Abstract
Transforming growth factor beta (TGF-β) signaling pathway is involved in diverse cellular processes, including cell proliferation, differentiation, adhesion, apoptosis, and some human diseases including cancer. Smad proteins function as mediators of intracellular signal transduction of TGF-β. Following their phosphorylation by TGF-β receptor I, Smad2 and Smad3 form a heteromeric complex with Smad4 and then are translocated into the nucleus where they bind to other co-factors and regulate the expression of target genes. ERG (Ets Related Gene) belongs to the ETS family of transcriptional factors. Chromosomal rearrangement of TMPRSS2 gene and ERG gene has been found in the majority of prostate cancers. Over-expression of full length or truncated ERG proteins is associated with a higher rate of recurrence and unfavorable prognosis. In this review, we focus on recent understanding of regulation of TGF-β/Smads signaling pathway by ERG proteins in prostate cancer.
Keywords: TGF-β, Smad3, Phosphorylation, ERG, TMPRSS2-ERG
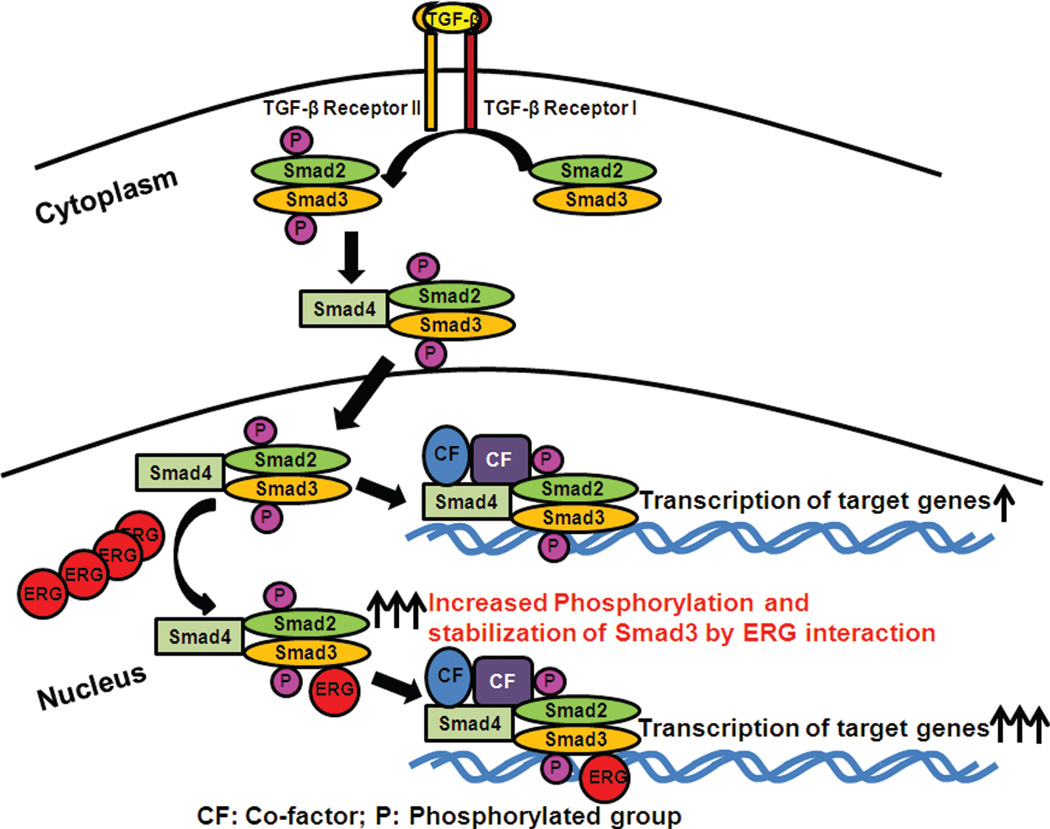
Since transforming growth factor-β1 (TGF-β1) was discovered in 1983, it is well accepted that TGF-β signaling pathway affects cell proliferation, differentiation, migration, adhesion, apoptosis, embryonic development, and is even involved in human diseases, including cardiovascular, fibrosis, reproductive, wounded healing disorders and cancer (Drabsch, 2012; Larsson, 2005; Sakaki-Yumoto, 2013). TGF-β1 isoform is expressed abundantly and ubiquitously in all cells and is secreted as a complex with many proteins into extra-cellular matrix. It is not very well known how TGF-β activates downstream signaling pathways. Once activated, the TGF-β ligands regulate cellular processes through binding to cell surface receptors (Elliott, 2005; Kang, 2009). TGF-β1 signaling involves its binding to the TGF-β receptor type II (TGF-βRII) (Fig. 1), which allows it to recruit TGF-β receptor I (TGF-βRI) and assemble it as a heterodimeric receptor complex. TGF-βRII phosphorylates TGF-βRI, which, inturn, phosphorylates R-Smads (Receptor-regulated Smads, including Smad2 and Smad3 proteins). Phosphorylated Smad2 and Smad3 interact with common Smad (Smad4) to form a complex and then translocate into the nucleus where they bind to transcriptional coactivators or corepressors and regulate transcription of target genes (Fig. 1) (Feng, 2005; Massague, 2012). Smads contain a highly conserved N-terminal domain (MH1) and a conserved C-terminal domain (MH2). The conserved domain MH1 functions as a DNA-binding domain whereas MH2 domain transiently interacts with TGF-βRI. Activated TGF-β receptor complex phosphorylates R-Smads at the sequence SSXS in the C-terminal tail of the MH2 domain. This phosphorylation of R-Smads is required for their activation. Smad4 is not phosphorylated because it lacks SSXS motif (Yue, 2001). In order for the Smad4-R-Smad complexes to bind to specific target genes, they need to recruit transcriptional coactivators, corepressors, and chromatin remodeling factors. Through this complex interaction, they regulate hundreds of target genes at once (Massague, 2008).
Figure 1.
Phosphortlated Smad3 is stabilized by ERG in ERG-positive prostate cancer cells. Smad3 is phosphorylated by TGF-β binding to receptor. Phosphorylated Smad3 forms a complex with phosphorylated Smad2 and the common Smad (Smad4) and translocates to the nucleus. Phosphorylated Smad3 is stabilized by binding to ERG in the nucleus.
Ubiquitin-dependent protein degradation plays a key role in various biological processes. Smad proteins are degraded by the ubiquitin-proteasome pathway. After TGF-β binding to membrane receptor, phosphorylated R-Smad and Smad4 complexes are formed and translocated into nucleus, where they activate or repress target genes. Heterologous Smad complex can remain for several hours in the nucleus, and then R-Smads are dephosphorylated at a low rate. This dephosphorylation results in the dissociation from Smad4. In the nucleus, Smad3 interacts with a RING finger protein, ROC1, through its C-terminal MH2 domain. Smad3 bound to ROC1-SCFFbw1a (an E3 ubiquitin ligase complex) is then exported from the nucleus to the cytoplasm where it is subjected to proteasomal degradation (Fukuchi, 2001; Ten Dijke, 2004). This ubiquitin-proteasome mediated degradation of Smads regulates the levels of Smads in cell (Derynck, 2003). Despite the fact that Smads are not enzymes they still function as key regulators of TGF-β signaling and, thereby show profound effect on cellular responses with small changes in Smad protein levels (de Caestecker, 2000).
Drs. Reddy and Rao have identified the ERG gene (ETS Related Gene) that belongs to the ETS family of transcriptional factors (Rao, 1987; Reddy, 1987) and have shown that ERG functions as a sequence specific transcriptional activator (Siddique, 1993). ERG gene was also involved in chromosome translocations in Ewing family of tumors as well as in leukemias (Liu, 2013; Ohno and Prasad, 1994; Rao, 1988; Sreenath and Tsuzuki, 2011). It has also been shown to be a common genetic alteration of a recurrent gene fusion between ERG and the androgen responsive gene TMPRSS2 (transmembrane protease, serine 2) on chromosome 21 in prostate cancer (Tomlins, 2005). Approximately 50% of prostate cancer patients have a fusion of TMPRSS2 and ERG genes (Furusato, 2008; Shah, 2009). In these prostate cancers, ERG gene expression is significantly up regulated by the androgen-responsive promoter of TMPRSS2. To date, the role of TMPRSS2-ERG fusion protein in prostate cancer is not well understood (Brase, 2011; Hossain, 2013; Rosen, 2012). Recent results suggest that over-expression of ERG may be useful as a biomarker for prostate cancer diagnosis (Hossain, 2013).
ERG-positive patients have a low rate of high Gleason grade, poor differentiation, and African American ethnicity compared to ERG-negative patients (Hu, 2008). Consistent with this view, it was also shown that the frequency of ERG-positive tumors was significantly greater among Caucasian Americans than among African Americans (Rosen, 2012). Some studies also suggest a causal role of ERG protein in prostate cancers (Klezovitch, 2008). TMPRSS2-ERG gene fusions may be cancer-initiating, and expressed at both RNA and protein levels in prostate cancer stem cells (Klezovitch, 2008; Polson, 2013). Recently, Dr. Reddy’s group has shown that an anti-epileptic drug targets ERG-positive prostate cancer cells through the activation of tumor suppressors and nuclear receptors (Fortson, 2011). Similar results were also observed in the Ewing family of tumors (Kayarthodi and Reddy et al., unpublished observations).
TGF-β/Smad signaling plays an important role in the regulation of growth of normal and cancer cells (de Caestecker, 2000; Tian, 2011; Yue, 2001). This signaling pathway has been acknowledged to have a dual role in tumor progression, which is a tumor suppressor for normal epithelial and early stages of cancer cells. It is also a tumor promoter in the last steps of the metastatic disease (Kocic and Miles, 2012). However, it is not clear how this signaling pathway plays a role in ERG-positive prostate cancers, and if there is crosstalk between ERG onco-protein and TGF-β/Smads signaling pathway. Recent studies have shown that ERG protein regulates TGF-β/Smads pathway (Fang and Reddy unpublished observations). We find that ERG can enhance the activity of Smad3 in absence or presence of TGF-β (Fang and Reddy unpublished observations). Furthermore, these results revealed that ERG onco-protein physically interacts with P-Smad3, and stabilized phospho-Smad3 protein levels (Fig. 1). Possible implications of the above mechanism are: first, ERG binds to P-Smad3 and make latter not to bind to other proteins especially involved in ubiquitination pathway and thereby reduce the amount of ubiquited Smad3 and, secondly, ERG bind to P-Smad3 and, thereby inhibits the dephosphorylation of P-Smad3, which leads to inhibition of export of Smad3 from nucleus to cytoplasm. The above-mentioned two novel possibilities may result in an increased amount of phosphorylated-Smad3 in the nucleus and enhance the activity of TGF-β/Smads (Fig. 1). These results provide the first direct evidence that ERG onco-protein contributes to prostate cancer progression by enhancing TGF-β/Smads-signaling pathway in ERG-positive prostate cancers (Fang and Reddy unpublished observations). Therefore, it is possible that therapeutic agents that interfere with the interaction of Smad3 and ERG onco-protein can be used to treat ERG-positive prostate cancers.
Acknowledgments
We thank all the members of Reddy and Rao laboratories. This study was funded by in part by the U.S. Army Medical Research and Materiel Command under W81XWH-08-1-0628, W81XWH-09-1-0236, W81XWH-10-1-0418 (E. Shyam P. Reddy) and the Georgia Cancer Coalition Distinguished Cancer Scholar award (E. Shyam P. Reddy and Veena N. Rao), NIH 2U54CA118948, 3U54CA118638-05S1, U54/56 More-house School of Medicine/University of Alabama at Birmingham/Tuskegee University Partnership Grant (U54 CA118638) and the Research Centers in Minority Institutions (G-12-RR003034).
REFERENCES
- Brase JC, Johannes M, Mannsperger H, Falth M, Metzger J, Kacprzyk LA, Andrasiuk T, Gade S, Meister M, Sirma H, Sauter G, Simon R, Schlomm T, Beissbarth T, Korf U, Kuner R, Sultmann H. TMPRSS2-ERG-specific transcriptional modulation is associated with prostate cancer biomarkers and tgf-beta signaling. BMC Cancer. 2011;11:507. doi: 10.1186/1471-2407-11-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Caestecker MP, Piek E, Roberts AB. Role of transforming growth factor-β signaling in cancer. J. Natl. Cancer Inst. 2000;92:1388–1402. doi: 10.1093/jnci/92.17.1388. [DOI] [PubMed] [Google Scholar]
- Derynck R, Zhang YE. Smad-dependent and smad-independent pathways in TGF-β family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- Drabsch Y, ten Dijke P. TGF-β signalling and its role in cancer progression and metastasis. Cancer Metastasis Rev. 2012;31:553–568. doi: 10.1007/s10555-012-9375-7. [DOI] [PubMed] [Google Scholar]
- Elliott RL, Blobe GC. Role of transforming growth factor beta in human cancer. J. Clin. Oncol. 2005;23:2078–2093. doi: 10.1200/JCO.2005.02.047. [DOI] [PubMed] [Google Scholar]
- Feng XH, Derynck R. Specificity and versatility in TGF-β signaling through smads. Annu. Rev. Cell Dev. Biol. 2005;21:659–693. doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- Fortson WS, Kayarthodi S, Fujimura Y, Xu H, Matthews R, Grizzle WE, Rao VN, Bhat GK, Reddy ES. Histone deacetylase inhibitors, valproic acid and trichostatin-a induce apoptosis and affect acetylation status of p53 in ERG-positive prostate cancer cells. Int. J. Oncol. 2011;39:111–119. doi: 10.3892/ijo.2011.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuchi M, Imamura T, Chiba T, Ebisawa T, Kawabata M, Tanaka K, Miyazono K. Ligand-dependent degradation of smad3 by a ubiquitin ligase complex of ROC1 and associated proteins. Mol. Biol. Cell. 2001;12:1431–1443. doi: 10.1091/mbc.12.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furusato B, Gao CL, Ravindranath L, Chen Y, Cullen J, McLeod DG, Dobi A, Srivastava S, Petrovics G, Sesterhenn IA. Mapping of TMPRSS2-ERG fusions in the context of multi-focal prostate cancer. Mod. Pathol. 2008;21:67–75. doi: 10.1038/modpathol.3800981. [DOI] [PubMed] [Google Scholar]
- Hossain D, Bostwick DG. Significance of the TMPRSS2:ERG gene fusion in prostate cancer. BJU Int. 2013;111:834–835. doi: 10.1111/bju.12120. [DOI] [PubMed] [Google Scholar]
- Hu Y, Dobi A, Sreenath T, Cook C, Tadase AY, Ravindranath L, Cullen J, Furusato B, Chen Y, Thangapazham RL, Mohamed A, Sun C, Sesterhenn IA, McLeod DG, Petrovics G, Srivastava S. Delineation of TMPRSS2-ERG splice variants in prostate cancer. Clin. Cancer Res. 2008;14:4719–4725. doi: 10.1158/1078-0432.CCR-08-0531. [DOI] [PubMed] [Google Scholar]
- Kang JS, Liu C, Derynck R. New regulatory mechanisms of TGF-β receptor function. Trends. Cell Biol. 2009;19:385–394. doi: 10.1016/j.tcb.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Klezovitch O, Risk M, Coleman I, Lucas JM, Null M, True LD, Nelson PS, Vasioukhin V. A causal role for ERG in neoplastic transformation of prostate epithelium. Proc. Natl. Acad. Sci. USA. 2008;105:2105–2110. doi: 10.1073/pnas.0711711105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocic J, Bugarski D, Santibanez JF. Smad3 is essential for transforming growth factor-β1-induced urokinase type plasminogen activator expression and migration in transformed keratinocytes. Eur. J. Cancer. 2012;48:1550–1557. doi: 10.1016/j.ejca.2011.06.043. [DOI] [PubMed] [Google Scholar]
- Larsson J, Karlsson S. The role of smad signaling in hematopoiesis. Oncogene. 2005;24:5676–5692. doi: 10.1038/sj.onc.1208920. [DOI] [PubMed] [Google Scholar]
- Liu F, Gao L, Jing Y, Xu YY, Ding Y, Zhou MH, Ma C, Li MY, Sun JZ, Wang LL, Yu L. Detection and clinical significance of gene rearrangements in chinese patients with adult acute lymphoblastic leukemia. Leuk Lymphoma. 2013;54:1521–1526. doi: 10.3109/10428194.2012.754888. [DOI] [PubMed] [Google Scholar]
- Massague J. TGFβ signalling in context. Nat. Rev. Mol. Cell. Biol. 2012;13:616–630. doi: 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massague J. TGFβ in cancer. Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles FL, Tung NS, Aguiar AA, Kurtoglu S, Sikes RA. Increased TGF-β1-mediated suppression of growth and motility in castrate-resistant prostate cancer cells is consistent with smad2/3 signaling. Prostate. 2012;72:1339–1350. doi: 10.1002/pros.22482. [DOI] [PubMed] [Google Scholar]
- Ohno T, Ouchida M, Lee L, Gatalica Z, Rao VN, Reddy ES. The EWS gene, involved in ewing family of tumors, malignant melanoma of soft parts and desmoplastic small round cell tumors, codes for an RNA binding protein with novel regulatory domains. Oncogene. 1994;9:3087–3097. [PubMed] [Google Scholar]
- Polson ES, Lewis JL, Celik H, Mann VM, Stower MJ, Simms MS, Rodrigues G, Collins AT, Maitland NJ. Monoallelic expression of TMPRSS2/ERG in prostate cancer stem cells. Nat. Commun. 2013;4:1623. doi: 10.1038/ncomms2627. [DOI] [PubMed] [Google Scholar]
- Prasad DD, Ouchida M, Lee L, Rao VN, Reddy ES. TLS/FUS fusion domain of TLS/FUS-erg chimeric protein resulting from the t(16;21) chromosomal translocation in human myeloid leukemia functions as a transcriptional activation domain. Oncogene. 1994;9:3717–3729. [PubMed] [Google Scholar]
- Rao VN, Modi WS, Drabkin HD, Patterson DO, S J, Papas TS, Reddy ES. The human erg gene maps to chromosome 21, band q22: Relationship to the 8; 21 translocation of acute myelogenous leukemia. Oncogene. 1988;3:497–500. [PubMed] [Google Scholar]
- Rao VN, Papas TS, Reddy ES. Erg, a human ets-related gene on chromosome 21: Alternative splicing, polyadenylation, and translation. Science. 1987;237:635–639. doi: 10.1126/science.3299708. [DOI] [PubMed] [Google Scholar]
- Reddy ES, Rao VN, Papas TS. The erg gene: A human gene related to the ets oncogene. Proc. Natl. Acad. Sci. USA. 1987;84:6131–6135. doi: 10.1073/pnas.84.17.6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen P, Pfister D, Young D, Petrovics G, Chen Y, Cullen J, Bohm D, Perner S, Dobi A, McLeod DG, Sesterhenn IA, Srivastava S. Differences in frequency of ERG oncoprotein expression between index tumors of caucasian and african american patients with prostate cancer. Urology. 2012;80:749–753. doi: 10.1016/j.urology.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen P, Sesterhenn IA, Brassell SA, McLeod DG, Srivastava S, Dobi A. Clinical potential of the ERG oncoprotein in prostate cancer. Nat. Rev. Urol. 2012;9:131–137. doi: 10.1038/nrurol.2012.10. [DOI] [PubMed] [Google Scholar]
- Sakaki-Yumoto M, Katsuno Y, Derynck R. TGF-β family signaling in stem cells. Biochim. Biophys. Acta. 2013;1830:2280–2296. doi: 10.1016/j.bbagen.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah RB, Chinnaiyan AM. The discovery of common recurrent transmembrane protease serine 2 (TMPRSS2)-erythroblastosis virus E26 transforming sequence (ETS) gene fusions in prostate cancer: Significance and clinical implications. Adv. Anat. Pathol. 2009;16:145–153. doi: 10.1097/PAP.0b013e3181a12da7. [DOI] [PubMed] [Google Scholar]
- Siddique HR, Rao VN, Lee L, Reddy ES. Characterization of the DNA binding and transcriptional activation domains of the erg protein. Oncogene. 1993;8:1751–1755. [PubMed] [Google Scholar]
- Sreenath TL, Dobi A, Petrovics G, Srivastava S. Oncogenic activation of ERG: A predominant mechanism in prostate cancer. J. Carcinog. 2011;10:37. doi: 10.4103/1477-3163.91122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijke PT, Hill CS. New insights into TGF-β-smad signalling. Trends Biochem. Sci. 2004;29:265–273. doi: 10.1016/j.tibs.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Tian M, Neil JR, Schiemann WP. Transforming growth factor-β and the hallmarks of cancer. Cell Signal. 2011;23:951–962. doi: 10.1016/j.cellsig.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–648. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- Tsuzuki S, Taguchi O, Seto M. Promotion and maintenance of leukemia by ERG. Blood. 2011;117:3858–3868. doi: 10.1182/blood-2010-11-320515. [DOI] [PubMed] [Google Scholar]
- Yue J, Mulder KM. Transforming growth factor-β signal transduction in epithelial cells. Pharmacol. Ther. 2001;91:1–34. doi: 10.1016/s0163-7258(01)00143-7. [DOI] [PubMed] [Google Scholar]