor bed, while the paclitaxel-only and paclitaxel followed by XRT (P/XRT) groups received an intracranial implant of paclitaxel polymer; the XRT-only and XRT followed by paclitaxel (XRT/P) groups underwent 20-Gy single-fraction XRT. On Day 10, the order of treatments for the combination treatment groups was reversed such that the P/XRT group underwent XRT while the XRT/P group received an intracranial implant of paclitaxel polymer. Animal survival was assessed for 150 days.
Discussion
Innovative chemoradiotherapy modalities are clearly needed to improve local disease control and overall survival in patients suffering from malignant gliomas. The clinical utility of these regimens has been hindered by the BBB’s low permeability to most chemotherapeutic agents. Often the onset of dose-limiting systemic toxicity occurs and treatment must be halted before effective therapeutic dosing can be reached. The development and application of carmustine-loaded polymers have validated the safety and efficacy of local chemotherapy against malignant gliomas in experimental models3,5,30,35 and clinical trials.4,32,37 Controlled-release polymers have demonstrated improved patient survival and quality of life4,32,37 and due to advances in research in this field, the prognosis of malignant glioma has become far more promising. The median survival of patients with malignant gliomas has increased from less than 9 months to 21 months.23 Carmustine (BCNU, Gliadel), a controlled-release polymer formulation, was the first chemotherapeutic agent approved by the FDA for the treatment of malignant glioma in 2002, as the first drug to be approved for this indication in 23 years.26 Significant drug delivery advantages exist when controlled-release, biodegradable polymers are implanted, including zero-order release kinetics and polymer degradation. This eliminates the need for surgical removal posttreatment and allows for a safe distribution of the drug to the site of the tumor without reaching dose-limiting toxicity.26
Aside from carmustine, other pharmaceutical agents, including paclitaxel, have also been experimentally incorporated into controlled-release polymers and used in preclinical models of efficacy.20,30,35 In vivo application of convection-enhanced delivery of paclitaxel has been shown to be effective in prolonging survival in animal models,33 and convection-enhanced delivery of paclitaxel to patients with gliomas has been promising, with a 73% patient response rate in a clinical study of 15 patients.22 However, there remains a need for optimization of delivery both for increased efficacy and for reduced toxicity.22 In vivo tumor growth inhibition has been shown using liquid crystalline cubic phases, discs, and microparticles to carry paclitaxel to the tumor mass.25,34 Progress in mediated release of paclitaxel was demonstrated in laser-regulated paclitaxel nanospheres in animal models.40 Thermal gel depots (Oncogel) have also been shown to be effective modes of local delivery and treatment in animal models of both brain tumors30 and metastatic tumor in spinal models.31
Radiotherapy is a main treatment adjunct in the management of malignant gliomas. In response to ionizing irradiation, cells display characteristic variation in survival as a function of cell cycle phase, with the G2-M phase exhibiting the greatest sensitivity and the S and late G1 phases showing maximum resistance to radiation.38 Paclitaxel exerts its pleiotropic antineoplastic effects by binding to microtubules and interfering with their dynamic instability and thereby inducing arrested mitosis, disrupting the function of the microtubule cytoskeleton, and modulating the activity of apoptosis-regulating proteins.1 Because paclitaxel induces cytokinetic promotion of cells into the G2-M phase of the cell cycle, it is an attractive candidate as a radiosensitizer.29 In this study, we confirmed a time- and concentration-dependent increase in the distribution of malignant glioma cells in the G2-M phase of the cell cycle. With continued paclitaxel exposure, there was evidence of aberrant DNA histogram changes indicative of nuclear fragmentation and cell death (Fig. 1). Our results illustrate that paclitaxel exerts potent activity against human and rat malignant glioma cell lines in the nanomolar concentration range, and confirm our previous observation that human glioma cell lines are more sensitive to paclitaxel than rodent cell lines.7 Accumulation of glioma cells in the G2-M phase corresponded with increasing radiosensitivity. This was demonstrated by a synergistic reduction in colony formation when paclitaxel preceded radiation exposure, as opposed to when the cells were exposed to either agent alone or to radiation followed by paclitaxel. The radiosensitizing effect persisted for 6–36 hours after exposure in vitro.
Combined-modality protocols using paclitaxel and radiotherapy have been successful against various solid tumors.8 With malignant glioma, however, the results have been less promising, and positive results have been observed less often. This problem is due to paclitaxel’s poor BBB penetration.13,15,19 After systemic administration, paclitaxel is bound tightly to plasma albumin,18 and its accumulation in the CNS is hampered by the activity of multidrug resistance protein P-glycoprotein,28 which is highly expressed in the luminal surface of brain capillaries27 and tumor cells, including 9L and U373.21,39 Local delivery of paclitaxel as a radiosensitizing agent shows more promise. Administration of paclitaxel-loaded lipid nanocapsules through convection-enhanced delivery has shown efficacy in conjunction with radiotherapy in an animal model.33 However, there remains much to be explored in terms of administration, optimization, and the overall effects that local delivery of paclitaxel can have with respect to radiosensitization.
We have demonstrated the safety and efficacy of a biodegradable paclitaxel microsphere preparation for local CNS chemotherapy.20 Paclitaxel release from this formulation, at a 5–10 nM range, is detectable 5–7 mm from the implantation site 30 days after implantation, with histological evidence of paclitaxel’s activity persisting for more than 120 days. The concentrations of paclitaxel achievable in vivo correspond favorably with the paclitaxel concentrations necessary to achieve radiosensitivity in vitro. We therefore hypothesized that the microsphere formulation may overcome paclitaxel’s limitations as a systemically administered radiation sensitizer and tested it in an animal model of glioma.
Our results with 9L gliosarcoma indicate that while both XRT and paclitaxel improved animal survival when used as single agents, there was a significant benefit to the use of combined paclitaxel and XRT modalities (Fig. 4). Median survival in the paclitaxel-only and XRT-only groups was increased by 62% compared with control animals (21 days vs 13 days). When XRT was administered prior to paclitaxel, the median survival was more than doubled in relation to either monotherapy (45 days vs 21 days), and 3 animals survived to the end of the study. In contrast, when paclitaxel was used as pretreatment for XRT, median survival was never reached (> 150 days) and 8 of 13 animals survived to the end of the study. Also, the synergistic survival benefit due to paclitaxel pretreatment before XRT was clinically and statistically significant in relation to the additive effect observed with the use of XRT prior to paclitaxel (> 150 days vs 45 days). It is encouraging that the synergy between paclitaxel and XRT becomes evident when paclitaxel is administered as pretreatment for XRT, since this paradigm resembles the clinical scenario for chemoradiotherapy regimens. Specifically, our animal models used high-dose single-fraction radiotherapy, indicating that paclitaxel microspheres might be optimally used in the clinical setting as a radiosensitizer preceding single-fraction radiosurgery or other hypofractionated radiotherapy modalities.
Conclusions
We have demonstrated that paclitaxel can arrest human and rodent glioma cell lines at G2-M, rendering these cell lines more sensitive to ionizing radiation as measured by clonogenic assay. Using a novel biodegradable microsphere formulation of paclitaxel, we extend these observations to the preclinical 9L gliosarcoma model and demonstrate that pretreatment with locally delivered paclitaxel microspheres prior to XRT synergistically improves survival. Our findings support the continued development of novel paclitaxel formulations for use against malignant brain tumors. Future directions for advancing paclitaxel chemoradiotherapy in the treatment of malignant gliomas include using genetically engineered models of glioma, improving drug delivery kinetics, and optimizing radiotherapy regimens.
Abbreviations used in this paper
- BBB
blood-brain barrier
- DMSO
dimethyl sulfoxide
- FACS
fluorescence-activated cell sorting
- IC50
half maximal inhibitory concentration
- LD50
median lethal dose
- PPE
polyphosphoester
- SEM
standard error of the mean
- XRT
radiation treatment
Footnotes
Disclosure
This work was supported by Research Scholar Grant 116293-RSG-08-119-01-CCE from the American Cancer Society (B. Tyler), National Cancer Institute (NCI) U19 CA52857 (H. Brem), and National Institute of Neurologic Disease and Stroke (NINDS) K08 NS046461 (K. Walter).
Author contributions to the study and manuscript preparation include the following. Conception and design: Gabikian, Walter. Acquisition of data: Tyler, Gabikian, Walter. Analysis and interpretation of data: Tyler, Gabikian, Li. Drafting the article: Gabikian. Critically revising the article: all authors. Reviewed submitted version of manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: Tyler. Statistical analysis: Tyler, Gabikian, Brem.
References
- 1.Blagosklonny MV, Fojo T. Molecular effects of paclitaxel: myths and reality (a critical review) Int J Cancer. 1999;83:151–156. doi: 10.1002/(sici)1097-0215(19991008)83:2<151::aid-ijc1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 2.Boockvar JA, Tsiouris AJ, Hofstetter CP, Kovanlikaya I, Fralin S, Kesavabhotla K, et al. Safety and maximum tolerated dose of superselective intraarterial cerebral infusion of bevacizumab after osmotic blood-brain barrier disruption for recurrent malignant glioma. Clinical article. J Neurosurg. 2011;114:624–632. doi: 10.3171/2010.9.JNS101223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brem H, Gabikian P. Biodegradable polymer implants to treat brain tumors. J Control Release. 2001;74:63–67. doi: 10.1016/s0168-3659(01)00311-x. [DOI] [PubMed] [Google Scholar]
- 4.Brem H, Piantadosi S, Burger PC, Walker M, Selker R, Vick NA, et al. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. Lancet. 1995;345:1008–1012. doi: 10.1016/s0140-6736(95)90755-6. [DOI] [PubMed] [Google Scholar]
- 5.Brem S, Tyler B, Li K, Pradilla G, Legnani F, Caplan J, et al. Local delivery of temozolomide by biodegradable polymers is superior to oral administration in a rodent glioma model. Cancer Chemother Pharmacol. 2007;60:643–650. doi: 10.1007/s00280-006-0407-2. [DOI] [PubMed] [Google Scholar]
- 6.Burger PC, Dubois PJ, Schold SC, Jr, Smith KR, Jr, Odom GL, Crafts DC, et al. Computerized tomographic and pathologic studies of the untreated, quiescent, and recurrent glioblastoma multiforme. J Neurosurg. 1983;58:159–169. doi: 10.3171/jns.1983.58.2.0159. [DOI] [PubMed] [Google Scholar]
- 7.Cahan MA, Walter KA, Colvin OM, Brem H. Cytotoxicity of taxol in vitro against human and rat malignant brain tumors. Cancer Chemother Pharmacol. 1994;33:441–444. doi: 10.1007/BF00686276. [DOI] [PubMed] [Google Scholar]
- 8.Choy H. Taxanes in combined modality therapy for solid tumors. Crit Rev Oncol Hematol. 2001;37:237–247. doi: 10.1016/s1040-8428(00)00112-8. [DOI] [PubMed] [Google Scholar]
- 9.Combs SE, Zipp L, Rieken S, Habermehl D, Brons S, Winter M, et al. In vitro evaluation of photon and carbon ion radiotherapy in combination with chemotherapy in glioblastoma cells. Radiat Oncol. 2012;7:9. doi: 10.1186/1748-717X-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeAngelis LM. Brain tumors. N Engl J Med. 2001;344:114–123. doi: 10.1056/NEJM200101113440207. [DOI] [PubMed] [Google Scholar]
- 11.Doolittle ND, Petrillo A, Bell S, Cummings P, Eriksen S. Blood-brain barrier disruption for the treatment of malignant brain tumors: The National Program. J Neurosci Nurs. 1998;30:81–90. doi: 10.1097/01376517-199804000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Fellner S, Bauer B, Miller DS, Schaffrik M, Fankhänel M, Spruss T, et al. Transport of paclitaxel (Taxol) across the blood-brain barrier in vitro and in vivo. J Clin Invest. 2002;110:1309–1318. doi: 10.1172/JCI15451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fetell MR, Grossman SA, Fisher JD, Erlanger B, Rowinsky E, Stockel J, et al. Preirradiation paclitaxel in glioblastoma multiforme: efficacy, pharmacology, and drug interactions. J Clin Oncol. 1997;15:3121–3128. doi: 10.1200/JCO.1997.15.9.3121. [DOI] [PubMed] [Google Scholar]
- 14.Gallia GL, Brem S, Brem H. Local treatment of malignant brain tumors using implantable chemotherapeutic polymers. J Natl Compr Canc Netw. 2005;3:721–728. doi: 10.6004/jnccn.2005.0042. [DOI] [PubMed] [Google Scholar]
- 15.Glantz MJ, Choy H, Kearns CM, Mills PC, Wahlberg LU, Zuhowski EG, et al. Paclitaxel disposition in plasma and central nervous systems of humans and rats with brain tumors. J Natl Cancer Inst. 1995;87:1077–1081. doi: 10.1093/jnci/87.14.1077. [DOI] [PubMed] [Google Scholar]
- 16.Hall WA, Rustamzadeh E, Asher AL. Convection-enhanced delivery in clinical trials. Neurosurg Focus. 2003;14(2):E2. doi: 10.3171/foc.2003.14.2.3. [DOI] [PubMed] [Google Scholar]
- 17.Jordan MA, Wendell K, Gardiner S, Derry WB, Copp H, Wilson L. Mitotic block induced in HeLa cells by low concentrations of paclitaxel (Taxol) results in abnormal mitotic exit and apoptotic cell death. Cancer Res. 1996;56:816–825. [PubMed] [Google Scholar]
- 18.Kumar GN, Walle UK, Bhalla KN, Walle T. Binding of taxol to human plasma, albumin and alpha 1-acid glycoprotein. Res Commun Chem Pathol Pharmacol. 1993;80:337–344. [PubMed] [Google Scholar]
- 19.Langer CJ, Ruffer J, Rhodes H, Paulus R, Murray K, Movsas B, et al. Phase II radiation therapy oncology group trial of weekly paclitaxel and conventional external beam radiation therapy for supratentorial glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2001;51:113–119. doi: 10.1016/s0360-3016(01)01597-8. [DOI] [PubMed] [Google Scholar]
- 20.Li KW, Dang W, Tyler BM, Troiano G, Tihan T, Brem H, et al. Polilactofate microspheres for Paclitaxel delivery to central nervous system malignancies. Clin Cancer Res. 2003;9:3441–3447. [PubMed] [Google Scholar]
- 21.Liang BC. Effects of hypoxia on drug resistance phenotype and genotype in human glioma cell lines. J Neurooncol. 1996;29:149–155. doi: 10.1007/BF00182138. [DOI] [PubMed] [Google Scholar]
- 22.Lidar Z, Mardor Y, Jonas T, Pfeffer R, Faibel M, Nass D, et al. Convection-enhanced delivery of paclitaxel for the treatment of recurrent malignant glioma: a phase I/II clinical study. J Neurosurg. 2004;100:472–479. doi: 10.3171/jns.2004.100.3.0472. [DOI] [PubMed] [Google Scholar]
- 23.McGirt MJ, Than KD, Weingart JD, Chaichana KL, Attenello FJ, Olivi A, et al. Gliadel (BCNU) wafer plus concomitant temozolomide therapy after primary resection of glioblastoma multiforme. Clinical article. J Neurosurg. 2009;110:583–588. doi: 10.3171/2008.5.17557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mielke S, Sparreboom A, Mross K. Peripheral neuropathy: a persisting challenge in paclitaxel-based regimes. Eur J Cancer. 2006;42:24–30. doi: 10.1016/j.ejca.2005.06.030. [DOI] [PubMed] [Google Scholar]
- 25.Naraharisetti PK, Ong BYS, Xie JW, Lee TKY, Wang CH, Sahinidis NV. In vivo performance of implantable biodegradable preparations delivering Paclitaxel and Etanidazole for the treatment of glioma. Biomaterials. 2007;28:886–894. doi: 10.1016/j.biomaterials.2006.09.044. [DOI] [PubMed] [Google Scholar]
- 26.Raza SM, Pradilla G, Legnani FG, Thai QA, Olivi A, Weingart JD, et al. Local delivery of antineoplastic agents by controlled-release polymers for the treatment of malignant brain tumours. Expert Opin Biol Ther. 2005;5:477–494. doi: 10.1517/14712598.5.4.477. [DOI] [PubMed] [Google Scholar]
- 27.Seetharaman S, Barrand MA, Maskell L, Scheper RJ. Multidrug resistance-related transport proteins in isolated human brain microvessels and in cells cultured from these isolates. J Neurochem. 1998;70:1151–1159. doi: 10.1046/j.1471-4159.1998.70031151.x. [DOI] [PubMed] [Google Scholar]
- 28.Sparreboom A, van Asperen J, Mayer U, Schinkel AH, Smit JW, Meijer DK, et al. Limited oral bioavailability and active epithelial excretion of paclitaxel (Taxol) caused by P-glycoprotein in the intestine. Proc Natl Acad Sci U S A. 1997;94:2031–2035. doi: 10.1073/pnas.94.5.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tishler RB, Geard CR, Hall EJ, Schiff PB. Taxol sensitizes human astrocytoma cells to radiation. Cancer Res. 1992;52:3495–3497. [PubMed] [Google Scholar]
- 30.Tyler B, Fowers KD, Li KW, Recinos VR, Caplan JM, Hdeib A, et al. A thermal gel depot for local delivery of paclitaxel to treat experimental brain tumors in rats. Laboratory investigation. J Neurosurg. 2010;113:210–217. doi: 10.3171/2009.11.JNS08162. [DOI] [PubMed] [Google Scholar]
- 31.Tyler BM, Hdeib A, Caplan J, Legnani FG, Fowers KD, Brem H, et al. Delayed onset of paresis in rats with experimental intramedullary spinal cord gliosarcoma following intratumoral administration of the paclitaxel delivery system Onco-Gel. Laboratory investigation. J Neurosurg Spine. 2012;16:93–101. doi: 10.3171/2011.9.SPINE11435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valtonen S, Timonen U, Toivanen P, Kalimo H, Kivipelto L, Heiskanen O, et al. Interstitial chemotherapy with carmustine-loaded polymers for high-grade gliomas: a randomized double-blind study. Neurosurgery. 1997;41:44–49. doi: 10.1097/00006123-199707000-00011. [DOI] [PubMed] [Google Scholar]
- 33.Vinchon-Petit S, Jarnet D, Paillard A, Benoit JP, Garcion E, Menei P. In vivo evaluation of intracellular drug-nanocarriers infused into intracranial tumours by convection-enhanced delivery: distribution and radiosensitisation efficacy. J Neurooncol. 2010;97:195–205. doi: 10.1007/s11060-009-0012-4. [DOI] [PubMed] [Google Scholar]
- 34.von Eckardstein KL, Reszka R, Kiwit JC. Intracavitary chemotherapy (paclitaxel/carboplatin liquid crystalline cubic phases) for recurrent glioblastoma—clinical observations. J Neurooncol. 2005;74:305–309. doi: 10.1007/s11060-004-7559-x. [DOI] [PubMed] [Google Scholar]
- 35.Walter KA, Cahan MA, Gur A, Tyler B, Hilton J, Colvin OM, et al. Interstitial taxol delivered from a biodegradable polymer implant against experimental malignant glioma. Cancer Res. 1994;54:2207–2212. [PubMed] [Google Scholar]
- 36.Walter KA, Tamargo RJ, Olivi A, Burger PC, Brem H. Intratumoral chemotherapy. Neurosurgery. 1995;37:1128–1145. [PubMed] [Google Scholar]
- 37.Westphal M, Hilt DC, Bortey E, Delavault P, Olivares R, Warnke PC, et al. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro Oncol. 2003;5:79–88. doi: 10.1215/S1522-8517-02-00023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Withers HR, Mason K, Reid BO, Dubravsky N, Barkley HT, Jr, Brown BW, et al. Response of mouse intestine to neutrons and gamma rays in relation to dose fractionation and division cycle. Cancer. 1974;34:39–47. doi: 10.1002/1097-0142(197407)34:1<39::aid-cncr2820340107>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 39.Yamashima T, Ohnishi T, Nakajima Y, Terasaki T, Tanaka M, Yamashita J, et al. Uptake of drugs and expression of P-glyco-protein in the rat 9L glioma. Exp Brain Res. 1993;95:41–50. doi: 10.1007/BF00229652. [DOI] [PubMed] [Google Scholar]
- 40.You J, Shao R, Wei X, Gupta S, Li C. Near-infrared light triggers release of Paclitaxel from biodegradable microspheres: photothermal effect and enhanced antitumor activity. Small. 2010;6:1022–1031. doi: 10.1002/smll.201000028. [DOI] [PMC free article] [PubMed] [Google Scholar]