Abstract
Neurogenesis persists in the adult subventricular zone (SVZ) of the mammalian brain. During aging, the SVZ neurogenic capacity undergoes a progressive decline, which is attributed to a decrease in the population of neural stem cells (NSCs). However, the behavior of the NSCs that remain in the aged brain is not fully understood. Here we performed a comparative ultrastructural study of the SVZ niche of 2-month-old and 24-month-old male C57BL/6 mice, focusing on the NSC population. Using thymidine-labeling, we showed that residual NSCs in the aged SVZ divide less frequently than those in young mice. We also provided evidence that ependymal cells are not newly generated during senescence, as others studies suggest. Remarkably, both astrocytes and ependymal cells accumulated a high number of intermediate filaments and dense bodies during aging, resembling reactive cells. A better understanding of the changes occurring in the neurogenic niche during aging will allow us to develop new strategies for fighting neurological disorders linked to senescence.
Keywords: subventricular zone, neural stem cells, astrocytes, ependymal cells, aging, ultrastructure
Introduction
Adult neurogenesis persists in the adult mammalian brain throughout life. The subventricular zone (SVZ) of the lateral ventricles is the largest source of neural stem cells (NSCs) in the neurogenic process (Alvarez-Buylla and Garcia-Verdugo, 2002; Doetsch et al., 1997). In this region, NSCs are identified as a subpopulation of astrocytes called B1 astrocytes, which reside adjacent to the ependymal cells (type E cells) that separate the SVZ from the ventricle. Additionally, the SVZ presents a subpopulation of non-neurogenic astrocytes (B2 astrocytes) that are located at the underlying striatal parenchyma and have higher number of intermediate filaments (Doetsch et al., 1999a; Gil-Perotin et al., 2009; Ihrie and Alvarez-Buylla, 2008; Morrens et al., 2012). Contrary to B2 astrocytes, B1 astrocytes extend an apical process between ependymal cells to make contact with the ventricle. This cellular process ends in a primary cilium that is essential for proliferation (Doetsch et al., 1997; Han et al., 2008; Mirzadeh et al., 2008; Morrens et al., 2012). In rodents, B1 astrocytes proliferate and give rise to rapidly dividing progenitor cells (type C cells), which generate neuroblasts (type A cells) that differentiate into neurons (Doetsch et al., 1999b; Imayoshi et al., 2008; Lazarini and Lledo, 2011; Luskin et al., 1997; Ponti et al., 2013). The different cellular components of the SVZ interact with each other to regulate the neurogenic process and are intimately associated with the extracellular matrix and blood vessels of the microenvironment (Ihrie and Alvarez-Buylla, 2011; Kazanis et al., 2010; Lim et al., 2000; Shen et al., 2008; Tavazoie et al., 2008).
Several studies demonstrate that the neurogenic potential of the SVZ declines during aging due to a progressive loss of primary neural precursors (Bouab et al., 2011; Capilla-Gonzalez et al., 2013a,b; Luo et al., 2006; Maslov et al., 2004). However, the behavior of the NSCs remaining in the aged SVZ is poorly understood. For instance, the ability of these residual NSCs to divide is still a subject of debate. While some reports suggest that NSCs present increased proliferation during aging (Shook et al., 2012; Stoll et al., 2011), others indicate that they divide less frequently (Ahlenius et al., 2009; Bouab et al., 2011; Encinas and Sierra, 2012; Gritti et al., 2009). Moreover, it was suggested that NSCs can modify their traditional cell differentiation process to produce new ependymal cells during aging (Luo et al., 2008). In this study, we focus on the age-related changes in the astrocytes and ependymal cells within the SVZ. We demonstrate that (1) B1 astrocytes (NSCs) in the aged SVZ divided less frequently than those in young mice; (2) ependymal cells were not found to proliferate or regenerate during aging; and (3) remaining astrocytes and ependymal cells acquire a reactive phenotype during aging. This study provides new information about the effects of aging on the SVZ neurogenic niche.
Materials and Methods
Animals
Male C57BL/6 mice (2 and 24-months-old) from National Institute on Aging (Baltimore, MD) and Charles River Laboratory (Barcelona, Spain) were used in all experiments (nyoung=17, naged=19). Animals were housed under a 12 h light/dark cycle with food and water available ad libitum. Mice were treated according to the European Communities Council (86/609/EEC) and the Johns Hopkins Animal Care and Use Committee guidelines and followed standard animal care and use protocols.
Administration of 5-bromo-2-deoxyuridine
The 5-bromo-2’-deoxyuridine (BrdU, Sigma Aldrich, Dorset, UK) is an exogenous marker which is incorporated into newly synthesized DNA of replicating cells during the S phase. To study proliferation, animals received a single intraperitoneal injection of BrdU (50 mg/kg b.wt.) and were euthanized after 2 h (nyoung=4, naged=6).
Tritiated Thymidine Administration
For ultrastructural identification of the proliferative cells and newly generated cells, animals received a single daily dose of 1.67 μL/g b.wt. of 1mCi tritiated thymidine (3H-Thy) (specific activity 5 Ci/mmol) (Amersham Biosciences, Uppsala, Sweden) for 10 consecutive days and were euthanized 6 weeks after the last injection (nyoung=4, naged=4).
Brain Tissue Fixation
Animals were anesthetized by an intraperitoneal injection of 2:1 ketamine/xylazine (5 μL/g of weight) and subjected to an intracardiac perfusion using a peristaltic pump. As fixative we used either 2% paraformaldehyde and 2.5% glutaraldehyde for electron microscopy or 4% paraformaldehyde for immunohistochemistry. Prior to brain dissection, heads were removed and post-fixed in the same fixative overnight.
Transmission Electron Microscopy
After post-fixation, brains were washed in 0.1 M phosphate buffer (PB) (pH 7,4), cut into 200 μm sections with a VT 1000M vibratome (Leica, Wetzlar, Germany) and treated with 2% osmium tetraoxide in 0.1M PB for 2 h. Then sections were rinsed, dehydrated through a series of ethanol solutions and stained in 2% uranyl acetate at 70% ethanol. Following dehydration, slices were embedded in araldite (Durcupan, Fluka BioChemika, Ronkokoma, NY). To study the SVZ cell organization, serial 1.5 μm semithin sections were cut using a diamond knife and stained with 1% toluidine blue. To identify individual cell types, 60–70 nm ultrathin sections were cut with a diamond knife, stained with lead citrate, and examined under a Spirit transmission electron microscope (FEI Tecnai, Hillsboro, OR). To quantify the SVZ cells in the ventricular wall, we examined the dorsal horn and the entire length of the lateral wall, taking into account the first 20 μm adjacent to the ventricle (0–1 mm anterior to bregma). The quantification was performed in relation to length of the SVZ (cells/mm) and using three different levels per animal (nyoung=6, naged=6). The analysis was performed with Image Tool software (Evans Technology, Roswell, GA).
Tritiated Thymidine Autoradiography
Brains treated with 3H-Thy was processed for transmission electron microscopy as described above. Subsequently, semithin sections were dipped in LM-1 hypercoat emulsion (Amersham Biosciences), dried in the dark, and stored at 4°C for 1 month (Doetsch et al., 1997). Autoradiography was developed using standard methods and counterstained with 1% toluidine blue. Selected semithin sections, with a total of 40 labeled cells, were processed for ultrathin sections to be analyzed using electron microscopy. Cell reconstruction was performed when required.
Scanning Electron Microscopy
Animals (nyoung=3, naged=3) were euthanized and the lateral walls of the lateral ventricles were removed, fixed by immersion in 2.5% glutaraldehyde and 2% paraformaldehyde for 1 h, washed in 0.1M PB, and incubated for 2 h in 2% osmium tetroxide in 0.1M PB. The tissue was then dehydrated through an ethanol series, dried with liquid CO2, mounted, sputter-coated, and examined under an S-4100 scanning electron microscope (Hitachi, Tokyo, Japan).
Immunohistochemistry
After post-fixation, brains were washed in 0.1 M PB and cut into serial 10-μm thick coronal sections using a cryostat (Leica, CM 1900). One series (five to eight sections) from each animal was used in each immunostaining. Sections were incubated in blocking solution for 1 h at room temperature, followed by overnight incubation at 4°C with primary antibodies (see Table 1). Then, sections were washed and incubated with the appropriate secondary antibodies conjugated with either biotin or fluorophores. After the secondary biotinylated antibody, sections were incubated with ABC Elite complex (Vector, Burlingame, CA) and treated with diaminobenzidine (DAB, 0.05%; Sigma–Aldrich). Measurement of BrdU incorporation during DNA synthesis was carried out in coronal sections by quantification of BrdU+ cells within the SVZ, under an Eclipse E200 light microscope (Nikon, Tokyo, Japan), and were expressed as cells/mm. Fluorescence samples were examined under an Olympus IX81 confocal microscope and imaged using the Olympus Fluoview software version 3.1(Center Valley, PA).
TABLE 1.
Primary Antibodies used in this Study
| Antibody | Specie, type | Dilution | Antigen | Cat. Number, manufacturer |
Specificity |
|---|---|---|---|---|---|
| BrdU | mouse monoclonal, IgG |
1:150 | Bromodeoxyuridine conjugated to bovine serum albumin. |
MO744, Dako (Glostrup, Denmark) |
Cells in S-phase |
| BrdU | rat monoclonal, IgG | 1:200 | The details of the anti- gen for this antibody are not available |
AB6326, Abcam (Cambridge, MA, USA) |
Cells in S-phase |
| Dcx | goat polyclonal, IgG | 1:200 | Peptide mapping at the C-terminus of Dcx of human origin. |
SC-8066, Santa Cruz Biotechnology (Santa Cruz, CA, USA) |
Young neurons |
| GFAP | mouse monoclonal, IgG |
1:500 | Purified GFAP from porcine spinal cord |
MAB360, Millipore (Billerica, MA, USA) |
Astrocytes |
| GFAP | rabbit polyclonal, IgG |
1:500 | GFAP isolated from cow spinal cord. |
Z0334 Dako (Glostrup, Denmark) |
Astrocytes |
| Nestin | Mouse monoclonal, IgG |
1:100 | Rat (E15) spinal cord extracts |
556309, BD Pharmigen (San Jose, California USA) |
Stem cells |
| S100 | rabbit polyclonal, IgG |
1:1 | S-100 protein isolated from bovine brain. |
22520, ImmunoStar, Inc (Hudson, WI, USA) |
Ependymal and glial cells |
BrdU, 5-bromo-2’-deoxyuridine; DCX, doublecortin; GFAP, Glial fibrillary acidic protein.
Statistical Analysis
Data were expressed as mean±SEM. After testing for normal distribution with Shapiro-Wilke Test, a Student’s t test was performed using SigmaPlot 11.0 software (Jandel Scientific, San Rafael, CA). For samples that were not normally distributed the non-parametric Mann Whitney U test was used. Differences were considered significant at a P value <0.05.
Results
The Main Cellular Populations of the SVZ are Decreased in the Aged Mice
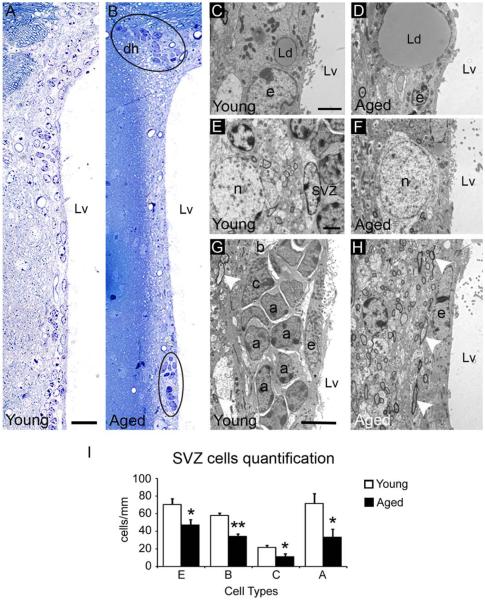
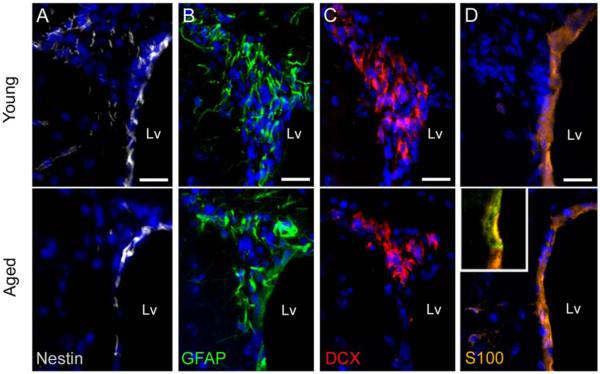
To examine the age-related changes in the cellular organization of the SVZ, we used light and electron microscopy. The ventricular wall (dorsal horn plus lateral wall) of aged mice (24-month old) presented reduced number of SVZ cells compared to young mice (2-month old) (Young 232.2±12.3 cells/mm vs. Aged 135.6±16.48 cells/mm, P=0.003). While the dorsal horn of the aged SVZ preserved groups of cells similar to young mice, the lateral wall presented dispersed cells that rarely formed groups (Fig. 1A,B). During aging, ependymal cells displayed larger lipid droplets (Fig. 1C,D) and neurons and axons from the neuropil were displaced next to the ependymal layer (Fig. 1E–H). Although the aged SVZ preserved all the main cell types, they were significantly decreased in number (E cells: Young 70.2±6.8 cells/mm vs. Aged 47.4±5.9 cells/mm, P=0.046; B cells: Young 57.7±2.9 cells/mm vs. Aged 34.5±2.3 cells/mm, P<0.001; C cells: Young 21.5±2.5 cells/mm vs. Aged 11.6±2.9 cells/mm, P=0.043; A cells: Young 71.6±11.1 cells/mm vs. Aged 33.5±8.7 cells/mm, P=0.036). Nevertheless, the proportions of each cell type in the SVZ (dorsal horn plus lateral wall) were not affected by age (Fig. 1I and Supp. Info. Table S1). Next, we verified the loss of cells in the neurogenic niche by immunohistochemistry, using the molecular markers Nestin (NSCs marker), Glial fibrillary acidic protein (GFAP, astrocytes marker), doublecortin (DCX, neuroblasts maker), and S100 (ependymal cells marker). We observed a decrease in Nestin+, GFAP+, and DCX+ cells in the aged SVZ, which suggests a reduction in the number of cells and confirms the previous cell quantification (Fig. 2A–C). In addition, the number of cells coexpressing Nestin and GFAP was reduced and mainly restricted to the dorsal horn of the aged SVZ (Supp. Info. Fig. S1). Remaining GFAP+ cells appeared to be more intensely labeled (Fig. 2B). We further observed a flattening of the ependymal layer in the aged SVZ, where cells coexpressing both S100 and GFAP markers were surprisingly found (Fig. 2D).
FIGURE 1.
Aging reduces the main cellular components of the SVZ niche. Light and transmission electron microscopy analysis of the SVZ. (A) Typical organization of the lateral wall in young mice with SVZ cells homogeneously distributed. (B) The aged SVZ showed a loss of cells. Remaining cells appeared forming groups (black circles) and were mainly located in the dorsal horn. (C) The young SVZ showed small lipid droplets in the ependymal layer. (D) Detail of a large lipid droplet in the ependymal layer of the aged SVZ. (E) The young mice typically presented neurons in the neuropil next to the SVZ. (F) Detail of a neuron close to the ventricle wall in the aged SVZ. (G) Typical neurogenic niche showing the SVZ cells next to the ependymal layer in a young mouse. Note the presence of myelin axons (white arrowhead) in the neuropil. (H) Lateral ventricle wall of an aged mouse displaying a depletion of SVZ cells. Note the presence of myelin axons (white arrowheads) next to the ependymal layer. (I) Cell quantification using electron microscopy showed a number reduction for type E, B, A, and C cells. a, neuroblast; b, astrocyte; c, type C cell; dh, dorsal horn; e, ependymal cell; Lp, lipid droplet; Lv, lateral ventricle; n, neuron. Scale bar: A–B 20 μm, C–F 2 μm, G–H 20 μm. *P<0.05, **P<0.01. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
FIGURE 2.
The molecular markers characteristic of the main SVZ cell types are altered during aging. Immunoassay against Nestin (white), GFAP (green), DCX (red), and S100 (orange) in the dorsal horn of the lateral ventricles. (A) Nestin expression was reduced in the aged SVZ. (B) GFAP expression was reduced in aged mice, but remaining GFAP+ cells were more intensely labeled, compared to the young brain. (C) DCX expression was diminished during aging. (D) Cells expressing S100 marker showed flattened morphology in the aged SVZ, compared to the young SVZ. Some S100+ cells located in the ependymal layer of aged mice co-expressed the GFAP marker (inset in D). Lv, lateral ventricle. Scale bar 20 μm. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Ultrastructural Changes in Astrocytic and Ependymal Cells Within the Aged SVZ
When evaluating in detail the ultrastructural characteristics of the SVZ cells, we did not observe differences for type C and A cells between young and aged mice. However, there were notable differences in astrocytic and ependymal cells, e.g., accumulation of dense bodies and intermediate filaments during aging (Table 2).
TABLE 2.
Age-related Changes in the Ultrastructure of Astrocytes and Ependymal Cells in the SVZ
| Features | Young | Aged |
|---|---|---|
| ASTROCYTES | ||
| Dense bodies | Rarely, dense bodies are found in the cyto- plasm of astrocytes. |
Dense bodies were frequently found in the astrocytes of aged mice. |
| Intermediate filaments | Astrocytes are characterized by the presence of intermediate filaments in the cytoplasm. |
With aging, astrocytes showed a higher num- ber of intermediate filaments. |
| Mitochondria | Mitochondria were distributed around the nucleus of astrocytes in young mice. The matrix is dense and contains high number of cristae. |
Mitochondria were dispersed in the cytosol of aged astrocytes. The matrix was lighter and presented fewer cristae, which were more dilated. |
| EPENDYMAL CELLS | ||
| Morphology | Ependymal cells have a cubical morphology and form an epithelial monolayer separating the SVZ from the ventricular cavity. |
Ependymal cells adopted a flattened and elongated morphology in aging SVZ, form- ing a thin multilayer in some regions. |
| Processes | These cells present lateral processes between them that are heavily interdigitated. |
Ependymal cells presented long processes parallel to ventricle wall, which established cell junctions between them and formed up to 4–5 layers that contributed to the thin multilayer. |
| Intermediate filaments | Some intermediate filaments can be found in the cytoplasm of ependymal cells. |
Cytoplasm of ependymal cells showed higher number of intermediate filaments in aged mice. |
| Dense bodies | Dense bodies are rarely found in the ependy- mal cell under physiological conditions. |
Frequently, dense bodies were observed in the cytoplasm of ependymal cells of the aged SVZ. |
| Lipid droplet | Lipid droplets are unique to ependymal cells. | Ependymal cells in aged SVZ presented larger lipid droplets. |
| Cilia and microvilli | A typical characteristic of this cell type is the presence of cilia in the apical surface, where they appear homogeneously distributed. |
Cilia were concentrated in a limited area of the apical surface and they appeared tangled. |
Young: 2-months old, Aged: 24-months old.
Astrocytic Cells
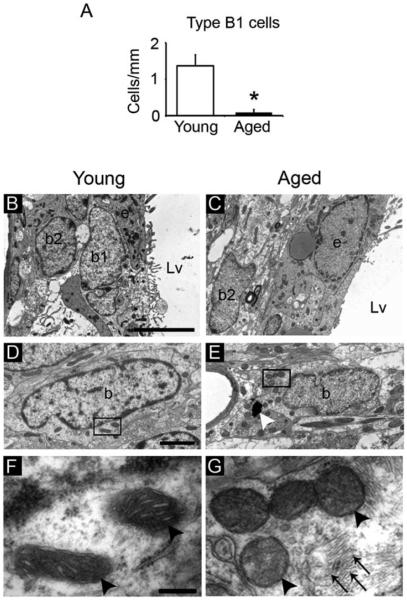
To evaluate the astrocytic population we considered both B1 and B2 astrocytes. Although both astrocytic populations have similar cytoplasmic contents, they present some ultrastructural differences a part of a different location. For instance, B2 astrocytes have higher number of intermediate filaments than B1 cells. B1 cells present microvilli and B2 do not. Both B1 and B2 astrocytes present a primary cilium. However, while the primary cilium of B1 astrocytes contacts the ventricle, the cilium of B2 cells is in parallel to the ventricle surface and does not contact the ventricle. In our study, B1 astrocytes were observed between ependymal cells and contacting the ventricle of young mice, as previously described (Doetsch et al., 1997), but they were rarely found in the aged SVZ (Young 1.37±0.3 cells/mm vs. Aged 0.09±0.0.08 cells/mm, P=0.005). Hence, SVZ astrocytes of aged mice were mostly identified as B2 astrocytes, which were located at the underlying striatal parenchyma (Fig. 3A–C). Astrocytes presented a different distribution of mitochondria during aging. While the mitochondria appeared dispersed in the cytoplasm of the astrocytes in the aged SVZ, this organelle presented perinuclear distribution in young mice (Fig. 3D,E). We also observed that the matrix density and the number of cristae were decreased in the mitochondria of aged astrocytes (Fig. 3F,G). Moreover, we found that astrocytes of aged SVZ had dense bodies in the cytosol and increased number of intermediate filaments (Fig. 3E,G and Supp. Info. Fig. S2). Contrarily to young mice, the astrocytes observed in the aged SVZ did not have invaginations of the nuclear membrane (Supp. Info. Fig. S2C,D), which is a typical finding in young SVZ astrocytes (Gil-Perotin et al., 2009).
FIGURE 3.
Aging alters the ultrastructure of the SVZ astrocytes. Electron microscopy analysis of the astrocytes within the SVZ. (A) Cell quantification of B1 astrocytes revealed a reduction of this subpopulation of cells in the aged SVZ. (B) Both B1 astrocytes (NSCs) and B2 astrocytes (non-neurogenic astrocytes) were frequently found in the SVZ of the young brain. (C) Astrocytes in the aged SVZ were mainly close to the underlying striatal parenchyma, corresponding to B2 astrocytes. (D) Astrocyte with light cytoplasm in the SVZ of young mice showed perinuclear mitochondria. (E) Astrocyte in the aged SVZ presented mitochondria distributed in a dispersed manner and dense bodies (white arrowhead) in the cytosol. (F) Detail of mitochondria (black arrowheads) in the cytoplasm of an astrocyte in a young mouse, with increased number of cristae and dense matrix. (G) Detail of mitochondria (black arrowheads) in the cytosol of an astrocyte in the aged SVZ with less developed cristae. Note the presence of abundant intermediate filaments (arrows). b, astrocyte; b1, B1 astrocyte; b2, B2 astrocyte; e, ependymal cell; Lv, lateral ventricle. Scale bar: B–C 5 μm, D–E 1 μm, F–G 500 nm. *P<0.01.
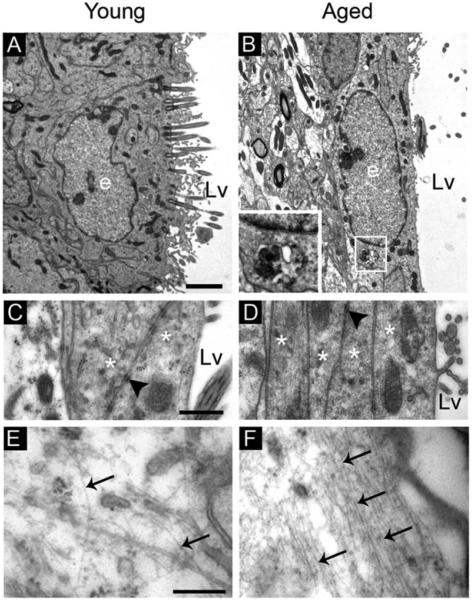
Ependymal Cells
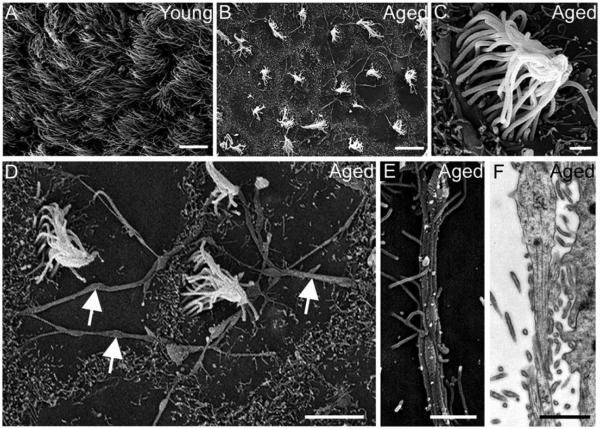
The young SVZ presents a monolayer of ependymal cells with cubical morphology, but these cells assume an elongated morphology during aging. Aged ependymal cells had long processes parallel to the ventricle wall that formed a thin multilayer of up to four to five sheets, which were connected by frequent cell junctions (Fig. 4A–D). Ultrastructural analysis revealed frequent dense bodies and increased number of intermediate filaments in the cytosol of ependymal cells during aging (Fig. 4A,B and E,F). The accumulation of intermediate filaments in these cells was also observed in the SVZ immunostained with S100 and GFAP makers, where cells located in the ependymal layer were found to co-express both markers (see inset in Fig. 2D). Moreover, ependymal cells displayed differences in their cilia. While cilia were located across the apical surface in young mice, they were rarely observed in the aged SVZ or appeared concentrated in a limited area of the apical surface, which frequently was eccentric (Fig. 4A,B). To examine the cilia in detail, we used scanning electron microscopy. The analysis of the ventricular wall in the aged mice revealed that the ependymal cilia tufts were more separated than in young mice, resulting in extended areas that were devoid of cilia (Fig. 5A,B). At higher magnifications, we found that cilia from the tufts were tangled in the aged brain, whereas they appeared untangled in young mice (Fig. 5C). Consistent with transmission electron microscopy images, cilia tufts were frequently displaced from the center of the cell surface (Fig. 5D). Interestingly, cilia-devoid areas revealed structures resembling axons forming an extensive network on the ventricular surface of aged mice, which were also observed using transmission electron microscopy (Fig. 5D–F). Although we only found these axons in aged mice, it is not a particular characteristic of aging, since these axons have been previously described in the ventricles of rat and marmoset (Lorez and Richards, 1982; Sawamoto et al., 2011).
FIGURE 4.
Aging alters the ultrastructure of the ependymal cells. Electron microscopy analysis of ependymal cells in the ventricle wall. (A) Typical ependymal cell in the young SVZ with cubical morphology and multiple cilia in the apical surface. (B) Elongated ependymal cell in the aged SVZ with dense bodies (box) in the cytosol. (C) Detail of cell junctions (black arrowhead) between two ependymal cells (asterisks), which constitute the monolayer. (D) Thin multilayer constituted by 4 different ependymal processes (asterisks), which were connected by cell junctions (black arrowheads). (E) Detail of an ependymal cytosol in the young mouse showing intermediate filaments (arrows). (F) Detail of an ependymal cytoplasm in the aged SVZ showing an increase in intermediate filaments (arrows). e, ependymal cell; Lv, lateral ventricle. Scale bar: A–B 1 μm, C–D 500 nm, E–F 200 nm.
FIGURE 5.
The ventricular wall presents cilia-devoid patches during aging. (A–E) Scanning electron microscopy images. (A) In young mice, the ventricular wall was completely covered by cilia of ependymal cells. (B) Cilia-devoid patches were abundant in the aged brain. (C) Detail of tangled cilia in an ependymal cell of aged mice. (D) Ventricular surface in the aged brain showed an extensive network of structures resembling axons (white arrows). (E) Detail of structure-like axons observed over the lateral wall of the aged brain. (F) Detail of structure-like axons observed over the lateral wall of the aged brain using transmission electron microscopy. Scale bar A–B 10μm, C, E, F 1 μm, D 5 μm.
Aging Induces Changes in the Cytoarchitecture of the SVZ Microenvironment
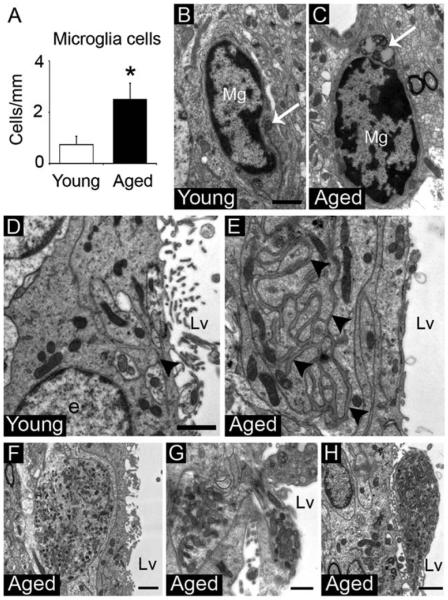
Previous studies demonstrated that the SVZ microenvironment can affect the neurogenic capacity (Ihrie and Alvarez-Buylla, 2011; Kazanis et al., 2010; Lim et al., 2000; Shen et al., 2008; Tavazoie et al., 2008; Thored et al., 2009). Here, we found that the number of microglial cells located in the SVZ niche was significantly increased in aged mice (Young 0.72±0.35 cells/mm vs. Aged 2.5±0.62 cells/mm, P=0.047) (Fig. 6A and Supp. Info. Table S1). Microglia cells presented dark nuclei with clumped chromatin and dark cytoplasm with long cisternae of the rough endoplasmic reticulum and dense bodies. In the aged brain, microglia cells had a less ramified round shape and accumulated high numbers of dense bodies in the cytoplasm, suggesting that they were activated (Fig. 6B,C) (Streit et al., 1999). Additionally, the SVZ showed networks of basal lamina, also named fractons (Kerever et al., 2007), between ependymal, astrocytic or endothelial cells. These fractons were larger and more penetrative in the extracellular space during aging, but differences in thickness or electron-density was not observed between groups (Fig. 6D,E).
Figure 6.
The cytoarchitecture of the SVZ microenvironment changes during aging. (A) Cell quantification of microglia cells in the SVZ. (B) Microglia cell in the young SVZ showing few dense bodies (arrow) in the cytoplasm. (C) Microglia cell in the aged SVZ showing round shape and abundant dense bodies (arrow) in the cytoplasm. (D) Small fractons (arrowhead) located between ependymal cells in the young SVZ. (E) Large penetrative fractons (arrowheads) located between ependymal cells in the aged SVZ. (F) Neuron-like cytosol with a high number of mitochondria, vesicles, and microtubules within the aged SVZ. (G) Neuron-like cytosol through the ependymal layer in the aged SVZ. (H) Neuron-like cytosol on the ventricle wall of the aged brain. e; ependymal cell, Lv; lateral ventricle, Mg; microglia cell. Scale bar: B,C 1 μm, D,E 10 μm, F, H 1μm, G 500 nm. *P<0.05.
Interestingly, we found cytosols that presented a high number of mitochondria, pleomorphic vesicles, and microtubules. These structures were observed through the ependymal layer and on the ventricular wall of the aged SVZ (Fig. 6F–H). The ultrastructural features of these cytosols were similar to those described for neurons of the rodent spinal cord (Alfaro-Cervello et al., 2012; Marichal et al., 2009). Although we cannot confirm that the cytosols in the aged SVZ are neurons, we referred to them as neuron-like cells based on these previous findings.
The Aged SVZ Maintains Proliferative Astrocytes Which Divide Less Than Those in Young Mice
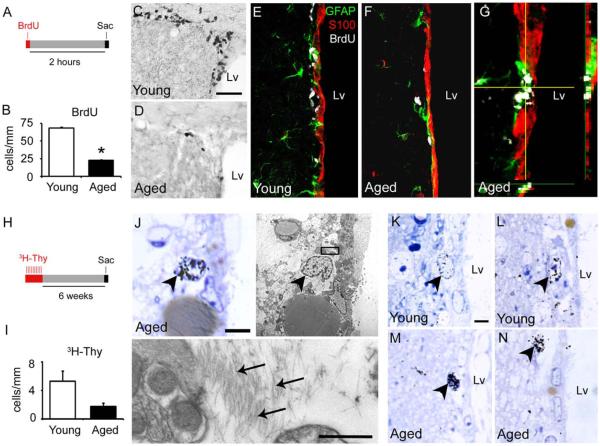
To study the remaining proliferative cells in the aged SVZ, animals received a single dose of BrdU 2 h before sacrifice (Fig. 7A). Consistent with previous studies (Luo et al., 2006; Maslov et al., 2004; Tatar et al., 2013), we found a declined proliferative capacity in the SVZ of aged male C57BL/6 mice, with 70% less cells expressing BrdU (Young 68.1±1.7 cells/mm vs. Aged 22.5±1.3 cells/mm, P<0.001) (Fig. 7B). BrdU+ cells were distributed in a dispersed manner, isolated or paired along the lateral wall of aged mice, while they formed large clusters and were homogeneously distributed in young animals (Fig. 7C,D). To determine the identity of these proliferative cells, we performed triple staining against BrdU, GFAP and S100 markers. All the proliferative cells were BrdU+/GFAP+/S100− or BrdU+/GFAP−/S100− cells. BrdU incorporation on S100+ cell was not observed in any experimental group (Fig. 7E–G). These results imply that the aged SVZ maintains dividing astrocytes and non-proliferative ependymal cells.
Figure 7.
Aged astrocytes preserve their proliferative capacity but divide less frequently. (A) Timeline for the BrdU administration protocol. Animals received a single dose of BrdU 2 h before euthanize. (B) Bar graph depicting a significant reduction of BrdU+ cells in the aged SVZ. (C,D) Immunoassay against BrdU in coronal brain sections. (C) Young mice presented a high number of BrdU+ cells in the dorsal horn of the SVZ. (D) Aged mice presented a drastic reduction in proliferating cells. (E–G) Immunoassay against GFAP (green), S100 (red), and BrdU (white) in coronal brain sections. BrdU+ cells coexpressed GFAP, but not S100 in both young (E) and aged (F) mice. (G) Detail of a BrdU/GFAP+ cells close to ependymal layer in aged mice. (H) Timeline for the 3H-Thy administration protocol. Animals received 10 doses of 3H-Thy (1 dose per day) 6 weeks before euthanized. (I) Bar graph depicting a trend to decrease the number of 3H-Thy+ cells in the aged SVZ. (J–N) Ultrastructural analysis of SVZ cells incorporating 3H-Thy. (J) Labeled astrocytes (arrowhead) were identified by the presence of intermediate filaments (arrows) in the cytosol. (K,L) The young SVZ showed astrocytes with low intensity of the 3H-Thy marker. (M,N) In the aged brain, astrocytes displayed an intense 3H-Thy labeling. Lv, lateral ventricle; sac, sacrifice. Scale bar A,B 50 μm, C,D 20 μm, J–N 5 μm, detail in J 500 nm. *P<0.01. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
To evaluate the proliferative potential of SVZ cells in a longer period of time and to determine the fate of the newly generated cells by ultrastructural analysis, a group of mice was injected with 3H-Thy over a 10-day period (1 dose per day) and euthanized after 6 weeks (Fig. 7H). In line with the reduction on proliferation, the overall number of cells labeled with 3H-Thy showed a decreasing trend 6 weeks after treatment in aged mice (Young 5.3±1.4 cells/mm vs. Aged 1.76±0.49 cells/mm, P<0.1) (Fig. 7I). However, the differences were likely not significant due to the limited number of labeled cells that are found in semithin sections. On the basis of morphological observations, we found astrocytes and microglia cells labeled with 3H-Thy (Fig. 7J and Supp. Info. Fig. S3). During aging, astrocytes presented a strong intensity of the radioactive marker, suggesting that these cells might proliferate, but less frequently than in the young brain, where the labeling was more diluted (Fig. 7K–N). We did not observe any 3H-Thy+ cells corresponding to type C cells or neuroblasts at the ultrastructural level, possibly due to their migration away from the SVZ, as previously described (Capilla-Gonzalez et al., 2013a). Supporting the results from our BrdU assay, the radioactive marker was not detected in ependymal cells identified by the presence of cilia or deuterosomes, which are involved in the cilia formation. These results suggest that, in addition to be non-proliferative, ependymal cells are not newly generated in the aged brain.
Discussion
In this study, we described age-related changes in the SVZ neurogenic niche. Our principal findings were that the aged SVZ maintains proliferative B1 astrocytes (NSCs), but they divide less frequently than in the young brain. We provided new evidence supporting the hypothesis that ependymal cells are not newly generated during aging. Also, we found that the astrocytes and ependymal cells within the aged SVZ display ultrastructural changes by accumulation of intermediate filaments and dense bodies, resembling reactive cells. These effects could be contributing to the disruption of the neurogenic process during aging.
The Aged SVZ Preserves Some B1 Astrocytes that Proliferate Less Frequently
In line with previous studies, we found a partial depletion of SVZ cells during aging (Capilla-Gonzalez et al., 2013a; Luo et al., 2006; Maslov et al., 2004). When we focused in the astrocytic population, we noted that most remaining astrocytes in the aged SVZ were located in the underlying striatal parenchyma and did not contact the ventricle. These astrocytes presented a high number of intermediate filaments. Moreover, mitochondria of remaining astrocytes were dispersedly distributed and displayed a light matrix with dilated cristae, similar to those in mature cells (Chen et al., 2008; Facucho-Oliveira and St John, 2009; Lonergan et al., 2006). These features correspond to B2 astrocytes, which are in a more mature stage compared to B1 astrocytes. This data supports the hypothesis that the number of NSCs decreases over time.
Currently, the mitotic capacity of remaining NSCs in the aged SVZ is subject of debate. While some reports indicate that NSCs divide less frequently during aging (Ahlenius et al., 2009; Bouab et al., 2011; Encinas and Sierra, 2012; Gritti et al., 2009), other studies suggest that they are highly proliferative (Shook et al., 2012; Stoll et al., 2011). Most of these studies rely on the use of immunostaining to draw their conclusions, but aging can alter the molecular patterns expressed by the cells (McGinn et al., 2012). Hence, to accurately identify the dividing cells, we supplemented our immunostaining findings with electron microscopy analysis of 3H-Thy-labeled cells. We found that a subset of the astrocytes that remain in the aged SVZ preserved the ability to divide. Additionally, the intensity of the radioactive marker in those cells was stronger compared to that in young mice, suggesting that proliferative astrocytes that remain in the aged SVZ divide less frequently. However, it is known that factors such as strains or sex are determinants for SVZ proliferative capacity (Tatar et al., 2013), which could explain the differences found in the literature regarding this issue.
Ependymal Cells do not Regenerate in the Aged Brain
The role of the ependymal cells in the process of neurogenesis is controversial. Some investigators suggest that these cells are the bona fide NSCs, since it was observed that ependymal cells might act as NSCs under pathological conditions (Batiz et al., 2011; Carlen et al., 2009; Johansson et al., 1999). Additionally, it has been suggested that the B1 astrocytes can modify their traditional B-C-A path to generate new ependymal cells and mediate ependymal-repair during aging (Luo et al., 2008; Mokry and Karbanova, 2006). In our study, we did not find dividing ependymal cells in the aged brain, using double immunostaining against BrdU and S100 markers 2 h after BrdU administration. Likewise, we did not observe any proliferative or newly generated ependymal cells when animals were given 3H-Thy for 10 days and sacrificed after 6 weeks, supporting previous findings (Capela and Temple, 2002; Del Carmen Gomez-Roldan et al., 2008; Spassky et al., 2005). These differences could be due to the use of different techniques to track the newly generated cells. B1 astrocytes could be difficult to distinguish from ependymal cells if they are integrated in the ependymal layer. The use of electron microscopy solves this difficulty, providing a more accurate interpretation of our results. Moreover, during the differentiation process, ependymal cells can resemble astrocytic cells, since they lack cilia at early developmental stages. We confirmed that 3H-Thy+ astrocytes were not ependymal cells because they did not have cilia or deuterostomes in their cytoplasm, a structure associated with the formation of cilia (Spassky et al., 2005). These findings support the hypothesis that ependymal cells do not proliferate and/or regenerate during aging.
Astrocytes and Ependymal Cells Acquire a Reactive Phenotype During Aging
Under pathological conditions, astrocytes can acquire a reactive phenotype, increasing the number of intermediate filaments and their content of dense bodies (Hatten et al., 1991; Robel et al., 2011; Schiffer et al., 1986; Young et al., 2012). This phenomenon can also be observed in astrocytes and ependymal cells of the SVZ as a response to stroke or Parkinson’s disease (L’Episcopo et al., 2012; Young et al., 2012). In our study, we found that astrocytes and ependymal cells assume a reactive phenotype in the non-pathological SVZ during aging by accumulating dense bodies and long processes rich in intermediate filaments. These features resemble the hypocellular gap layer of the adult human SVZ, where neurogenic capacity and neuroblast migration is also reduced (Guerrero-Cazares et al., 2011; Quinones-Hinojosa et al., 2006; Sanai et al., 2011, 2004). Furthermore, we found that the ependymal layer of the aged SVZ presented cells coexpressing GFAP and S100 markers. This finding was previously described in elderly mice, suggesting that astrocytes could transform into ependymal cells to mediate ependymal repair (Luo et al., 2008). However, our results indicate that these GFAP/S100 positive cells correspond indeed to ependymal cells that acquired a reactive phenotype during aging.
Cilia in the Ventricle Surface are Subject to Change During Aging
Ependymal cells play an important role in the neurogenic process since the beating of ependymal cilia is required for the directional migration of the neuroblasts toward the OB (Sawamoto et al., 2006). In our study, we describe large areas that were cilia-devoid in the ventricle wall of the aged brain. Moreover, ependymal cells displayed tangled cilia during aging, which likely present difficulties for beating. These age-related changes in the ependymal cilia could contribute to the neurogenic decline and induce a failure in neuroblasts migration, as previously described (Capilla-Gonzalez et al., 2013a). A similar cilia organization was observed in young mice that were exposed to N-ethyl-N-nitrosourea, which presented impaired OB neurogenesis and odor discrimination (Capilla-Gonzalez et al., 2010, 2012). Moreover, cilia of ependymal cells were disrupted in young mice after stroke and cerebrospinal fluid flow was slower and more turbulent. These effects were suggested to be due to morphological changes in the ependymal cells post stroke (Young et al., 2012). In line with this report, we found that the ependymal cells became elongated and extended long processes in the aged brain, which could explain the changes in cilia distribution. The shape of ependymal cells can be influenced by the distention of the ventricle walls that is associated with senescence (Batiz et al., 2011; Conover and Shook, 2011) or by the extensive network of axons on the ventricle wall (Lorez and Richards, 1982).
Aging Induces Changes in the Microenvironment of the Neurogenic Niche
The microenvironment of the SVZ niche plays an important role in modulating its proliferative and neurogenic processes (Ihrie and Alvarez-Buylla, 2011; Kazanis et al., 2010; Lim et al., 2000; Shen et al., 2008; Tavazoie et al., 2008). During aging, the SVZ microenvironment can be subject to changes. For instance, chronic inflammation and oxidative stress are hallmarks of the aged brain, which are combated by increasing the reactive microglia (Henry et al., 2009; Njie et al., 2012; Qin et al., 2013). Consistent with these studies, we found an increase in microglia cells in the aged SVZ niche that seemed to be activated (i.e., less ramified round shape and an accumulation of dense bodies in the cytoplasm) (Streit et al., 1999). Microglia cells are known to be involved in molecular pathways that may regulate neurogenesis, for example by affecting cytokines expression (Garden and Moller, 2006; Gonzalez-Perez et al., 2012; Kang et al., 2012; L’Episcopo et al., 2013; Logan et al., 2013; Pluchino et al., 2008; Tonchev, 2011; Walton et al., 2006; Yan et al., 2006). Likewise, the basal lamina of the blood vessels close to the SVZ also presents a role in proliferation in the neurogenic niche by providing cytokines and growth factors (Kazanis et al., 2010; Kerever et al., 2007; Shen et al., 2008). This basal lamina forms a deep network between the interdigitations of ependymal cells or astrocytes, which was larger and more penetrative in the aged SVZ. The changes in the basal lamina could be a response to the reduction of proliferation that occurs during aging. This phenomenon was described in the spinal cord of the rodent (Alfaro-Cervello et al., 2012) or in experimental models with a reduced SVZ proliferative capacity, such as in mice exposed to radiation or N-ethyl-N-nitrosourea (Achanta et al., 2012; Capilla-Gonzalez et al., 2012). Another common characteristic found in periventricular regions with low proliferation is the presence of neuron-like cells protruding toward the ventricle, which were described in the rodent spinal cord (Alfaro-Cervello et al., 2012; Marichal et al., 2009) and the monkey SVZ (Sawamoto et al., 2011). In our study we demonstrated for the first time that these structures resembling neurons were also found in the SVZ of aged mice. These findings raise the possibility that the neuron-like cells could be a reservoir of immature neurons in “standby mode,” which may respond under some pathologies (Marichal et al., 2009), or even in the non-pathological aged brain. Together, these findings provide new information regarding how age-related changes in the microenvironment can affect the neurogenic capacity of the SVZ.
Conclusion
In summary, the principal findings of this study are that (i) B1 astrocytes within the aged SVZ maintain their proliferate capacity but divide less frequently than in the young mice, at least in male C57BL/6 mice; (ii) ependymal cells in the aged brain are non-proliferative and are not newly generated; (iii) astrocytes and ependymal cells acquire a reactive phenotype during aging; and (iv) the microenvironment of the SVZ presents changes in the aged brain. These findings provide new insight into the events occurring in the SVZ niche during aging.
Supplementary Material
Acknowledgment
Grant sponsor: National Institutes of Health; Grant number: RO1 NS070024; Grant sponsor: Prometeo Grant; Grant number: GVPROMETEO-2009/011; Grant sponsor: Spanish MINECO Grants; Grant numbers: SAF2012–33683; AP2010-4264; Grant sponsor: Maryland Stem Cell Research Fund; Robert Wood Johnson Foundation; Howard Hughes Medical Institute; Red de TerapiaCelularTerCel (RETICS) from Instituto de Salud Carlos III.
The authors thank Julia Litzky for her technical help and Alejandro Martin-Montalvo for his proof and critical reading of the manuscript.
Footnotes
Additional Supporting Information may be found in the online version of this article.
References
- Achanta P, Capilla-Gonzalez V, Purger D, Reyes J, Sailor K, Song H, Garcia-Verdugo JM, Gonzalez-Perez O, Ford E, Quinones-Hinojosa A. Subventricular zone localized irradiation affects the generation of proliferating neural precursor cells and the migration of neuroblasts. Stem Cells. 2012;30:2548–2560. doi: 10.1002/stem.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlenius H, Visan V, Kokaia M, Lindvall O, Kokaia Z. Neural stem and progenitor cells retain their potential for proliferation and differentiation into functional neurons despite lower number in aged brain. J Neurosci. 2009;29:4408–4419. doi: 10.1523/JNEUROSCI.6003-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfaro-Cervello C, Soriano-Navarro M, Mirzadeh Z, Alvarez-Buylla A, Garcia-Verdugo JM. Biciliated ependymal cell proliferation contributes to spinal cord growth. J Comp Neurol. 2012;520:3528–3552. doi: 10.1002/cne.23104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Garcia-Verdugo JM. Neurogenesis in adult subventricular zone. J Neurosci. 2002;22:629–634. doi: 10.1523/JNEUROSCI.22-03-00629.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batiz LF, Jimenez AJ, Guerra M, Rodriguez-Perez LM, Toledo CD, Vio K, Paez P, Perez-Figares JM, Rodriguez EM. New ependymal cells are born postnatally in two discrete regions of the mouse brain and support ven tricular enlargement in hydrocephalus. Acta Neuropathol. 2011;121:721–735. doi: 10.1007/s00401-011-0799-x. [DOI] [PubMed] [Google Scholar]
- Bouab M, Paliouras GN, Aumont A, Forest-Berard K, Fernandes KJ. Aging of the subventricular zone neural stem cell niche: Evidence for quiescence-associated changes between early and mid-adulthood. Neuroscience. 2011;173:135–149. doi: 10.1016/j.neuroscience.2010.11.032. [DOI] [PubMed] [Google Scholar]
- Capela A, Temple S. LeX/ssea-1 is expressed by adult mouse CNS stem cells, identifying them as nonependymal. Neuron. 2002;35:865–875. doi: 10.1016/s0896-6273(02)00835-8. [DOI] [PubMed] [Google Scholar]
- Capilla-Gonzalez V, Cebrian-Silla A, Guerrero-Cazares H, Garcia-Verdugo JM, Quinones-Hinojosa A. The generation of oligodendroglial cells is preserved in the rostral migratory stream during aging. Front Cell Neurosci. 2013a;7:147. doi: 10.3389/fncel.2013.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capilla-Gonzalez V, Gil-Perotin S, Ferragud A, Bonet-Ponce L, Canales JJ, Garcia-Verdugo JM. Exposure to N-ethyl-N-nitrosourea in adult mice alters structural and functional integrity of neurogenic sites. PLoS One. 2012;7:e29891. doi: 10.1371/journal.pone.0029891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capilla-Gonzalez V, Gil-Perotin S, Garcia-Verdugo JM. Postnatal exposure to N-ethyl-N-nitrosurea disrupts the subventricular zone in adult rodents. Eur J Neurosci. 2010;32:1789–1799. doi: 10.1111/j.1460-9568.2010.07450.x. [DOI] [PubMed] [Google Scholar]
- Capilla-Gonzalez V, Guerrero-Cazares H, Bonsu JM, Gonzalez-Perez O, Achanta P, Wong J, Garcia-Verdugo JM, Quinones-Hinojosa A. The subventricular zone is able to respond to a demyelinating lesion after localized radiation. Stem Cells. 2013b;32:59–69. doi: 10.1002/stem.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlen M, Meletis K, Goritz C, Darsalia V, Evergren E, Tanigaki K, Amendola M, Barnabe-Heider F, Yeung MS, Naldini L, Honjo T, Kokaia Z, Shupliakov O, Cassidy RM, Lindvall O, Frisen J. Forebrain ependymal cells are Notch-dependent and generate neuroblasts and astrocytes after stroke. Nat Neurosci. 2009;12:259–267. doi: 10.1038/nn.2268. [DOI] [PubMed] [Google Scholar]
- Chen CT, Shih YR, Kuo TK, Lee OK, Wei YH. Coordinated changes of mitochondrial biogenesis and antioxidant enzymes during osteogenic differentiation of human mesenchymal stem cells. Stem Cells. 2008;26:960–968. doi: 10.1634/stemcells.2007-0509. [DOI] [PubMed] [Google Scholar]
- Conover JC, Shook BA. Aging of the subventricular zone neural stem cell niche. Aging Dis. 2011;2:49–63. [PMC free article] [PubMed] [Google Scholar]
- Del Carmen Gomez-Roldan M, Perez-Martin M, Capilla-Gonzalez V, Cifuentes M, Perez J, Garcia-Verdugo JM, Fernandez-Llebrez P. Neuroblast proliferation on the surface of the adult rat striatal wall after focal ependymal loss by intracerebroventricular injection of neuraminidase. J Comp Neurol. 2008;507:1571–1587. doi: 10.1002/cne.21618. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999a;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Regeneration of a germinal layer in the adult mammalian brain. Proc Natl Acad Sci USA. 1999b;96:11619–11624. doi: 10.1073/pnas.96.20.11619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encinas JM, Sierra A. Neural stem cell deforestation as the main force driving the age-related decline in adult hippocampal neurogenesis. Behav Brain Res. 2012;227:433–439. doi: 10.1016/j.bbr.2011.10.010. [DOI] [PubMed] [Google Scholar]
- Facucho-Oliveira JM, St John JC. The relationship between pluripotency and mitochondrial DNA proliferation during early embryo development and embryonic stem cell differentiation. Stem Cell Rev. 2009;5:140–158. doi: 10.1007/s12015-009-9058-0. [DOI] [PubMed] [Google Scholar]
- Garden GA, Moller T. Microglia biology in health and disease. J Neuroimmune Pharmacol. 2006;1:127–137. doi: 10.1007/s11481-006-9015-5. [DOI] [PubMed] [Google Scholar]
- Gil-Perotin S, Alvarez-Buylla A, Garcia-Verdugo JM. Identification and characterization of neural progenitor cells in the adult mammalian brain. Adv Anat Embryol Cell Biol. 2009;203:1–101. [PubMed] [Google Scholar]
- Gonzalez-Perez O, Gutierrez-Fernandez F, Lopez-Virgen V, Collas-Aguilar J, Quinones-Hinojosa A, Garcia-Verdugo JM. Immunological regulation of neurogenic niches in the adult brain. Neuroscience. 2012;226:270–281. doi: 10.1016/j.neuroscience.2012.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritti A, Dal Molin M, Foroni C, Bonfanti L. Effects of developmental age, brain region, and time in culture on long-term proliferation and multipotency of neural stem cell populations. J Comp Neurol. 2009;517:333–349. doi: 10.1002/cne.22153. [DOI] [PubMed] [Google Scholar]
- Guerrero-Cazares H, Gonzalez-Perez O, Soriano-Navarro M, Zamora-Berridi G, Garcia-Verdugo JM, Quinones-Hinojosa A. Cytoarchitecture of the lateral ganglionic eminence and rostral extension of the lateral ventricle in the human fetal brain. J Comp Neurol. 2011;519:1165–1180. doi: 10.1002/cne.22566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YG, Spassky N, Romaguera-Ros M, Garcia-Verdugo JM, Aguilar A, Schneider-Maunoury S, Alvarez-Buylla A. Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat Neurosci. 2008;11:277–284. doi: 10.1038/nn2059. [DOI] [PubMed] [Google Scholar]
- Hatten ME, Liem RK, Shelanski ML, Mason CA. Astroglia in CNS injury. Glia. 1991;4:233–243. doi: 10.1002/glia.440040215. [DOI] [PubMed] [Google Scholar]
- Henry CJ, Huang Y, Wynne AM, Godbout JP. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1beta and anti-inflammatory IL-10 cytokines. Brain Behav Immun. 2009;23:309–317. doi: 10.1016/j.bbi.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihrie RA, Alvarez-Buylla A. Cells in the astroglial lineage are neural stem cells. Cell Tissue Res. 2008;331:179–191. doi: 10.1007/s00441-007-0461-z. [DOI] [PubMed] [Google Scholar]
- Ihrie RA, Alvarez-Buylla A. Lake-front property: A unique germinal niche by the lateral ventricles of the adult brain. Neuron. 2011;70:674–686. doi: 10.1016/j.neuron.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imayoshi I, Sakamoto M, Ohtsuka T, Takao K, Miyakawa T, Yamaguchi M, Mori K, Ikeda T, Itohara S, Kageyama R. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci. 2008;11:1153–1161. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- Johansson CB, Momma S, Clarke DL, Risling M, Lendahl U, Frisen J. Identification of a neural stem cell in the adult mammalian central nervous system. Cell. 1999;96:25–34. doi: 10.1016/s0092-8674(00)80956-3. [DOI] [PubMed] [Google Scholar]
- Kang SS, Keasey MP, Arnold SA, Reid R, Geralds J, Hagg T. Endogenous CNTF mediates stroke-induced adult CNS neurogenesis in mice. Neurobiol Dis. 2012;49C:68–78. doi: 10.1016/j.nbd.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazanis I, Lathia JD, Vadakkan TJ, Raborn E, Wan R, Mughal MR, Eckley DM, Sasaki T, Patton B, Mattson MP, Hirschi KK, Dickinson ME, ffrench-Constant C. Quiescence and activation of stem and precursor cell populations in the subependymal zone of the mammalian brain are associated with distinct cellular and extracellular matrix signals. J Neurosci. 2010;30:9771–9781. doi: 10.1523/JNEUROSCI.0700-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerever A, Schnack J, Vellinga D, Ichikawa N, Moon C, Arikawa-Hirasawa E, Efird JT, Mercier F. Novel extracellular matrix structures in the neural stem cell niche capture the neurogenic factor fibroblast growth factor 2 from the extracellular milieu. Stem Cells. 2007;25:2146–2157. doi: 10.1634/stemcells.2007-0082. [DOI] [PubMed] [Google Scholar]
- L’Episcopo F, Tirolo C, Testa N, Caniglia S, Morale MC, Deleidi M, Serapide MF, Pluchino S, Marchetti B. Plasticity of subventricular zone neuroprogenitors in MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) mouse model of Parkinson’s disease involves cross talk between inflammatory and Wnt/beta-catenin signaling pathways: Functional consequences for neuroprotection and repair. J Neurosci. 2012;32:2062–2085. doi: 10.1523/JNEUROSCI.5259-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L’Episcopo F, Tirolo C, Testa N, Caniglia S, Morale MC, Impagnatiello F, Pluchino S, Marchetti B. Aging-induced Nrf2-ARE pathway disruption in the subventricular zone drives neurogenic impairment in parkinsonian mice via PI3K-Wnt/beta-catenin dysregulation. J Neurosci. 2013;33:1462–1485. doi: 10.1523/JNEUROSCI.3206-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarini F, Lledo PM. Is adult neurogenesis essential for olfaction? Trends Neurosci. 2011;34:20–30. doi: 10.1016/j.tins.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Lim DA, Tramontin AD, Trevejo JM, Herrera DG, Garcia-Verdugo JM, Alvarez-Buylla A. Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron. 2000;28:713–726. doi: 10.1016/s0896-6273(00)00148-3. [DOI] [PubMed] [Google Scholar]
- Logan TT, Villapol S, Symes AJ. TGF-beta superfamily gene expression and induction of the Runx1 transcription factor in adult neurogenic regions after brain injury. PLoS One. 2013;8:e59250. doi: 10.1371/journal.pone.0059250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonergan T, Brenner C, Bavister B. Differentiation-related changes in mitochondrial properties as indicators of stem cell competence. J Cell Physiol. 2006;208:149–153. doi: 10.1002/jcp.20641. [DOI] [PubMed] [Google Scholar]
- Lorez HP, Richards JG. Supra-ependymal serotoninergic nerves in mammalian brain: Morphological, pharmacological and functional studies. Brain Res Bull. 1982;9:727–741. doi: 10.1016/0361-9230(82)90179-4. [DOI] [PubMed] [Google Scholar]
- Luo J, Daniels SB, Lennington JB, Notti RQ, Conover JC. The aging neurogenic subventricular zone. Aging Cell. 2006;5:139–152. doi: 10.1111/j.1474-9726.2006.00197.x. [DOI] [PubMed] [Google Scholar]
- Luo J, Shook BA, Daniels SB, Conover JC. Subventricular zonemediated ependyma repair in the adult mammalian brain. J Neurosci. 2008;28:3804–3813. doi: 10.1523/JNEUROSCI.0224-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luskin MB, Zigova T, Soteres BJ, Stewart RR. Neuronal progenitor cells derived from the anterior subventricular zone of the neonatal rat forebrain continue to proliferate in vitro and express a neuronal phenotype. Mol Cell Neurosci. 1997;8:351–366. doi: 10.1006/mcne.1996.0592. [DOI] [PubMed] [Google Scholar]
- Marichal N, Garcia G, Radmilovich M, Trujillo-Cenoz O, Russo RE. Enigmatic central canal contacting cells: Immature neurons in “standby mode”? J Neurosci. 2009;29:10010–10024. doi: 10.1523/JNEUROSCI.6183-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslov AY, Barone TA, Plunkett RJ, Pruitt SC. Neural stem cell detection, characterization, and age-related changes in the subventricular zone of mice. J Neurosci. 2004;24:1726–1733. doi: 10.1523/JNEUROSCI.4608-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinn MJ, Colello RJ, Sun D. Age-related proteomic changes in the subventricular zone and their association with neural stem/progenitor cell proliferation. J Neurosci Res. 2012;90:1159–1168. doi: 10.1002/jnr.23012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzadeh Z, Merkle FT, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. 2008;3:265–278. doi: 10.1016/j.stem.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokry J, Karbanova J. Foetal mouse neural stem cells give rise to ependymal cells in vitro. Folia Biol (Praha) 2006;52:149–155. [PubMed] [Google Scholar]
- Morrens J, Van Den Broeck W, Kempermann G. Glial cells in adult neurogenesis. Glia. 2012;60:159–174. doi: 10.1002/glia.21247. [DOI] [PubMed] [Google Scholar]
- Njie EG, Boelen E, Stassen FR, Steinbusch HW, Borchelt DR, Streit WJ. Ex vivo cultures of microglia from young and aged rodent brain reveal age-related changes in microglial function. Neurobiol Aging. 2012;33:195 e1–12. doi: 10.1016/j.neurobiolaging.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluchino S, Muzio L, Imitola J, Deleidi M, Alfaro-Cervello C, Salani G, Porcheri C, Brambilla E, Cavasinni F, Bergamaschi A, Garcia-Verdugo JM, Comi G, Khoury SJ, Martino G. Persistent inflammation alters the function of the endogenous brain stem cell compartment. Brain. 2008;131:2564–2578. doi: 10.1093/brain/awn198. Part 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponti G, Obernier K, Guinto C, Jose L, Bonfanti L, Alvarez-Buylla A. Cell cycle and lineage progression of neural progenitors in the ventricular-subventricular zones of adult mice. Proc Natl Acad Sci USA. 2013;110:E1045–E1054. doi: 10.1073/pnas.1219563110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Liu Y, Hong JS, Crews FT. NADPH oxidase and aging drive microglial activation, oxidative stress, and dopaminergic neurodegeneration following systemic LPS administration. Glia. 2013;61:855–868. doi: 10.1002/glia.22479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinones-Hinojosa A, Sanai N, Soriano-Navarro M, Gonzalez-Perez O, Mirzadeh Z, Gil-Perotin S, Romero-Rodriguez R, Berger MS, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and cytoarchitecture of the adult human subventricular zone: A niche of neural stem cells. J Comp Neurol. 2006;494:415–434. doi: 10.1002/cne.20798. [DOI] [PubMed] [Google Scholar]
- Robel S, Berninger B, Gotz M. The stem cell potential of glia: Lessons from reactive gliosis. Nat Rev Neurosci. 2011;12:88–104. doi: 10.1038/nrn2978. [DOI] [PubMed] [Google Scholar]
- Sanai N, Nguyen T, Ihrie RA, Mirzadeh Z, Tsai HH, Wong M, Gupta N, Berger MS, Huang E, Garcia-Verdugo JM, Rowitch DH, Alvarez-Buylla A. Corridors of migrating neurons in the human brain and their decline during infancy. Nature. 2011;478:382–386. doi: 10.1038/nature10487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanai N, Tramontin AD, Quinones-Hinojosa A, Barbaro NM, Gupta N, Kunwar S, Lawton MT, McDermott MW, Parsa AT, Garcia-Verdugo JM, Berger MS, Alvarez-Buylla A. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427:740–744. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- Sawamoto K, Hirota Y, Alfaro-Cervello C, Soriano-Navarro M, He X, Hayakawa-Yano Y, Yamada M, Hikishima K, Tabata H, Iwanami A, Nakajima K, Toyama Y, Itoh T, Alvarez-Buylla A, Garcia-Verdugo JM, Okano H. Cellular composition and organization of the subventricular zone and rostral migratory stream in the adult and neonatal common marmoset brain. J Comp Neurol. 2011;519:690–713. doi: 10.1002/cne.22543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawamoto K, Wichterle H, Gonzalez-Perez O, Cholfin JA, Yamada M, Spassky N, Murcia NS, Garcia-Verdugo JM, Marin O, Rubenstein JL, Tessier-Lavigne M, Okano H, Alvarez-Buylla A. New neurons follow the flow of cerebrospinal fluid in the adult brain. Science. 2006;311:629–632. doi: 10.1126/science.1119133. [DOI] [PubMed] [Google Scholar]
- Schiffer D, Giordana MT, Migheli A, Giaccone G, Pezzotta S, Mauro A. Glial fibrillary acidic protein and vimentin in the experimental glial reaction of the rat brain. Brain Res. 1986;374:110–118. doi: 10.1016/0006-8993(86)90399-9. [DOI] [PubMed] [Google Scholar]
- Shen Q, Wang Y, Kokovay E, Lin G, Chuang SM, Goderie SK, Roysam B, Temple S. Adult SVZ stem cells lie in a vascular niche: A quantitative analysis of niche cell–cell interactions. Cell Stem Cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shook BA, Manz DH, Peters JJ, Kang S, Conover JC. Spatiotemporal changes to the subventricular zone stem cell pool through aging. J Neurosci. 2012;32:6947–6956. doi: 10.1523/JNEUROSCI.5987-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spassky N, Merkle FT, Flames N, Tramontin AD, Garcia-Verdugo JM, Alvarez-Buylla A. Adult ependymal cells are postmitotic and are derived from radial glial cells during embryogenesis. J Neurosci. 2005;25:10–18. doi: 10.1523/JNEUROSCI.1108-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll EA, Habibi BA, Mikheev AM, Lasiene J, Massey SC, Swanson KR, Rostomily RC, Horner PJ. Increased re-entry into cell cycle mitigates age-related neurogenic decline in the murine subventricular zone. Stem Cells. 2011;29:2005–2017. doi: 10.1002/stem.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ, Walter SA, Pennell NA. Reactive microgliosis. Prog Neurobiol. 1999;57:563–581. doi: 10.1016/s0301-0082(98)00069-0. [DOI] [PubMed] [Google Scholar]
- Tatar C, Bessert D, Tse H, Skoff RP. Determinants of central nervous system adult neurogenesis are sex, hormones, mouse strain, age, and brain region. Glia. 2013;61:192–209. doi: 10.1002/glia.22426. [DOI] [PubMed] [Google Scholar]
- Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, Garcia-Verdugo JM, Doetsch F. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thored P, Heldmann U, Gomes-Leal W, Gisler R, Darsalia V, Taneera J, Nygren JM, Jacobsen SE, Ekdahl CT, Kokaia Z, Lindvall O. Long-term accumulation of microglia with proneurogenic phenotype concomitant with persistent neurogenesis in adult subventricular zone after stroke. Glia. 2009;57:835–849. doi: 10.1002/glia.20810. [DOI] [PubMed] [Google Scholar]
- Tonchev AB. Brain ischemia, neurogenesis, and neurotrophic receptor expression in primates. Arch Ital Biol. 2011;149:225–231. doi: 10.4449/aib.v149i2.1368. [DOI] [PubMed] [Google Scholar]
- Walton NM, Sutter BM, Laywell ED, Levkoff LH, Kearns SM, Marshall GP, II, Scheffler B, Steindler DA. Microglia instruct subventricular zone neurogenesis. Glia. 2006;54:815–825. doi: 10.1002/glia.20419. [DOI] [PubMed] [Google Scholar]
- Yan YP, Sailor KA, Vemuganti R, Dempsey RJ. Insulin-like growth factor-1 is an endogenous mediator of focal ischemia-induced neural progenitor proliferation. Eur J Neurosci. 2006;24:45–54. doi: 10.1111/j.1460-9568.2006.04872.x. [DOI] [PubMed] [Google Scholar]
- Young CC, van der Harg JM, Lewis NJ, Brooks KJ, Buchan AM, Szele FG. Ependymal ciliary dysfunction and reactive astrocytosis in a reorganized subventricular zone after stroke. Cereb Cortex. 2012;23:647–659. doi: 10.1093/cercor/bhs049. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.