Abstract
Analysis of centrosome number and structure has become one means of assessing the potential for aberrant chromosome segregation and aneuploidy in tumor cells. Centrosome amplification directly causes multipolar catastrophic mitoses in mouse embryonic fibroblasts (MEFs) deficient for the tumor suppressor genes Brca1 or Trp53. We observed supernumerary centrosomes in cell lines established from aneuploid, but not from diploid, colorectal carcinomas, however, multipolar mitoses were never observed. This discrepancy prompted us to thoroughly characterize the centrosome abnormalities in these and other cancer cell lines with respect to both structure and function. The most striking result was that supernumerary centrosomes in aneuploid colorectal cancer cell lines were unable to nucleate microtubules despite the presence of γ-tubulin, pericentrin, PLK1 and AURKA. Analysis by scanning electron microscopy revealed that these supernumerary structures are devoid of centrioles, a result significantly different from observations in aneuploid pancreatic cancer cell lines and in Trp53 or Brca1 deficient MEFs. Thus, multipolar mitoses are dependent upon the ability of extra γ-tubulin containing structures to nucleate microtubules, and this correlated with the presence of centrioles. The assessment of centrosome function with respect to chromosome segregation must therefore take into consideration the presence of centrioles and the capacity to nucleate microtubules.
Keywords: Centrosome, aneuploidy, chromosome instability, gene expression
Significance
The patterns and mechanisms of chromosomal aberrations in hematologic malignancies and solid tumors are fundamentally different. The former is characterized by specific chromosome translocations, whose consequence is the activation of oncogenes. Most carcinomas, however, reveal variations in the nuclear DNA content. The observed genomic imbalances and gross variations in chromosome number can result from unequal chromosome segregation during mitotic cell division. It is therefore fundamental to elucidate mechanisms involved in distribution of the genome to daughter cells. Prior to cell division, the centrosome organizes microtubules and the mitotic spindle. Deciphering the consequences of alterations in centrosome number, structure and function is an important step towards understanding how a diploid genome is maintained. Although extra centrosomes have now been observed in carcinomas and were correlated with aneuploidy, a careful functional investigation of these structures and their role in generating chromosome imbalances may lead to the identification of distinct mechanistic pathways of genomic instability. Understanding these pathways will also be important in determining whether they are potential molecular targets of therapeutic intervention.
Introduction
Over a century ago Theodor Boveri coined the term centrosome to describe the sub-cellular structures originally observed by Van Beneden in 1876. He postulated that due to their role in cell division, deviation in number from the two centrosomes normally present in each cell could have dire consequences with respect to the partitioning of chromosomes into the daughter cells. Subsequent chromosome missegregation could result in the loss of tumor inhibiting, and the gain of tumor promoting, chromosomes and that this served as the genetic basis of malignant transformation [Boveri 1929]. The development of molecular cytogenetic techniques [Kallioniemi et al. 1992; Schröck et al. 1996], and their recent adaptation to higher resolution platforms including whole genome sequencing [Dufva 2009; Pettersson et al. 2009], has enabled the comprehensive characterization of chromosomal aberrations in cancer genomes. It has become clear through the use of these techniques that solid tumors originating in epithelial cells of different organs are characterized by a distribution of specific and distinct genomic imbalances, that whole chromosome gains and losses are early events in tumorigenesis [Ried et al. 1999] and that strong selection for these events may have a causative role in tumorigenesis. Accordingly, Boveri's hypothesis with respect to chromosome segregation errors and the role of centrosomes in this process has been revisited [Brinkley and Goepfert 1998; Pihan and Doxsey 1999; Pihan et al. 1998].
The centrosome is positioned in the cytoplasm adjacent to the nucleus and its duplication is concurrent with replication of the genome during S phase. At the beginning of M phase, the two centrosomes separate to opposite poles of the nucleus where they nucleate the formation of mitotic spindles containing α- and β-tubulin, many of which eventually connect to kinetochore proteins associated with the centromere of each chromosome. A dynamic interplay between the microtubules, mitotic kinesins, and cytokinesins is necessary for the coordinated separation of sister chromatids, their migration to opposite poles and generation of the cleavage furrow at the end of mitosis [Bowerman 2004; Brinkley 2001; Neef et al. 2006; Petronczki et al. 2007; Rogers et al. 2004; Stearns 2001].
Centrosomes consist of centrioles and the pericentriolar matrix (PCM). The centrioles contain a protein known as centrin while the PCM contains γ-tubulin, pericentrin (PCNT), hGCP2, GCP3/HsSpc98 and AKAP450 among other proteins too numerous to list [Brinkley 2001; Stearns 2001; Alieva and Uzbekov 2008]. Two protein families shown to regulate centrosome duplication and/or separation are the polo-like kinases (e.g. PLK1) and aurora family kinases (e.g., AURKA) [Glover et al. 1995; Lane and Nigg 1996]. Overexpression of these proteins results in supernumerary centrosomes, while abrogating expression causes a failure of centrosome migration [Lane and Nigg 1996; Zhou et al. 1998]. The Cdk2-cyclin E (Cdk2-E) complex has been shown to regulate centrosome duplication in Xenopus cell extracts [Hinchcliffe et al. 1999]. A positive feedback loop between the Xenopus Plk1 protein (Plx1) and a downstream protein kinase target xPlkk1 has also been demonstrated [Erikson et al. 2004; Qian et al. 1998a; Qian et al. 1998b], implicating yet another phosphorylation pathway regulating the centrosome cycle.
We and others have previously demonstrated the presence of supernumerary centrosomes in primary tumors and tumor cell lines of different origins [Ghadimi et al. 2000; Lingle et al. 1998; Pihan et al. 1998]. These findings have been touted as proof that extra centrosomes can cause aneuploidy through their direct role in mis-segregation of chromosomes during mitosis. In only a very few instances, however, has this mechanism been proven by direct visualization of aberrant mitotic figures [Fukasawa et al. 1996; Xu et al. 1999]. In the present study we have identified differences with respect to the type of centrosome aberrations occurring in tumorigenesis. Our results suggest that the failure of certain centrosomes to nucleate microtubules and organize the mitotic spindle could be due to the absence of centrioles. This is the first report to our knowledge of γ-tubulin structures lacking nucleation capacity in mammalian cells.
Experimental Procedures
Cell lines and RNA Isolation
The following colorectal cancer cell lines were used in this study: DLD-1, HCT116, p53HCT116, SW48, and LoVo (near-diploid); SW480, SW837, HT-29, T84, Colo 201 for immunocytochemistry and nucleation assays. For gene expression analysis Colo 320DM, LS411N, SK-CO-1, NCI-H508, and NCI-H716 (aneuploid) were also utilized. The pancreatic tumor cell lines included AsPC-1, BxPC-3, Capan-1, Capan-2, CFPac-1, Hs766T, Mia PaCa-2, Panc-1, SU 86.86. All of the aforementioned cell lines were obtained from the ATCC (American Type Culture Collection) and cultured following their recommendations, except p53HCT116, a derivative of HCT116 with a homozygous disruption of TP53 [Bunz et al. 1998], which was kindly provided by Dr. Curtis C. Harris of the National Cancer Institute, NIH. Control fibroblasts were cultured from human foreskin. p53−/− mouse embryonic fibroblasts (MEFs) were obtained from Andre Nussenzweig of the National Cancer Institute, NIH.
RNA was extracted from the cell lines and primary tumors [Camps et al. In Press] following standard procedures (http://www.riedlab.nci.nih.gov/protocols.asp). Nucleic acid quantification was determined using the Nanodrop ND-1000 UV-VIS spectrophotometer (Nanodrop, Rockland, DE) and RNA quality was assessed using the Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA). Normal colon RNA isolated post-mortem from five different donors without a history of colorectal cancer was purchased from Ambion (Applied Biosystems, Foster City, CA).
Antibodies
Mouse monoclonal antibodies were used to detect γ-tubulin (Sigma-Aldrich, St Louis, MO, T6557; diluted 1:2000) and α-tubulin (Sigma-Aldrich, T9026; diluted 1:1000). Anti-PCNT rabbit polyclonal antibodies were obtained from Berkley Ab Company, Berkley, CA (PRB-432C; diluted 1:100). Anti-PLK1 and anti-AURKA rabbit polyclonal antibodies were produced by injection of peptide [Hamanaka et al. 1995]. Secondary antibodies used for immunocytochemistry were purchased from Vector Laboratories, Burlingame, CA (Goat anti-rabbit-TR, TI-1000, diluted 1:1000) and Boehringer Mannheim, Indianapolis, IN (Goat anti-mouse-FITC, diluted 1:200).
Immunocytochemistry
Cells were grown on Falcon chamber slides (Becton & Dickinson, Bedford, MA), rinsed once each in PBS and PHEM buffer [PIPES (60mM), HEPES (25mM), EGTA (10mM), MgCl2 (2mM), pH 6.9], fixed in ice cold methanol for 10 min and washed 4× with PBS. Slides were blocked with 5% normal goat serum (NGS), 1% BSA in PBS for 30 min at 37°C. Primary antibodies were diluted (as indicated above) in 1% NGS, 1% BSA in PBS and incubated for 45 min at 37°C followed by three washes in PBS. The primary antibodies were detected with Goat-anti-rabbit-TR and Goat anti-mouse-FITC followed by three washes in PBS. Cells were counterstained with DAPI and mounted with antifade [p-phenylene-diamine (5.52mM), 77% glycerol, 0.1×PBS, to pH 8.0 with carbonate/bicarbonate buffer (pH 9.0)]. Images were acquired using Leica Q-FISH software (Leica Imaging Systems, Cambridge, UK). A minimum of 50 mitotic figures and 300 interphase nuclei were evaluated for centrosome number and organization.
Nucleation Assays
Cell lines were grown on Falcon culture slides (Becton & Dickinson). Cells were then incubated with the microtubule destabilizing drug nocodazole (10 µg/ml) for 1.5 hour at 37°C, and washed two times with PBS at room temperature and allowed to recover by incubation in media for 5 – 10 min. Slides were then rinsed once in PBS, once with PHEM buffer and then fixed in −20°C methanol. Tubulin structures were detected by incubating cells with a monoclonal α-tubulin (Sigma-Aldrich, 1:1000) and rabbit polyclonal γ-tubulin (Sigma-Aldrich, 1:2000) antibodies for 45 min. Following three PBS washes, the primary antibodies were detected with a FITC labeled goat anti-mouse and a TRITC labeled goat anti-rabbit antibody (Sigma-Aldrich, 1:200 each) for 45 min and cells were counterstained with DAPI.
Standard Transmission Electron Microscopy and Immunoelectron Microscopy
Cultured cells were processed in situ and embedded for electron microscopy as described [Gonda et al. 1976]. Briefly, cells were cultured in a T-75 flask, rinsed once with PBS and fixed with sodium cacodylate buffer (0.1M, pH 7.2) [Electron Microscope Sciences, Fort Washington, PA] containing 2% glutaraldehyde [Tousimis, Rockville, MD] for 1 hr at room temperature. The fixed cells were then rinsed with cacodylate buffer followed by post-fixation in cacodylate buffer + 1% osmium tetroxide [Electron Microscope Sciences, Fort Washington, PA] for 1 hr. The cells were dehydrated in an ethanol series (35%, 50%, 70%, 95%, and 100%) with three changes of absolute ethanol, embedded in pure Embed-812 epoxy resin [Electron Microscope Sciences, Fort Washington, PA] overnight and allowed to cure for 48 hrs at 55°C. Ultra thin-sections were cut and mounted on copper grids and stained with uranyl acetate followed by lead citrate. The sections were observed and photographed using a Hitachi H-7000 transmission electron microscope [Nissei Sanyo America, Ltd, Pleasanton, CA] operated at 75 kV.
For ICC/EM, cells were cultured on gridded glass coverslips (22×22 mm2) (Bellco Biotechnology, Vineland, NJ) and washed in PBS. The cells were fixed in cytoskeletal buffer (CSK) [PIPES (910mM), NaCl (100mM), Sucrose (300mM), EGTA (1mM), MgCl2 (3mM)] + 0.1% Triton X-100 for 30 min, CSK + 0.1% Triton X-100 + 1% glutaraldehyde for 2 min and CSK + 0.1% glutaraldehyde for 10 min. This was followed by two 15 min treatments with 0.1% NaBH4 in PBS. The coverslips were then processed for immunofluorescence as stated above using mouse mAb against γ-tubulin detected with goat anti-mouse-FITC and counterstained with DAPI. Images were acquired of cells with aberrant numbers of γ-tubulin staining bodies using a Nikon FXA fluorescence microscope and their coordinates recorded. The coverslips were floated off the slides and washed several times in PBS and then immersed in PBS overnight at 4°C. The cells were then washed 2 × 5 min in PBS and followed by 3 × 5 min washes in 0.1M sodium cacodylate buffer (pH 7.0). Cells were post fixed in 1% osmium potassium ferrocyanide for 1 hour at room temperature, washed 2 × 5 min in 0.1M sodium cacodylate and then 2 × 5 min in dH2O. Samples were stained with 2% aqueous uranyl acetate for 30 min. at room temp, washed 3 × 5 min in dH20, dehydrated in ethanol and infiltrated and embedded in Epon/Araldite. Thin sections were cut, cells relocated by their coordinates and examined using a Hitachi H-7000 transmission electron microscope [Nissei Sanyo America, Ltd, Pleasanton, CA] operated at 75 kV.
Gene Expression Microarrays and Data Analysis
The procedure followed for the hybridization and analysis of the gene expression arrays can be found elsewhere for the primary tumors [Camps et al. 2008] and the CRC cell lines (Camps et al., In Press). Briefly, one ug each of cell line or normal human colon RNA (Ambion, Austin, TX) and Universal Human Reference RNA (Stratagene) were amplified and labeled with Cy3 and Cy5, respectively, using a T7 RNA Polymerase (Low RNA Input Fluorescent Linear Amplification Kit, Agilent) according to the manufacturer's protocols, and hybridized to the 44K oligonucleotide-based Whole Human Genome Microarray G4112F (Agilent). Similarly, RNA from primary tumors and normal human colon were labeled with Cy3 and subjected to mono-channel hybridization onto 4×44K Whole Human Genome Microarray (G4112F, Agilent). Microarrays were washed and processed using an Agilent G2565BA scanner. Data were quality controled and extracted using Agilent Technologies’ Feature Extraction (version 9.1).
The analyses of the microarray experiments were performed with in-house developed software based on R version 2.6.2 (http://www.R-project.org). Gene expression data was obtained from 44K or 4×44K Agilent dual-channel arrays. Median per feature was used to summarize data when two or three technical replicates were available. The data were normalized using Linear & Lowess procedure in Agilent’s Feature Extraction software. Features for which signals were below background (as assessed by “gSurrogatedUsed” or “rSurrogatedUsed”) were forced to NA (not a number). The median measurement was used when more than 1 measurement was available per feature (i.e. median-summarization by array using “ProbeName”). The final CRC cell line dataset contained 20 samples (15 cell lines, and 5 normal colon samples), and 40380 features.
Results
Numerical Aberrations
Diploid and aneuploid colorectal tumor cell lines show striking differences with respect to both centrosome number and structure [Ghadimi et al. 2000]. Despite the presence of supernumerary centrosomes in the aneuploid tumors, we fail to observe a potential consequence of centrosome amplification, i.e. multipolar mitoses. We therefore began a thorough functional and structural analysis of centrosomes in normal cultured foreskin fibroblast cells, diploid tumors, and aneuploid tumors with and without multipolar mitoses. Based upon these analyses we identified distinct differences in the number, size and localization of the centrosomes in these cell types.
Normal fibroblasts, as well as the diploid colorectal cell lines (HCT116, DLD1, SW48), contained 1–2 γ-tubulin positive staining bodies resulting in the formation of a normal bipolar mitosis (Figs. 1A & 1D). The aneuploid colorectal cell lines (HT29, SW837, SW480, Colo201, T84) each had a subpopulation of cells containing ≥3 γ-tubulin positive bodies [Ghadimi et al. 2000], the size and morphology of which was variable (Fig. 1B: mononucleated or Fig. 1C: multinucleated cells). All of the observed mitoses were biplolar, however, with some exhibiting clusters of γ-tubulin at the poles (Fig. 2F). We therefore extended our analysis of centrosome defects to include aneuploid cancer cell lines of pancreatic origin. These lines also contained a percentage of cells with an abnormal number of γ-tubulin positive structures (Table I), however all were of equal size and morphology, a phenotype distinct from the colorectal cancer cell lines. In addition, we frequently observed multipolar mitoses (Fig. 2G).
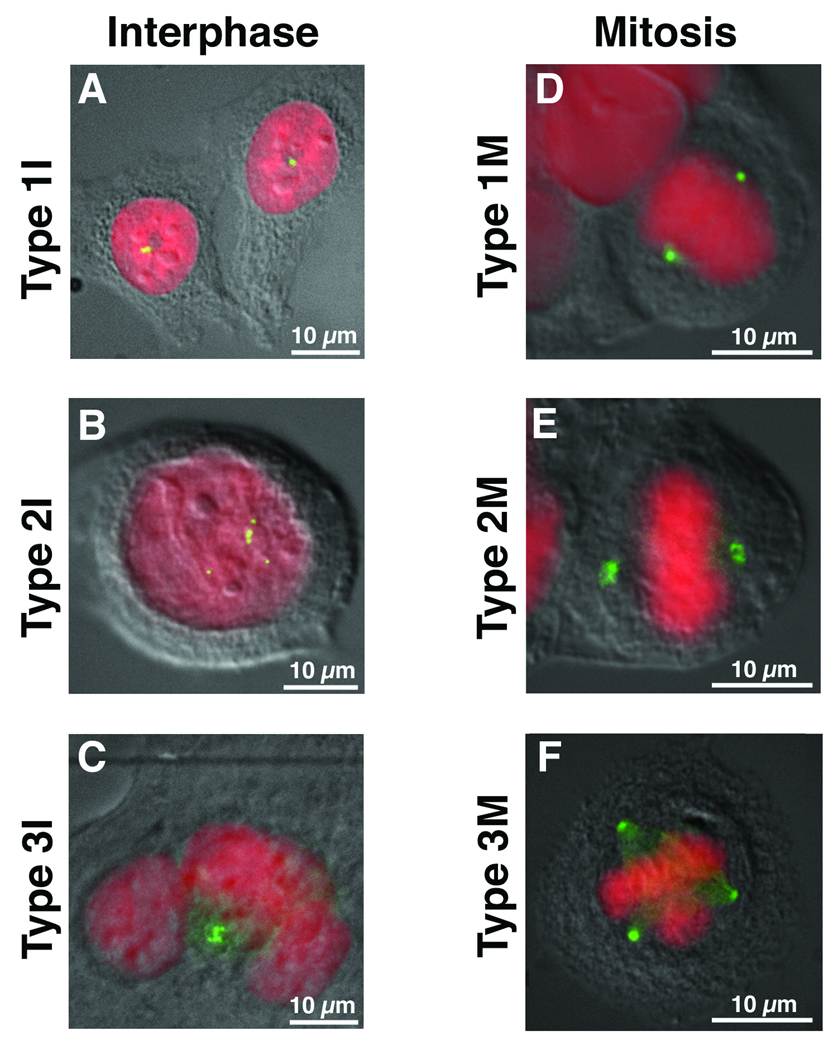
Figure 1.
Interphase cells (I) were categorized based on the number of their centrosomes (green) as detected with an anti-γ-tubulin antibody. DNA was counterstained with DAPI (red) and the cells visualized by differential interference contrast (DIC) microscopy. Type 1I (A) have 1–2 centrosomes. Type 2I (B) are mono-nucleated cells with multiple centrosomes while Type 3I (C) contain multiple centrosomes in a multi-nucleated cell. Mitotic cells (M) were categorized in a similar manner based on the number and orientation of their centrosomes with respect to the mitotic plate. Type 1M (D) have a bi-polar spindle with 1 centrosome on either side of the mitotic plate. Type 2M (E) mitoses are bi-polar but demonstrate coalescence of multiple centrosomes at either pole. Type 3M (F) are multipolar mitoses in which the chromosomes are pulled in more than two directions.
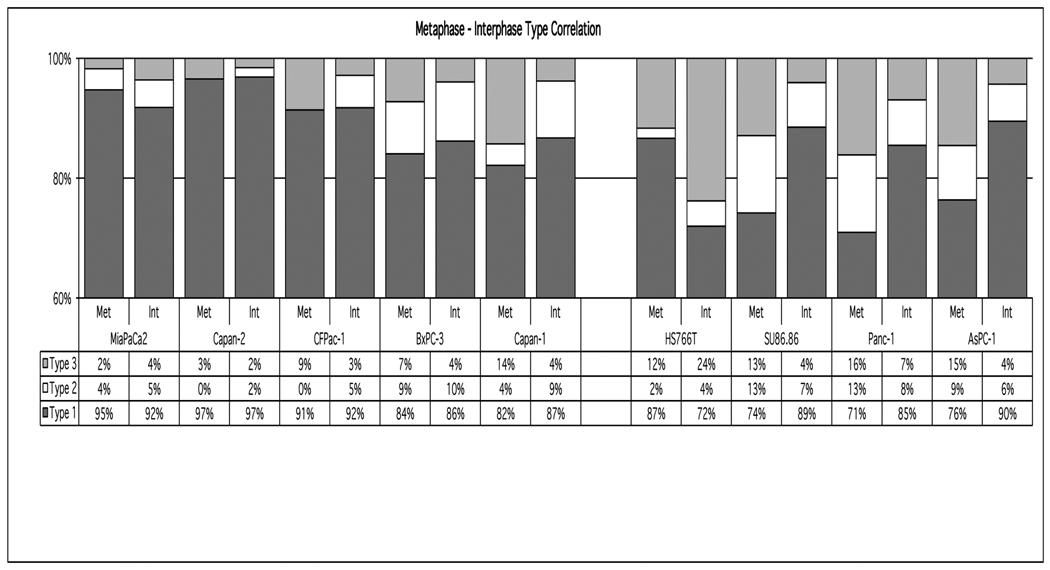
Figure 2.
Quantitation and comparison of interphase (Int) (n≥300) and metaphase (Met) (n≥50) pancreatic cancer cells based on the categories illustrated in Figure 1.
Table I.
Comparison of centrosome aberrations during mitosis and interphase in pancreatic cell lines.
| Interphase Nuclei |
Mitoses | |
|---|---|---|
| Cell Line |
Observed Abnormala |
Observed Multipolarb |
| AsPC-1 | 10% | 15% |
| BxPC-3 | 14% | 7% |
| Capan-1 | 13% | 9% |
| Capan-2 | 3% | 3% |
| CFPac-1 | 8% | 9% |
| Hs766T | 28% | 19% |
| Mia PaCa-2 | 8% | 2% |
| Panc-1 | 15% | 16% |
| SU86.86 | 11% | 13% |
Type2 and Type3 interphase cells combined.
Type3 metaphase cells.
Many of the aneuploid colorectal cancer cell lines contain inactivating TP53 mutations. The pattern of interphase centrosome aberrations we observed was consistent with the centrosome amplification seen in Trp53−/− or Brca1−/− MEFs [Fukasawa et al. 1996; Xu et al. 1999]. This caused us to wonder whether TP53 loss had a comparable consequence with respect to centrosome number in all cancer cell lines. In order to address this question, we took advantage of the diploid colorectal cancer cell line HCT116 made homozygously deficient for the TP53 protein (p53HCT116) [Waldman et al. 1996]. Unlike the TP53 wild-type parental cells, this cell line had centrosome amplification and multipolar mitoses akin to the Trp53−/− MEFs (see below).
Despite the absence of gross chromosome mis-segregation in the aneuploid colorectal cancer cell lines, we were interested in determining whether the interphase centrosome aberrations observed in the pancreatic cell lines could be directly correlated with events occurring during mitosis. A comparison of interphase and metaphase cell types in MiaPaCa2, Capan-2, CFPac-1, BxPC-3 and Capan-1 revealed that the percentage of interphase and metaphase cells with 1-2 centrosomes was similar (Fig. 2; Types 1I vs 1M). Thus, the population of interphase cells with normal centrosome numbers correlated with the percentage of metaphase cells containing two centrosomes and normal bi-polar mitotic spindles. This was not true, however, for HS766T, SU86.86, Panc-1 and AsPC-1 cells (Fig. 2).
Functional Aberrations
α-tubulin staining of mitotic spindles in Brca1−/− and Trp53−/− MEFs and in p53HCT116 and pancreatic cancer cell lines (Table 1 & [Sato et al. 2001]), revealed multiple nucleating centrosomes and multipolar mitosis, structures associated with gross chromosomal missegregation. Aberrant mitoses, however, were not observed in the aneuploid colorectal cell lines despite the presence of supernumerary γ-tubulin structures. This prompted us to assess whether all of the γ-tubulin structures in the aneuploid colorectal tumor cell lines were functionally capable of nucleating microtubules.
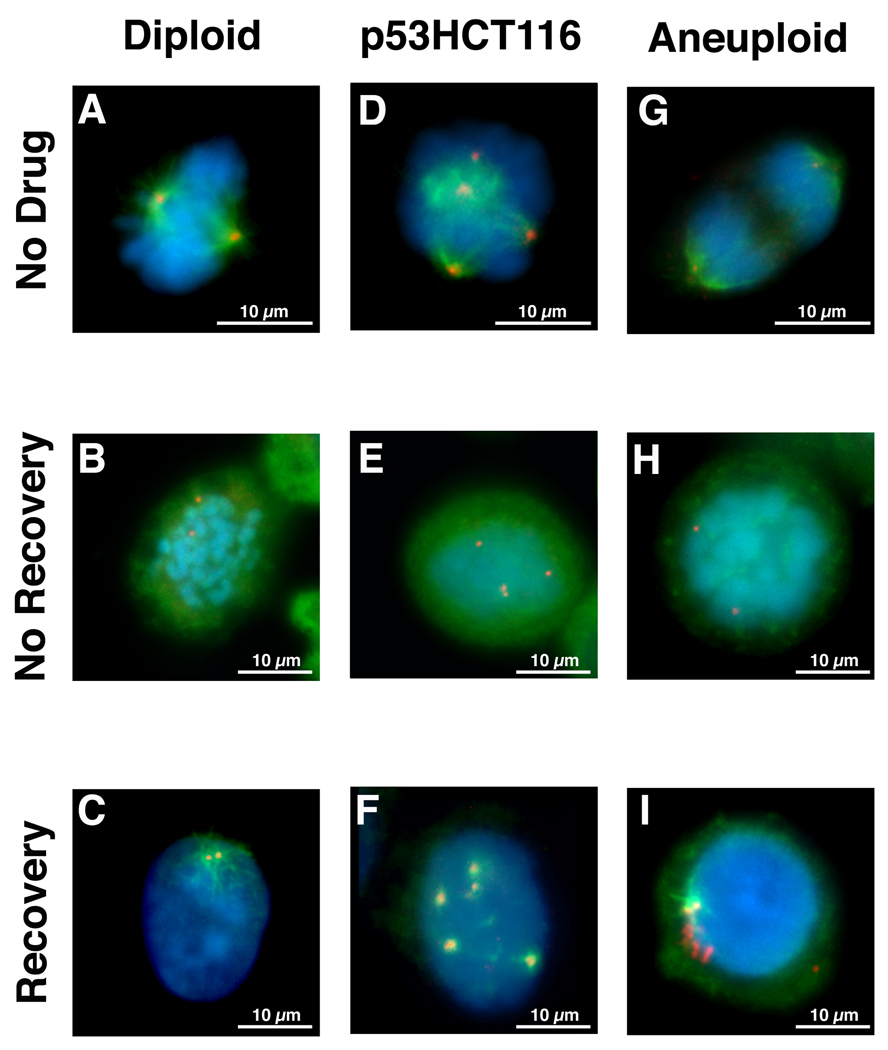
Nocodazole is a potent microtuble depolymerizing drug that causes cell cycle arrest at mitosis [Jordan et al. 1998]. The damage is reversible such that replacement with fresh medium allows repolymerization of the microtubules and the procession of mitosis. We therefore used nocodazole block and release to assess the ability of γ-tubulin structures to nucleate α-tubulin containing microtubules. Efficient microtubule nucleation from centrosomes was most readily observed in mitotic cells in the absence of nocodazole (Figs. 3A, D & G). The effectiveness of microtubule depolymerization by nocodazole can be seen in Figures 3B, E & H. In the diploid colorectal cell lines and normal fibroblasts (Fig. 3C), p53HCT116 (Fig. 3F), pancreatic cancer cell lines and p53−/− MEFs (data not shown), all γ-tubulin positive structures, regardless of their number, functioned as α-tubulin nucleating centers shortly after removal of nocodazole. This differed markedly from the aneuploid colorectal cancer cell lines in which, despite the presence of multiple γ-tubulin structures, only 1–2 centrosomes in each cell were observed to function as nucleating centers in the formation of normal mitotic spindles (Fig. 3I). Thus, the absence of multipolar mitoses in the aneuploid colorectal cancer cell lines correlated with the inability of supernumerary centrosomes to serve as nucleation centers for α-tubulin containing mitotic spindles.
Figure 3.
Nucleation assay and subsequent detection of both α-tubulin spindles (green) and γ-tubulin (red) in diploid (A–C) and aneuploid (G–I) colorectal cancer cell lines as well as p53HCT116 (D–F), a diploid colorectal cell line made homozygous deficient for TP53. Untreated cells (A, D, G) in mitosis reveal mitotic spindles nucleating from the centrosomes. Nocodazole treated cells (B, E, H) in mitosis. The absence of mitotic spindles demonstrates the microtubule depolymerizing activity of the drug. Cells allowed to briefly recover from nocodazole treatment (C, F, I) reveal nucleation of α-tubulin containing microtubules from all γ-tubulin structures in the diploid (C) and p53HCT116 (F) cells compared to only 1–2 γ-tubulin structures in the aneuploid colorectal cell lines (I).
Structural Aberrations
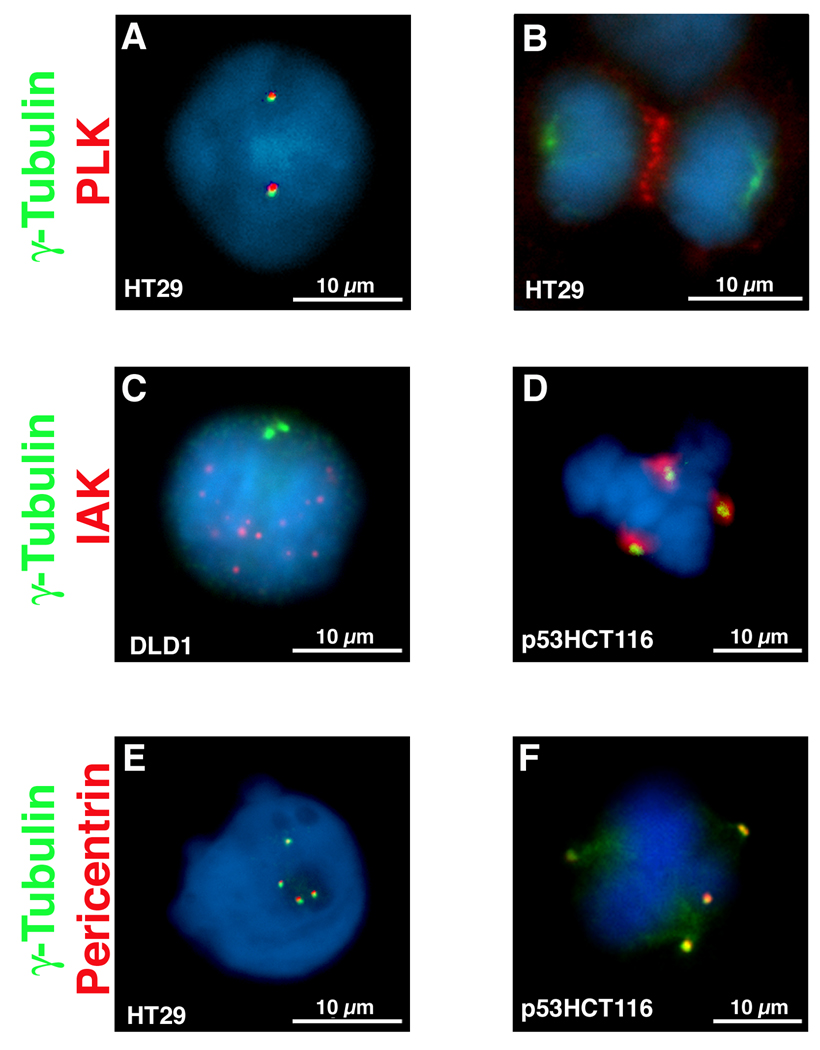
In an effort to determine why these supernumerary structures were not capable of giving rise to multipolar mitosis, co-localization studies of γ-tubulin with other centrosome-associated proteins were performed. PLK1 is normally associated with centrosomes throughout the cell cycle, until anaphase when it relocalizes to the metaphase plate and is distributed as two rings at the midbody during cytokinesis [Lane and Nigg 1996]. This pattern was recapitulated in both diploid (HCT116, p53HCT116, DLD1) and aneuploid (HT29, SW480, Colo201) CRC cell lines irrespective of the number of γ-tubulin positive structures. Representative images from different cell lines are shown in Figure 4A and 4B. In interphase, AURKA is distributed in multiple, non-centrosomal nuclear foci. Its association with centrosomes only occurs during mitosis. This redistribution pattern was also recapitulated in all of the CRC cell lines analyzed (Figs. 4C–D), with AURKA localized to every γ-tubulin positive structure. PCNT, as part of the pericentriolar matrix (PCM), always co-localizes with γ-tubulin in centrosomes [Doxsey et al. 1994], as was the case in all of the analyzed cell lines (Figs. 4E-F). Thus, we found no difference in the centrosome-associated localization pattern of PLK1, AURKA or PCNT proteins between diploid and aneuploid colorectal tumor cell lines. The presence of these centrosome-associated proteins was therefore insufficient to nucleate mitotic spindles and generate multipolar mitoses.
Figure 4.
Dual immunofluorescence for γ-tubulin and Polo-like kinase 1 (PLK1) (A &B), Aurora-kinase A (AURKA) (C & D) and PCNT (E & F). Interphase cells reveal co-localization of γ-tubulin with PLK1 (A) and PCNT (E) but not with AURKA (C). In mitosis, however, all four protein co-localize (D & E), however PLK1 remains at the metaphase plate during anaphase (B) and is found as two rings at the midbody after cytokinesis.
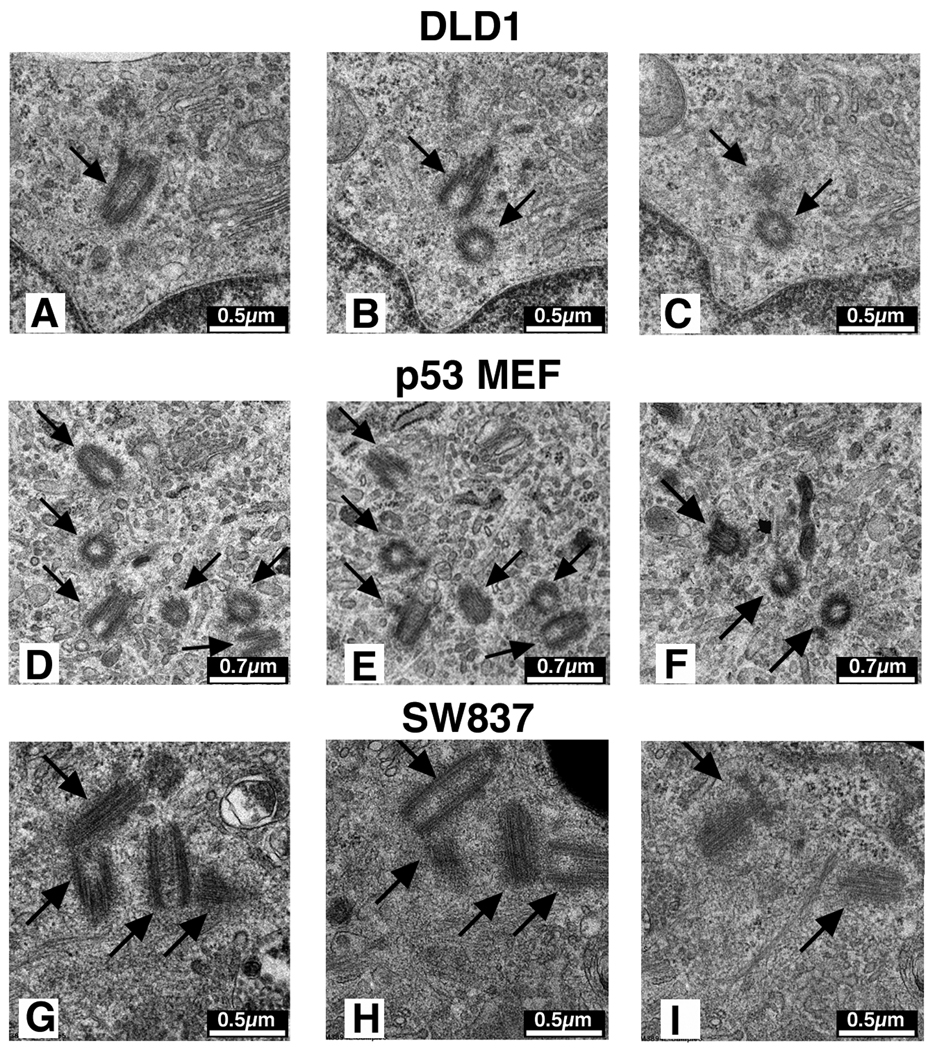
Each centrosome, as visualized by electron microscopy, consists of a pair of centrioles oriented at 90° relative to one another. Both fibroblasts and the diploid CRC cell line DLD1 contained 1–2 centrosomes, each with a pair of appropriately oriented centrioles (Figs. 5A–C). Many of the Trp53−/− MEFs and p53HCT116 cells contained multiple centriole pairs, often found in clusters (Figs. 5D–F). This was not surprising given the normal association of PCNT, PLK1 and AURKA with all of the supernumerary γ-tubulin structures (Fig. 4) and the presence of multipolar mitoses in these cells (Fig. 3D & Fig.4F). Examination of the aneuploid colorectal cancer cell lines revealed the presence of only 1–2 centriole pairs in greater than 95% of the cells (Fig. 5G–I). In the remaining cells, clusters of centrioles were observed (Fig. 6L), however the perpendicular orientation between pairs of centrioles was sometimes lost (data not shown).
Figure 5.
Electron microscopic serial sections of centrioles. One centriole pair, in their normal perpendicular orientation, as illustrated in the diploid cell line DLD1 (A – C). In Trp53-deficient MEFs (D – F) and the TP53-deficient HCT116 cell line (data not shown), multiple pairs of centrioles were observed, sometimes in clusters. Three pairs of centrosomes are depicted here. In the aneuploid colorectal cancer cell lines, such as shown for SW837 (G – I), two pairs of centrosomes were also observed, even in cells with multiple γ-tubulin foci.
Figure 6.
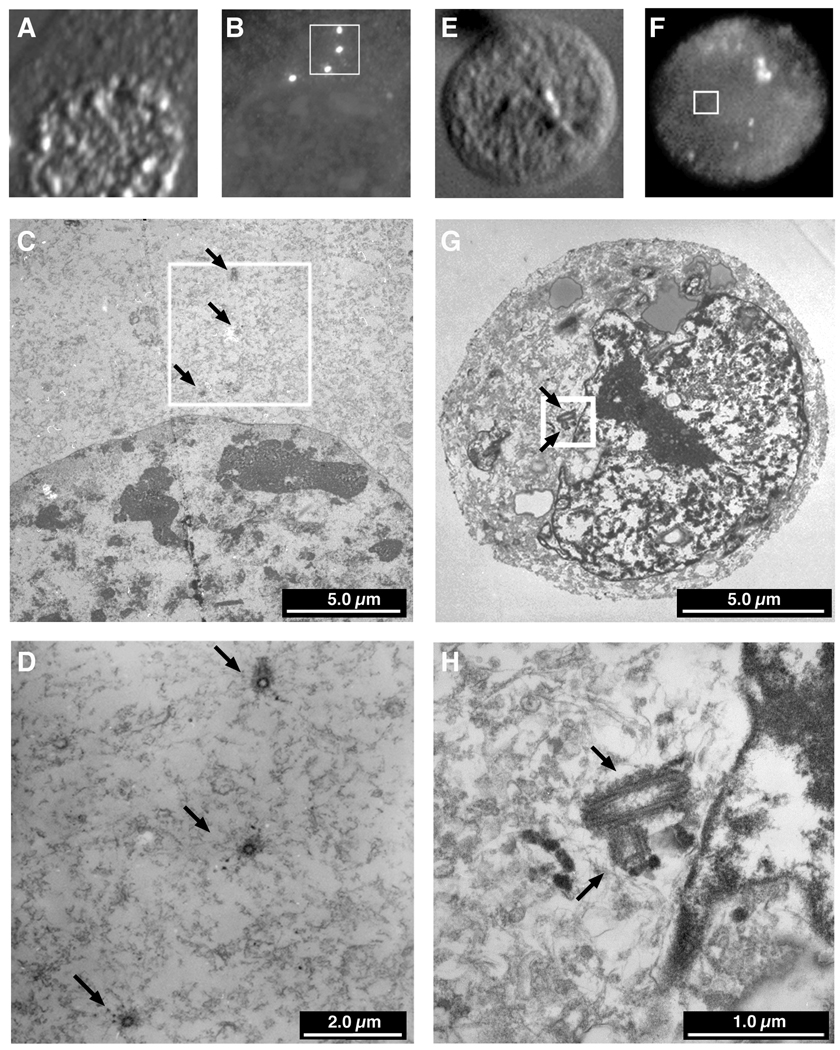
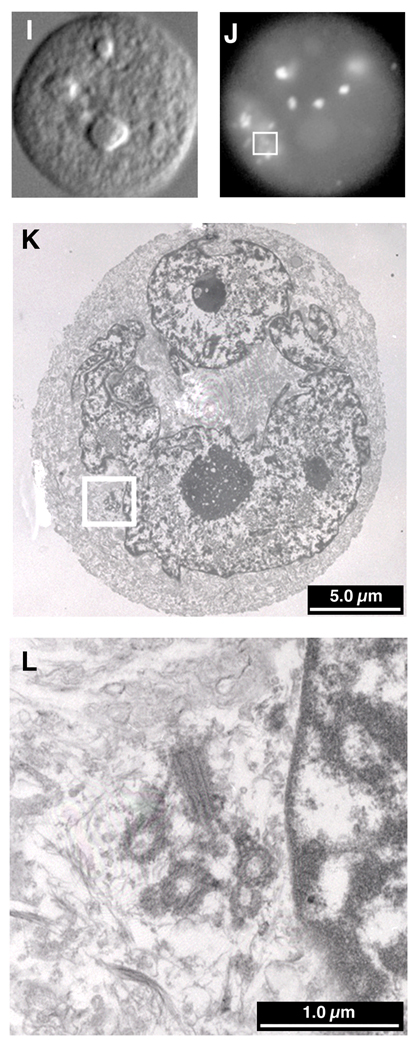
Immunocytochemistry - Electron microscopy (ICC-EM)
Differential interference contrast (DIC) (A, E & I) and immunofluorescence detection of γ-tubulin (B, F & J) was followed by preparation of serial sections for EM and relocalization of the cells with the aid of gridded coverslips. In Trp53-deficient MEFs (A-D) and the cell line p53HCT116 (data not shown), every pair of centrioles corresponded to a γ-tubulin structure. Aneuploid colorectal cells containing multiple γ-tubulin structures usually contained one or two correctly oriented centriole pairs (E-H). Very rarely, however, a cell was observed which contained a cluster of centrioles (I-L), but in association with only one of the many γ-tubulin structures, the others being devoid of centrioles.
This differed from our previous immunocytochemical observation that 30 – 40% of the SW837 cells contained multiple γ-tubulin structures. We therefore grew the cells on gridded coverslips, performed immunocytochemistry, identified those cells with multiple γ-tubulin structures and recorded their coordinates. The cells were then embedded for electron microscopy, serial sections cut and the cells relocated based on their coordinates. This afforded us the opportunity to directly correlate the presence of γ-tubulin and centrioles. As anticipated, all of the supernumerary centrosomes in the Trp53−/− MEFs contained a perpendicularly oriented pair of centrioles (Fig. 6A–D). In the aneuploid SW837 cancer line, cells with multiple γ-tubulin structures were found to contain only one or two pairs of centrioles (Fig. 6E–H), despite analysis of serial sections through the cells. Cells containing clusters of centrioles (Fig. 6I–L) did not always aggregate all of their γ-tubulin in one location. Thus, we were greatly surprised to find that the supernumerary structures containing γ-tubulin, PCNT and PLK in the aneuploid colorectal cancer cell lines were actually devoid of centrioles, thereby correlating the presence of centrioles with the nucleation capacity of γ-tubulin structures and the propensity for catastrophic multipolar mitoses.
Gene Expression Analysis
One approach to query the large number of genes currently known to be involved in centrosome regulation and function is global gene expression analysis. We expanded our set of CRC cell lines to include an additional five aneuploid CRC cell lines as well as five biopsies of normal colon epithelia. These results were also compared to a previous analysis consisting of 23 primary colon carcinomas [Camps et al. In Press]. Of the 131 genes spotted on the array that we queried based on their known or potential connection to the development of aneuploidy through an association with the centromere, centrosome or spindles, 11 genes were significantly deregulated (P<0.0001) in both the diploid and aneuploid cell lines relative to the mucosa, and of these seven were also of significance in primary colon tumors (Table 2). Only CSSP1 (1.48–1.96-fold decrease, P=0.009–0.0005), CETN2 (2.23-fold increase, P=0.000104), TTL4 (1.4-fold increase, P=0.009) and TUBGCP6 (1.52-fold decrease, P=0.0039) had an expression difference that reached any significance (P<0.01) in the aneuploid relative to the diploid cell lines.
Table 2.
| Gene Name | Map Position | DvM Linear Ratio |
DvM P- value |
AvM Linear Ratio |
AvM P value |
AvD Linear Ratio |
AvD P value |
TvM Linear Ratio |
TvM P value |
Description |
|---|---|---|---|---|---|---|---|---|---|---|
| ACTR1A | chr10:104229479-104229420 | 0.539647305 | 0.018696017 | 0.550153772 | 0.0010171 | 1.019469136 | 0.929200173 | 0.549205278 | 0.008497794 | Homo sapiens ARP1 actin-related protein 1 homolog A, centractin alpha (yeast) (ACTR1A), mRNA [NM_005736] |
| ACTR1A | chr10:104230605-104230546 | 1.04608995 | 0.818342947 | 0.648466369 | 0.008279034 | 0.61989542 | 0.042939928 | 0.699012881 | 0.00666194 | Homo sapiens ARP1 actin-related protein 1 homolog A, centractin alpha (yeast) (ACTR1A), mRNA [NM_005736] |
| ACTR1A | chr10:104235454-104235395 | 1.22501463 | 0.414176537 | 0.895646251 | 0.558158895 | 0.731131065 | 0.191056088 | 0.583951097 | 0.024949017 | Homo sapiens ARP1 actin-related protein 1 homolog A, centractin alpha (yeast) (ACTR1A), mRNA [NM_005736] |
| ACTR1B | chr2:97731657-97731598 | 0.659558652 | 0.038408023 | 0.843711821 | 0.311689899 | 1.279206661 | 0.173222468 | 0.783190459 | 0.350625827 | Homo sapiens ARP1 actin-related protein 1 homolog B, centractin beta (yeast) (ACTR1B), mRNA [NM_005735] |
| ASPM | chr1:193785170-193785111 | 10.77544985 | 4.44757E-06 | 7.890656212 | 0.000300598 | 0.732280909 | 0.459948772 | 8.31922161 | 3.8004E-06 | Homo sapiens asp (abnormal spindle)-like, microcephaly associated (Drosophila) (ASPM), mRNA [NM_018136] |
| ASPM | chr1:193844840-193844781 | 4.633452771 | 1.78125E-05 | 2.596709011 | 0.019904575 | 0.560426347 | 0.131647348 | 5.120339502 | 0.000480597 | Homo sapiens asp (abnormal spindle)-like, microcephaly associated (Drosophila) (ASPM), mRNA [NM_018136] |
| AURKA | chr20:54378586-54378527 | 5.033885486 | 0.000217951 | 5.096766692 | 2.44615E-05 | 1.012491585 | 0.963262042 | 8.378408936 | 1.93798E-09 | Homo sapiens aurora kinase A (AURKA), transcript variant 1, mRNA [NM_198433] |
| AURKAIP1 | chr1:1349349-1349193 | 0.875588444 | 0.432692593 | 0.892538657 | 0.491899499 | 1.019358654 | 0.889299865 | 1.344257482 | 0.19504244 | Homo sapiens aurora kinase A interacting protein 1 (AURKAIP1), mRNA [NM_017900] |
| AURKB | chr17:8051642-8051380 | 7.872273192 | 0.000490426 | 5.595317117 | 0.000234224 | 0.710762569 | 0.399696704 | 5.433402537 | 4.42256E-05 | Homo sapiens aurora kinase B (AURKB), mRNA [NM_004217] |
| AURKC | chr19:62435754-62435813 | 1.21624842 | 0.57975991 | 0.912809485 | 0.542426431 | 0.75051237 | 0.449794723 | 0.19721149 | 0.08925087 | Homo sapiens aurora kinase C (AURKC), transcript variant 1, mRNA [NM_001015878] |
| CCDC5 | chr18:41957282-41958798 | 2.693528404 | 0.001496512 | 2.034856415 | 0.018498786 | 0.75546128 | 0.264585612 | 0.978815227 | 0.897631526 | Homo sapiens coiled-coil domain containing 5 (spindle associated) (CCDC5), mRNA [NM_138443] |
| CEND1 | chr11:778062-778003 | 0.507068172 | 0.308536962 | 0.610988619 | 0.315812795 | 1.20494374 | 0.780149802 | 0.027157738 | 2.05961E-11 | Homo sapiens cell cycle exit and neuronal differentiation 1 (CEND1), mRNA [NM_016564] |
| CEND1 | chr11:778360-778301 | 0.968945488 | 0.660559255 | 1.278257171 | 0.104024766 | 1.319225062 | 0.086664261 | 1.425476559 | 0.006553738 | Homo sapiens cell cycle exit and neuronal differentiation 1 (CEND1), mRNA [NM_016564] |
| CENPA | chr2:26928565-26928624 | 14.66658432 | 2.16592E-05 | 11.34106026 | 8.24114E-05 | 0.77325845 | 0.526011482 | 9.892367239 | 1.58309E-06 | Homo sapiens centromere protein A (CENPA), transcript variant 1, mRNA [NM_001809] |
| CENPB | chr20:3713453-3713394 | 0.760030198 | 0.159655839 | 1.180472313 | 0.266016213 | 1.553191328 | 0.046935865 | 0.5537266 | 0.013168675 | Homo sapiens centromere protein B, 80kDa (CENPB), mRNA [NM_001810] |
| CENPB | chr20:3715043-3714984 | 1.160296316 | 0.363689859 | 1.185731237 | 0.340074488 | 1.021921057 | 0.845679736 | 0.73662732 | 0.001802347 | Homo sapiens centromere protein B, 80kDa (CENPB), mRNA [NM_001810] |
| CENPC1 | chr4:68169448-68169389 | 1.160612838 | 0.405485687 | 1.059114704 | 0.684977297 | 0.912547811 | 0.624082623 | 1.118880269 | 0.372545452 | Homo sapiens centromere protein C 1 (CENPC1), mRNA [NM_001812] |
| CENPC1 | chr4:68188382-68187437 | 1.006709055 | 0.934194201 | 0.876358785 | 0.358993464 | 0.870518429 | 0.332645481 | 0.851713879 | 0.289385268 | Homo sapiens centromere protein C 1 (CENPC1), mRNA [NM_001812] |
| CENPE | chr4:104385002-104384943 | 5.080925974 | 3.94403E-06 | 3.963894178 | 0.001133087 | 0.780151925 | 0.449791506 | 5.788419262 | 4.83071E-07 | Homo sapiens centromere protein E, 312kDa (CENPE), mRNA [NM_001813] |
| CENPF | chr1:211214634-211214693 | 13.31623126 | 6.81039E-06 | 8.593518485 | 1.4088E-05 | 0.645341638 | 0.139738593 | 9.487805522 | 0.000145524 | Homo sapiens centromere protein F, 350/400ka (mitosin) (CENPF), mRNA [NM_016343] |
| CENPF | chr1:211225703-211225762 | 15.29546784 | 1.87815E-07 | 8.370579827 | 0.00026139 | 0.547258829 | 0.147519816 | 10.40332935 | 1.61432E-06 | Homo sapiens centromere protein F, 350/400ka (mitosin) (CENPF), mRNA [NM_016343] |
| CENPH | chr5:68541405-68541464 | 9.107266658 | 0.000127541 | 7.245875049 | 9.04981E-05 | 0.79561468 | 0.503731509 | 6.864131023 | 0.001168224 | Homo sapiens centromere protein H (CENPH), mRNA [NM_022909] |
| CENPI | chrX:100193585-100201853 | 4.931762582 | 0.009120648 | 6.641217038 | 1.89721E-05 | 1.346621401 | 0.492460004 | 15.96776733 | 0.022712509 | Homo sapiens centromere protein I (CENPI), mRNA [NM_006733] |
| CENPI | chrX:100222701-100224023 | 5.244225042 | 0.002512297 | 5.004508063 | 0.002597057 | 0.954289342 | 0.893063222 | 12.55296847 | 0.042236629 | Homo sapiens centromere protein I (CENPI), mRNA [NM_006733] |
| CENPJ | chr13:24355465-24355406 | 1.922398211 | 0.015693791 | 2.160038313 | 0.02897501 | 1.123616481 | 0.722112884 | 3.433376648 | 0.001048035 | Homo sapiens centromere protein J (CENPJ), mRNA [NM_018451] |
| CENPJ | chr13:24361516-24361457 | 2.375437864 | 0.009754441 | 2.66206312 | 0.008230167 | 1.120662073 | 0.659930266 | 2.450784398 | 0.043428847 | Homo sapiens centromere protein J (CENPJ), mRNA [NM_018451] |
| CENPK | chr5:64849628-64849569 | 13.37245646 | 0.028684671 | 14.21561885 | 0.017558405 | 1.063052169 | 0.871131403 | 24.60140419 | 0.02444757 | Homo sapiens centromere protein K (CENPK), mRNA [NM_022145] |
| CENPL | chr1:170503839-170503780 | 8.302882521 | 3.66873E-05 | 7.378983166 | 3.66157E-05 | 0.888725469 | 0.704008813 | 10.18527728 | 0.026056405 | Homo sapiens centromere protein L (CENPL), mRNA [NM_033319] |
| CENPM | chr22:40666456-40665789 | 5.553492008 | 2.62399E-07 | 3.998953137 | 0.000247492 | 0.72007903 | 0.223876047 | 8.952540978 | 6.72703E-06 | Homo sapiens centromere protein M (CENPM), transcript variant 2, mRNA [NM_001002876] |
| CENPN | chr16:79598381-79603092 | 8.210107056 | 1.78164E-05 | 6.192759908 | 5.47473E-05 | 0.754284916 | 0.361160034 | 5.703264764 | 1.02245E-09 | Homo sapiens centromere protein N (CENPN), mRNA [NM_018455] |
| CENPN | chr16:79611372-79613909 | 5.821282605 | 3.28212E-05 | 4.440301964 | 0.000330328 | 0.762770383 | 0.379305925 | 6.851514614 | 4.41852E-12 | Homo sapiens centromere protein N (CENPN), mRNA [NM_018455] |
| CENPO | chr2:24952223-24952282 | 9.686741435 | 3.2314E-08 | 5.299169032 | 8.30294E-06 | 0.547053833 | 0.017438259 | 6.06202778 | 1.69889E-07 | Homo sapiens centromere protein O (CENPO), mRNA [NM_024322] |
| CENPP | chr9:92455060-92455119 | 2.88776894 | 0.000365082 | 2.467694847 | 0.005898249 | 0.854533343 | 0.581288593 | 3.776965241 | 3.8881E-05 | Homo sapiens centromere protein P (CENPP), mRNA [NM_001012267] |
| CENPQ | chr6:49564899-49564958 | 2.889926614 | 0.023577043 | 2.512399172 | 0.037472542 | 0.86936435 | 0.474572539 | 1.725225203 | 0.154183717 | Homo sapiens centromere protein Q (CENPQ), mRNA [NM_018132] |
| CENPT | chr16:66420143-66420084 | 1.022674924 | 0.788129804 | 1.175371187 | 0.304488725 | 1.149310655 | 0.321525946 | 1.824092922 | 0.017936524 | Homo sapiens centromere protein T (CENPT), mRNA [NM_025082] |
| CENPT | chr16:66421431-66421372 | 0.473186734 | 0.002043084 | 0.434525475 | 0.000711106 | 0.918295979 | 0.587202789 | 1.368268765 | 0.259911193 | Homo sapiens centromere protein T (CENPT), mRNA [NM_025082] |
| CENTA1 | chr7:711648-711589 | 1.861216881 | 0.100760409 | 2.566091142 | 0.022704163 | 1.378716885 | 0.239573316 | 2.914059777 | 0.021788012 | Homo sapiens centaurin, alpha 1 (CENTA1), mRNA [NM_006869] |
| CENTA2 | chr17:26309305-26309364 | 0.149897375 | 0.024326925 | 0.061964964 | 2.16546E-06 | 0.413382579 | 0.202638744 | 0.587337492 | 0.006963379 | Homo sapiens centaurin, alpha 2 (CENTA2), mRNA [NM_018404] |
| CENTA2 | chr17:26310276-26310335 | 0.4199129 | 0.249417153 | 0.066723052 | 6.24645E-06 | 0.158897361 | 0.039242308 | 0.58464795 | 0.065301568 | Homo sapiens centaurin, alpha 2 (CENTA2), mRNA [NM_018404] |
| CENTB1 | chr17:7191975-7192034 | 0.171301006 | 0.071517643 | 0.26397975 | 0.005963217 | 1.541028597 | 0.52189905 | 0.179123258 | 0.000434957 | Homo sapiens centaurin, beta 1 (CENTB1), mRNA [NM_014716] |
| CENTB2 | chr3:196477804-196477745 | 1.215686466 | 0.25098198 | 1.133828417 | 0.44386769 | 0.932665164 | 0.73061917 | 0.9186657 | 0.603597594 | Homo sapiens centaurin, beta 2 (CENTB2), mRNA [NM_012287] |
| CENTB2 | chr3:196480818-196480759 | 1.722939411 | 0.072313546 | 1.321469794 | 0.299997347 | 0.766985644 | 0.204891151 | 1.505341683 | 0.025996821 | Homo sapiens centaurin, beta 2 (CENTB2), mRNA [NM_012287] |
| CENTB5 | chr1:1269141-1268968 | 0.742843194 | 0.148353944 | 1.166081003 | 0.54117673 | 1.569753903 | 0.10821532 | 1.182467327 | 0.511673526 | Homo sapiens centaurin, beta 5 (CENTB5), mRNA [NM_030649] |
| CENTB5 | chr1:1269725-1269464 | 0.810471647 | 0.101784782 | 1.281318849 | 0.191679191 | 1.580954564 | 0.02038519 | 1.29718021 | 0.145506597 | Homo sapiens centaurin, beta 5 (CENTB5), mRNA [NM_030649] |
| CENTB5 | chr1:1275204-1275145 | 0.74540625 | 0.518379615 | 1.265706798 | 0.209817071 | 1.698009372 | 0.276853635 | 0.624373851 | 0.045615315 | Homo sapiens centaurin, beta 5 (CENTB5), mRNA [NM_030649] |
| CENTB5 | chr1:1277325-1275971 | 0.571072233 | 0.103770254 | 1.016373128 | 0.959436592 | 1.779762819 | 0.103733989 | 1.529327654 | 0.298978196 | Homo sapiens centaurin, beta 5 (CENTB5), mRNA [NM_030649] |
| CENTD1 | chr4:35890726-35890667 | 1.189865863 | 0.631663062 | 0.914683837 | 0.843478164 | 0.768728531 | 0.548536713 | 1.089223467 | 0.730138206 | Homo sapiens centaurin, delta 1 (CENTD1), transcript variant 1, mRNA [NM_015230] |
| CENTD2 | chr11:72073969-72073910 | 0.614070574 | 0.005408387 | 0.78701812 | 0.138614721 | 1.281641155 | 0.06546912 | 1.089927256 | 0.665474482 | Homo sapiens centaurin, delta 2 (CENTD2), transcript variant 1, mRNA [NM_139181] |
| CENTD2 | chr11:72074816-72074757 | 0.682167982 | 0.068049339 | 0.849583364 | 0.278080925 | 1.245416651 | 0.233612347 | 1.029541083 | 0.905644527 | Homo sapiens centaurin, delta 2 (CENTD2), transcript variant 1, mRNA [NM_139181] |
| CENTD3 | chr5:141013392-141013333 | 0.846143717 | 0.687687124 | 0.256973382 | 0.011218199 | 0.303699451 | 0.030830875 | 1.622820712 | 0.123428198 | Homo sapiens centaurin, delta 3 (CENTD3), mRNA [NM_022481] |
| CENTG2 | chr2:236815264-236815323 | 1.120771695 | 0.577704518 | 1.536917553 | 0.060203036 | 1.37130297 | 0.097805525 | 0.906022093 | 0.716329509 | Homo sapiens centaurin, gamma 2 (CENTG2), transcript variant 2, mRNA [NM_014914] |
| CENTG2 | chr2:236815985-236816043 | 0.878484028 | 0.448048591 | 1.023844397 | 0.897372888 | 1.165467287 | 0.221551269 | 1.214583463 | 0.39520102 | Homo sapiens centaurin, gamma 2 (CENTG2), transcript variant 1, mRNA [NM_001037131] |
| CENTG3 | chr7:150258174-150258233 | 0.647034429 | 0.019168303 | 0.56762896 | 0.003069559 | 0.877277829 | 0.311762193 | 0.628322942 | 0.116838387 | Homo sapiens centaurin, gamma 3 (CENTG3), transcript variant 2, mRNA [NM_001042535] |
| CENTG3 | chr7:150277356-150278121 | 1.247523599 | 0.330094239 | 1.686018723 | 0.050028366 | 1.351492449 | 0.253837315 | 1.170336749 | 0.506022938 | Homo sapiens centaurin, gamma 3 (CENTG3), transcript variant 1, mRNA [NM_031946] |
| CENTG3 | chr7:150279082-150279141 | 1.330930748 | 0.225453403 | 1.606891551 | 0.024721051 | 1.207344224 | 0.436969359 | 1.75815973 | 0.024665984 | Homo sapiens centaurin, gamma 3 (CENTG3), transcript variant 1, mRNA [NM_031946] |
| CEP110 | chr9:120938376-120940257 | 1.903514736 | 0.030865732 | 1.093647861 | 0.746872002 | 0.574541316 | 0.087998296 | 1.285331062 | 0.235830586 | Homo sapiens centrosomal protein 110kDa (CEP110), mRNA [NM_007018] |
| CEP110 | chr9:121015240-121015299 | 2.125447106 | 0.002437274 | 2.277011447 | 0.005969369 | 1.071309392 | 0.792661283 | 1.399054603 | 0.201796018 | Homo sapiens centrosomal protein 110kDa (CEP110), mRNA [NM_007018] |
| CEP135 | chr4:56740039-56740098 | 1.99879018 | 0.007840708 | 1.465343529 | 0.083959271 | 0.733115233 | 0.174405033 | 1.272069603 | 0.486322049 | Homo sapiens centrosomal protein 135kDa (CEP135), mRNA [NM_025009] |
| CEP152 | chr15:46818050-46817991 | 6.365304608 | 0.001010772 | 3.701976961 | 0.000342989 | 0.581586772 | 0.158150536 | 4.651734824 | 0.000154524 | Homo sapiens centrosomal protein 152kDa (CEP152), mRNA [NM_014985] |
| CEP152 | chr15:46835606-46835547 | 2.86387685 | 0.001660254 | 1.441871446 | 0.085179057 | 0.503468383 | 0.01677613 | 3.16611472 | 0.000112429 | Homo sapiens centrosomal protein 152kDa (CEP152), mRNA [NM_014985] |
| CEP152 | chr15:46876919-46876860 | 5.763911938 | 0.002469072 | 3.48895092 | 0.004439811 | 0.605309546 | 0.194190182 | 9.412200382 | 0.057854157 | Homo sapiens centrosomal protein 152kDa (CEP152), mRNA [NM_014985] |
| CEP164 | chr11:116787812-116788025 | 1.777902045 | 0.014046533 | 1.800247354 | 5.80363E-05 | 1.012568357 | 0.943495956 | 1.475793024 | 0.020343957 | Homo sapiens centrosomal protein 164kDa (CEP164), mRNA [NM_014956] |
| CEP164 | chr11:116788900-116788959 | 1.498076302 | 0.024992294 | 1.657991545 | 0.003670385 | 1.106747061 | 0.503469266 | 2.492997648 | 0.001292073 | Homo sapiens centrosomal protein 164kDa (CEP164), mRNA [NM_014956] |
| CEP170 | chr1:239615116-239615057 | 1.421950437 | 0.317851909 | 1.161020047 | 0.687824677 | 0.816498253 | 0.526860729 | 0.71943127 | 0.407049036 | Homo sapiens centrosomal protein 170kDa (CEP170), transcript variant alpha, mRNA [NM_014812] |
| CEP170 | chr1:239615751-239615693 | 1.238051333 | 0.482605789 | 1.440667912 | 0.222881189 | 1.163657656 | 0.530740462 | 0.922267283 | 0.861385217 | Homo sapiens centrosomal protein 170kDa (CEP170), transcript variant alpha, mRNA [NM_014812] |
| CEP170 | chr1:239615909-239615850 | 2.671694398 | 0.018583254 | 2.069706728 | 0.060888338 | 0.774679443 | 0.355862574 | 1.05375875 | 0.951032725 | Homo sapiens centrosomal protein 170kDa (CEP170), transcript variant alpha, mRNA [NM_014812] |
| CEP192 | chr18:13114695-13114754 | 2.388743046 | 0.008390604 | 1.985524549 | 0.002282799 | 0.831200556 | 0.446086642 | 1.84448137 | 0.021286845 | Homo sapiens centrosomal protein 192kDa (CEP192), transcript variant 2, mRNA [NM_018069] |
| CEP250 | chr20:33518300-33518590 | 1.644863155 | 0.039344698 | 1.832296492 | 0.002207924 | 1.113950718 | 0.635026044 | 2.367640393 | 0.00048323 | Homo sapiens centrosomal protein 250kDa (CEP250), transcript variant 1, mRNA [NM_007186] |
| CEP250 | chr20:33562995-33563054 | 1.388370642 | 0.051002882 | 1.424900352 | 0.058394494 | 1.026311209 | 0.881085598 | 3.035913597 | 0.000564547 | Homo sapiens centrosomal protein 250kDa (CEP250), transcript variant 1, mRNA [NM_007186] |
| CEP27 | chr15:40640846-40643251 | 4.880353597 | 6.12646E-06 | 3.564077959 | 3.7139E-06 | 0.730290928 | 0.021523266 | 2.216863674 | 3.22586E-06 | Homo sapiens centrosomal protein 27kDa (CEP27), mRNA [NM_018097] |
| CEP290 | chr12:86945362-86945303 | 2.482050252 | 0.001844159 | 1.719359227 | 0.005551278 | 0.692717331 | 0.077176961 | 1.560414439 | 0.022656252 | Homo sapiens centrosomal protein 290kDa (CEP290), mRNA [NM_025114] |
| CEP290 | chr12:86990086-86990027 | 2.13299736 | 0.046439019 | 1.240500022 | 0.228630892 | 0.581575976 | 0.114019801 | 1.161237974 | 0.40539539 | Homo sapiens centrosomal protein 290kDa (CEP290), mRNA [NM_025114] |
| CEP290 | chr12:87007554-87007495 | 1.775771373 | 0.014549972 | 1.146451569 | 0.305207578 | 0.645607642 | 0.03979151 | 1.335703384 | 0.15972627 | Homo sapiens centrosomal protein 290kDa (CEP290), mRNA [NM_025114] |
| CEP350 | chr1:176813814-176813873 | 0.602432222 | 0.031185402 | 0.750128266 | 0.121492348 | 1.245166242 | 0.180520516 | 0.793239701 | 0.36640114 | Homo sapiens centrosomal protein 350kDa (CEP350), mRNA [NM_014810] |
| CEP55 | chr10:95278744-95278803 | 15.24264981 | 5.10241E-06 | 9.267558577 | 0.000134993 | 0.608001803 | 0.250198277 | 9.489143833 | 4.0384E-09 | Homo sapiens centrosomal protein 55kDa (CEP55), mRNA [NM_018131] |
| CEP57 | chr11:95195182-95195241 | 3.754974244 | 0.002205218 | 3.483850989 | 0.002501611 | 0.927796241 | 0.735439741 | 0.966981407 | 0.907651468 | Homo sapiens centrosomal protein 57kDa, mRNA (cDNA clone MGC:47657 IMAGE:5415088), complete cds. [BC039711] |
| CEP57 | chr11:95204441-95204500 | 1.336414985 | 0.151671351 | 1.526372732 | 0.002837711 | 1.142139792 | 0.483345057 | 0.963759505 | 0.794560563 | Homo sapiens centrosomal protein 57kDa (CEP57), mRNA [NM_014679] |
| CEP63 | chr3:135708732-135708791 | 0.760254948 | 0.250754967 | 0.687965536 | 0.020752188 | 0.90491425 | 0.657505573 | 0.917784601 | 0.510706156 | Homo sapiens centrosomal protein 63kDa (CEP63), transcript variant 1, mRNA [NM_025180] |
| CEP63 | chr3:135751687-135751746 | 0.78571475 | 0.230862624 | 0.901251733 | 0.48501956 | 1.147046983 | 0.443665717 | 0.716637178 | 0.035471074 | Homo sapiens centrosomal protein 63kDa (CEP63), transcript variant 1, mRNA [NM_025180] |
| CEP68 | chr2:65211270-65211329 | 1.428281542 | 0.009524892 | 1.155090073 | 0.468734657 | 0.808727159 | 0.281613256 | 1.297912414 | 0.389265625 | Homo sapiens centrosomal protein 68kDa (CEP68), mRNA [NM_015147] |
| CEP70 | chr3:139702062-139702003 | 1.324438542 | 0.184212325 | 0.849397439 | 0.488493314 | 0.641326428 | 0.100758416 | 0.63599027 | 0.012980306 | Homo sapiens centrosomal protein 70kDa (CEP70), mRNA [NM_024491] |
| CEP70 | chr3:139771888-139738859 | 1.430083122 | 0.254485206 | 0.963065396 | 0.869853138 | 0.67343316 | 0.234549471 | 0.635790888 | 0.025818277 | Homo sapiens centrosomal protein 70kDa (CEP70), mRNA [NM_024491] |
| CEP72 | chr5:706323-706382 | 8.032716663 | 0.000101596 | 8.289461078 | 1.92135E-07 | 1.031962339 | 0.903396806 | 10.25139941 | 3.41397E-08 | Homo sapiens centrosomal protein 72kDa (CEP72), mRNA [NM_018140] |
| CEP76 | chr18:12664543-12663452 | 4.339251362 | 0.00224282 | 3.798982475 | 2.62339E-05 | 0.875492604 | 0.675830323 | 1.252748527 | 0.154761209 | Homo sapiens centrosomal protein 76kDa (CEP76), mRNA [NM_024899] |
| CEP78 | chr9:78115584-78115643 | 4.846245748 | 0.001425178 | 2.206902652 | 0.033235032 | 0.455383975 | 0.003817319 | 2.382707804 | 0.013801704 | Homo sapiens centrosomal protein 78kDa, mRNA (cDNA clone IMAGE:6194709), complete cds. [BC091515] |
| CETN1 | chr18:571466-571525 | n/a | n/a | n/a | n/a | n/a | n/a | 1.161456539 | n/a | Homo sapiens centrin, EF-hand protein, 1 (CETN1), mRNA [NM_004066] |
| CETN2 | chrX:151666808-151666749 | 0.501943496 | 0.008099263 | 1.119875238 | 0.58234511 | 2.231078295 | 0.000104316 | 1.049006507 | 0.776719386 | Homo sapiens centrin, EF-hand protein, 2 (CETN2), mRNA [NM_004344] |
| CETN3 | chr5:89730993-89730934 | 3.092503925 | 0.001160663 | 1.861321435 | 0.019098216 | 0.601881673 | 0.030064776 | 1.178904169 | 0.404238164 | Homo sapiens centrin, EF-hand protein, 3 (CDC31 homolog, yeast) (CETN3), mRNA [NM_004365] |
| CKAP1 | chr19:41308258-41308461 | 1.051873377 | 0.786514087 | 0.852142898 | 0.401057334 | 0.810119276 | 0.285933167 | 1.138465716 | 0.531417602 | Homo sapiens cytoskeleton associated protein 1 (CKAP1), mRNA [NM_001281] |
| CKAP2 | chr13:51933864-51933923 | 11.22581574 | 1.46171E-05 | 10.48840925 | 6.78574E-06 | 0.934311545 | 0.831813717 | 29.80248539 | n/a | Homo sapiens cytoskeleton associated protein 2 (CKAP2), mRNA [NM_018204] |
| CKAP2 | chr13:51948395-51948454 | 10.54431952 | 0.000178184 | 7.947936999 | 4.45488E-05 | 0.75376481 | 0.329337053 | 6.054208437 | 0.000299478 | Homo sapiens cytoskeleton associated protein 2 (CKAP2), mRNA [NM_018204] |
| CKAP2L | chr2:113212048-113211989 | 16.97870637 | 1.58193E-06 | 12.85249895 | 3.03004E-05 | 0.756977515 | 0.493586254 | 12.84382198 | 3.24863E-11 | Homo sapiens cytoskeleton associated protein 2-like (CKAP2L), mRNA [NM_152515] |
| CKAP4 | chr12:105134998-105134939 | 1.192424072 | 0.539105116 | 1.693828458 | 0.128720564 | 1.420491667 | 0.399268907 | 1.465653433 | 0.067533936 | Homo sapiens cytoskeleton-associated protein 4 (CKAP4), mRNA [NM_006825] |
| CKAP5 | chr11:46722100-46722041 | 2.263895189 | 1.42451E-05 | 2.165037942 | 3.07016E-06 | 0.956333116 | 0.678260735 | 2.619556866 | 3.08061E-05 | Homo sapiens cytoskeleton associated protein 5 (CKAP5), transcript variant 1, mRNA [NM_001008938] |
| CKAP5 | chr11:46728512-46728453 | 3.593201945 | 0.000428454 | 3.563956667 | 1.31413E-07 | 0.991860942 | 0.962482903 | 2.415876152 | 0.007136492 | Homo sapiens cytoskeleton associated protein 5 (CKAP5), transcript variant 1, mRNA [NM_001008938] |
| CNTROB | chr17:7791983-7792219 | 1.380090445 | 0.147794125 | 1.188513785 | 0.194224222 | 0.861185431 | 0.484869864 | 1.074462385 | 0.599837685 | Homo sapiens centrobin, centrosomal BRCA2 interacting protein (CNTROB), transcript variant 1, mRNA [NM_053051] |
| CNTROB | chr17:7793143-7793429 | 1.333007534 | 0.017272461 | 1.060273067 | 0.710207071 | 0.795399156 | 0.159160311 | 1.444629856 | 0.013689337 | Homo sapiens centrobin, centrosomal BRCA2 interacting protein (CNTROB), transcript variant 1, mRNA [NM_053051] |
| CSPP1 | chr8:68170424-68170483 | 1.85414209 | 9.72806E-05 | 1.250798595 | 0.041178529 | 0.674596948 | 0.000490814 | 1.822661654 | 3.73583E-06 | Homo sapiens centrosome and spindle pole associated protein 1 (CSPP1), mRNA [NM_024790] |
| CSPP1 | chr8:68206803-68206862 | 1.355004111 | 0.054170198 | 0.688085399 | 0.001863954 | 0.507810562 | 0.001200116 | 1.779930558 | 0.006905074 | Homo sapiens centrosome and spindle pole associated protein 1 (CSPP1), mRNA [NM_024790] |
| CSPP1 | chr8:68270186-68270245 | 2.714129926 | 0.00047888 | 1.768188301 | 0.008985281 | 0.651475187 | 0.009680708 | 2.553363272 | 0.000371821 | Homo sapiens centrosome and spindle pole associated protein 1 (CSPP1), mRNA [NM_024790] |
| CTGLF1 | chr10:45641249-45641190 | 1.714236368 | 0.125294337 | 1.107443101 | 0.7421465 | 0.646027072 | 0.171997553 | 1.113648586 | 0.731150986 | Homo sapiens centaurin, gamma-like family, member 1 (CTGLF1), mRNA [NM_133446] |
| CTGLF1 | chr10:45641325-45641266 | 1.35765382 | 0.237411476 | 1.11543456 | 0.594186436 | 0.821589822 | 0.327373296 | 1.558620468 | 0.156626762 | Homo sapiens centaurin, gamma-like family, member 1 (CTGLF1), mRNA [NM_133446] |
| CTGLF1 | chr10:45641778-45641719 | 1.251008149 | 0.1621321 | 1.021552801 | 0.87309074 | 0.81658365 | 0.123885274 | 1.468856065 | 0.113735649 | Homo sapiens centaurin, gamma-like family, member 1 (CTGLF1), mRNA [NM_133446] |
| CTGLF1 | chr10:45641831-45641772 | 1.141674539 | 0.44921962 | 0.922871213 | 0.631865769 | 0.808348773 | 0.137003791 | 1.574802573 | 0.132495227 | Homo sapiens centaurin, gamma-like family, member 1 (CTGLF1), mRNA [NM_133446] |
| CTGLF1 | chr10:45642218-45642159 | 1.190017741 | 0.345224548 | 1.156570148 | 0.381464493 | 0.971893198 | 0.8543206 | 1.498683585 | 0.284859853 | Homo sapiens centaurin, gamma-like family, member 1 (CTGLF1), mRNA [NM_133446] |
| CTGLF1 | chr10:45642232-45642173 | 1.174780573 | 0.312526032 | 1.2182892 | 0.204869199 | 1.037035535 | 0.761944006 | 1.360121165 | 0.324195659 | Homo sapiens centaurin, gamma-like family, member 1 (CTGLF1), mRNA [NM_133446] |
| CTGLF1 | chr10:45662203-45662144 | 1.217208374 | 0.444876035 | 1.268216579 | 0.353921386 | 1.041905894 | 0.795047887 | 2.354166807 | 0.047087627 | Homo sapiens centaurin, gamma-like family, member 1 (CTGLF1), mRNA [NM_133446] |
| ESPL1 | chr12:51950737-51950796 | n/a | n/a | n/a | n/a | 0.841553567 | 0.842833898 | 1.852540929 | n/a | Homo sapiens extra spindle poles like 1 (S. cerevisiae) (ESPL1), mRNA [NM_012291] |
| ESPL1 | chr12:51973015-51973376 | 3.254837903 | 0.041239758 | 1.932918036 | 0.20586329 | 0.59386 | 0.075112613 | 4.05140709 | 0.082693087 | Homo sapiens extra spindle poles like 1 (S. cerevisiae) (ESPL1), mRNA [NM_012291] |
| GCET2 | chr3:113328551-113325307 | 2.389227262 | 0.294056971 | 0.551039068 | 0.307032374 | 0.230634849 | 0.133085603 | 0.161110006 | n/a | Homo sapiens germinal center expressed transcript 2 (GCET2), transcript variant 2, mRNA [NM_001008756] |
| INCENP | chr11:61676318-61676377 | 3.697943337 | 1.33517E-05 | 2.832056481 | 0.000569571 | 0.76584637 | 0.235018719 | 4.941445961 | 3.98785E-10 | Homo sapiens inner centromere protein antigens 135/155kDa (INCENP), transcript variant 2, mRNA [NM_020238] |
| LOC645904 | chr22:22127982-22128041 | 0.441940165 | 0.282159047 | 2.182286412 | 0.320371214 | 4.937968039 | 0.058693174 | 0.248154139 | 0.023823843 | PREDICTED: Homo sapiens similar to Mitotic spindle assembly checkpoint protein MAD1 (Mitotic arrest deficient-like protein 1) (MAD1-like 1) (Mitotic checkpoint MAD1 protein-homolog) (HsMAD1) (hMAD1) (Tax-binding protein 181) (LOC645904), mRNA [XM_928876] |
| NUSAP1 | chr15:39455237-39455296 | 10.73924377 | 2.41311E-06 | 6.846925166 | 5.84109E-05 | 0.637561202 | 0.181184705 | 6.165005977 | 1.39808E-08 | Homo sapiens nucleolar and spindle associated protein 1 (NUSAP1), transcript variant 1, mRNA [NM_016359] |
| PCNT | chr21:46607847-46607906 | 1.995536761 | 0.003603067 | 1.147115076 | 0.438068366 | 0.574840363 | 0.01131725 | 1.599365964 | 0.034052472 | Homo sapiens pericentrin (kendrin) (PCNT), mRNA [NM_006031] |
| PCNT | chr21:46689818-46689877 | 2.13151517 | 0.009587076 | 1.535881154 | 0.029989851 | 0.720558397 | 0.164662802 | 1.74927465 | 0.025662446 | Homo sapiens pericentrin (kendrin) (PCNT), mRNA [NM_006031] |
| PLK1 | chr16:23608471-23608713 | 3.265872419 | 0.005250644 | 3.208935199 | 0.00025727 | 0.982566 | 0.956385248 | 7.3375786 | 0.000767782 | Homo sapiens polo-like kinase 1 (Drosophila) (PLK1), mRNA [NM_005030] |
| PLK1 | chr16:23609129-23609188 | 4.787465478 | 0.000781001 | 4.08903615 | 0.000354447 | 0.854112927 | 0.630617439 | 7.443472158 | 1.32082E-10 | Homo sapiens polo-like kinase 1 (Drosophila) (PLK1), mRNA [NM_005030] |
| PLK2 | chr5:57785809-57785750 | 3.488306828 | 0.0244796 | 1.521062725 | 0.117594995 | 0.436046139 | 0.09118439 | 1.02254131 | 0.887469567 | Homo sapiens polo-like kinase 2 (Drosophila) (PLK2), mRNA [NM_006622] |
| PLK3 | chr1:44940687-44940747 | 2.435368737 | 0.109707642 | 1.201460306 | 0.558596682 | 0.49333815 | 0.195117292 | 1.620874084 | 0.069446961 | Homo sapiens polo-like kinase 3 (Drosophila) (PLK3), mRNA [NM_004073] |
| PLK4 | chr4:129164616-129164675 | n/a | n/a | n/a | n/a | 0.895450209 | 0.60614004 | 5.199779736 | n/a | Homo sapiens polo-like kinase 4 (Drosophila) (PLK4), mRNA [NM_014264] |
| PLK4 | chr4:129173787-129173846 | 11.32166634 | 1.91662E-05 | 8.767602137 | 3.42365E-05 | 0.774409161 | 0.478940104 | 15.54431987 | 0.046930589 | Homo sapiens polo-like kinase 4 (Drosophila) (PLK4), mRNA [NM_014264] |
| SASS6 | chr1:100261677-100261618 | 3.04994683 | 0.001368359 | 2.739787511 | 0.002069993 | 0.898306647 | 0.609683684 | 3.516256585 | 0.014796998 | Homo sapiens spindle assembly 6 homolog (C. elegans) (SASS6), mRNA [NM_194292] |
| SERF1B | chr5:69356972-69357031 | 1.589652955 | 0.029834219 | 1.239638287 | 0.352283979 | 0.77981693 | 0.241576165 | 1.547922151 | 0.076344482 | Homo sapiens small EDRK-rich factor 1B (centromeric) (SERF1B), mRNA [NM_022978] |
| SERF1B | chr5:69374401-69374460 | 1.023819803 | 0.875568098 | 1.236393913 | 0.280545039 | 1.207628441 | 0.318605991 | 1.296395013 | 0.212592082 | Homo sapiens small EDRK-rich factor 1B (centromeric) (SERF1B), mRNA [NM_022978] |
| SFI1 | chr22:30337566-30338661 | 1.145225859 | 0.606425131 | 0.765941736 | 0.25362742 | 0.66881282 | 0.062296319 | 1.616553871 | 0.182497052 | Homo sapiens Sfi1 homolog, spindle assembly associated (yeast) (SFI1), transcript variant 1, mRNA [NM_001007467] |
| SFI1 | chr22:30338984-30339043 | 0.969564121 | 0.927280494 | 0.667412121 | 0.167391802 | 0.688363056 | 0.179544473 | 1.708216391 | 0.109655104 | Homo sapiens Sfi1 homolog, spindle assembly associated (yeast) (SFI1), transcript variant 1, mRNA [NM_001007467] |
| SMN2 | chr5:69398703-69398921 | 2.137039469 | 0.030982119 | 1.90124909 | 0.023116142 | 0.88966494 | 0.699783579 | 0.783099838 | 0.422866214 | Homo sapiens survival of motor neuron 2, centromeric (SMN2), transcript variant c, mRNA [NM_022877] |
| SPBC24 | chr19:11118053-11117994 | 2.986090864 | 0.000543131 | 2.931432497 | 0.00578461 | 0.981695678 | 0.951721418 | 5.353491369 | 0.000107404 | Homo sapiens spindle pole body component 24 homolog (S. cerevisiae) (SPBC24), mRNA [NM_182513] |
| SPBC25 | chr2:169553522-169553463 | 16.74630849 | 7.27785E-06 | 9.383383693 | 0.000302853 | 0.560325501 | 0.227108378 | 11.85878736 | 3.16569E-07 | Homo sapiens spindle pole body component 25 homolog (S. cerevisiae) (SPBC25), mRNA [NM_020675] |
| SYCE1 | chr10:135259492-135259189 | 1.184337701 | 0.068071939 | 1.286719934 | n/a | 1.086446824 | n/a | 0.199583466 | 0.10467662 | Homo sapiens synaptonemal complex central element protein 1 (SYCE1), transcript variant 2, mRNA [NM_201564] |
| THC2343415 | chr4:124009351-124009292 | 2.143457766 | 0.043669847 | 1.49395673 | 0.206816648 | 0.696984449 | 0.332591864 | 0.574745619 | n/a | Q5SUE1 (Q5SUE1) Centrin 4, partial (52%) [THC2343415] |
| 76P | chr15:41483980-41484039 | 5.950048853 | 0.000136909 | 4.643172054 | 0.000223346 | 0.780358644 | 0.147011559 | 1.881091083 | 0.003462753 | Homo sapiens gamma tubulin ring complex protein (76p gene) (76P), mRNA [NM_014444] |
| AA780798 | chr6:030799960-030799901 | 1.978180771 | 0.002137747 | 1.591348 | 0.039743857 | 0.804450242 | 0.302976729 | 1.600971506 | 0.036356944 | ag14d07.s1 Gessler Wilms tumor Homo sapiens cDNA clone IMAGE:1070317 3' similar to gb:J00314_rna2 TUBULIN BETA-1 CHAIN (HUMAN);, mRNA sequence [AA780798] |
| AI028577 | chr2:132074237-132074178 | 0.679517636 | 0.181584774 | 1.115795872 | 0.570128513 | 1.642041079 | 0.100541347 | 0.854957638 | 0.834643704 | AI028577 ow01h11.x1 Soares_testis_NHT Homo sapiens cDNA clone IMAGE:1645605 3' similar to gb:K00558 TUBULIN ALPHA-1 CHAIN (HUMAN);, mRNA sequence [AI028577] |
| AI608782 | chr12:47808298-47808357 | 1.255935486 | 0.237944754 | 1.18596674 | 0.435074234 | 0.944289538 | 0.806340361 | 1.459355627 | 0.130521468 | AI608782 tw94g05.x1 NCI_CGAP_HN6 Homo sapiens cDNA clone IMAGE:2267384 3' similar to gb:K00558 TUBULIN ALPHA-1 CHAIN (HUMAN);, mRNA sequence [AI608782] |
| AI911586 | chr10:104492950-104492891 | n/a | n/a | n/a | n/a | 0.720982417 | 0.283031701 | 18.86056627 | n/a | ty73h01.x1 NCI_CGAP_Kid11 Homo sapiens cDNA clone IMAGE:2284753 3' similar to SW:TTL_PIG P38160 TUBULIN--TYROSINE LIGASE ;, mRNA sequence [AI911586] |
| AW168145 | chr16:088529508-088529449 | 1.671060959 | 0.017452552 | 1.342976619 | 0.181410468 | 0.803667042 | 0.205957476 | 1.333594604 | 0.083987165 | xg60e01.x1 NCI_CGAP_Ut4 Homo sapiens cDNA clone IMAGE:2632728 3' similar to gb:X00734_cds1 TUBULIN BETA-5 CHAIN (HUMAN);, mRNA sequence [AW168145] |
| BC014971 | chr16:88690155-88690214 | 2.789518804 | 0.093886023 | 1.070134041 | 0.908832914 | 0.383626753 | 0.171254557 | 1.767978642 | 0.192001611 | Homo sapiens, Similar to tubulin, beta, 2, clone IMAGE:4873024, mRNA. [BC014971] |
| H2-ALPHA | chr2:132071974-132072033 | 0.969319995 | 0.888268077 | 1.088894578 | 0.72631665 | 1.123359245 | 0.584331437 | 0.854448924 | 0.495356593 | Homo sapiens alpha-tubulin isotype H2-alpha (H2-ALPHA), mRNA [NM_080386] |
| H2-ALPHA | chr2:132074162-132074221 | 1.105076099 | 0.604403333 | 0.963805346 | 0.83609939 | 0.872161969 | 0.250552949 | 0.734974792 | 0.104123911 | Homo sapiens alpha-tubulin isotype H2-alpha (H2-ALPHA), mRNA [NM_080386] |
| K-ALPHA-1 | chr12:47807959-47807900 | 0.857443642 | 0.560459713 | 1.185047241 | 0.604422153 | 1.382070125 | 0.325594025 | 1.174717108 | 0.580060146 | Homo sapiens alpha tubulin (K-ALPHA-1), mRNA [NM_006082] |
| MGC16703 | chr22:19688101-19688042 | 1.189996746 | 0.569120291 | 1.255005399 | 0.498103922 | 1.054629269 | 0.831504271 | 0.356314526 | 0.004960059 | Homo sapiens alpha tubulin-like (MGC16703), mRNA [NM_145042] |
| TBCA | chr5:77023014-77022955 | 1.628337401 | 0.015750817 | 1.207337195 | 0.333723591 | 0.741453948 | 0.188358639 | 0.971893895 | 0.869550385 | Homo sapiens tubulin-specific chaperone a (TBCA), mRNA [NM_004607] |
| TBCC | chr6:42820720-42820661 | 0.995875905 | 0.985422176 | 1.589501306 | 0.126759341 | 1.596083707 | 0.084494378 | 1.176170582 | 0.527720869 | Homo sapiens tubulin-specific chaperone c (TBCC), mRNA [NM_003192] |
| TBCC | chr6:42820889-42820830 | 1.003613754 | 0.984482864 | 1.67612549 | 0.063421457 | 1.670090195 | 0.031748746 | 1.041677783 | 0.878930352 | Homo sapiens tubulin-specific chaperone c (TBCC), mRNA [NM_003192] |
| TBCD | chr17:78477601-78478354 | 1.488387681 | 0.214609459 | 1.723997872 | 0.019360207 | 1.158298939 | 0.627420613 | 1.226622933 | 0.495345392 | Homo sapiens tubulin-specific chaperone d (TBCD), transcript variant 1, mRNA [NM_005993] |
| TBCD | chr17:78488452-78488511 | 1.399249127 | 0.175479398 | 1.213985031 | 0.173144039 | 0.86759749 | 0.56657747 | 1.748825524 | 0.007044583 | Homo sapiens tubulin-specific chaperone d (TBCD), transcript variant 1, mRNA [NM_005993] |
| TBCD | chr17:78493800-78493859 | 1.19735066 | 0.409177942 | 1.044632883 | 0.775196054 | 0.872453591 | 0.565410914 | 2.334565662 | 0.001132949 | Homo sapiens tubulin-specific chaperone d (TBCD), transcript variant 1, mRNA [NM_005993] |
| TBCE | chr1:231938185-231938244 | 1.70187682 | 0.008802425 | 1.923716165 | 0.000232169 | 1.130349825 | 0.468937133 | 1.674976767 | 0.003741768 | Homo sapiens tubulin-specific chaperone e (TBCE), mRNA [NM_003193] |
| THC2307581 | chr6:003169718-003169777 | 1.952910606 | 0.62434615 | 3.352015778 | 0.135931051 | 1.71642049 | 0.707633847 | 0.045570054 | 0.033095821 | B25437 tubulin beta-2 chain - mouse (fragment) {Mus musculus;}, partial (18%) [THC2307581] |
| THC2307600 | chr6:40075674-40075734 | 0.840555216 | 0.329703812 | 0.833605185 | 0.406911044 | 0.991731619 | 0.96364418 | 0.920517101 | 0.798113018 | Q9JJY6 (Q9JJY6) Beta-tubulin (Fragment), partial (46%) [THC2307600] |
| TTBK2 | chr15:40825647-40825588 | 0.775213323 | 0.265200427 | 1.243886247 | 0.349996464 | 1.604572844 | 0.024574108 | 0.710953596 | 0.31385279 | Homo sapiens tau tubulin kinase 2 (TTBK2), mRNA [NM_173500] |
| TTBK2 | chr15:40856649-40856590 | 2.339489963 | 0.047526696 | 2.276855802 | 0.044370793 | 0.973227429 | 0.935856907 | 0.884497395 | 0.622843256 | Homo sapiens tau tubulin kinase 2 (TTBK2), mRNA [NM_173500] |
| TTL | chr2:112968040-112968099 | 6.663981858 | 0.000240118 | 3.010395864 | 0.002774944 | 0.451741305 | 0.003176487 | 1.511421614 | 0.1010284 | Homo sapiens tubulin tyrosine ligase (TTL), mRNA [NM_153712] |
| TTL | chr2:112976906-112976965 | 14.4020585 | 2.891E-05 | 5.296386338 | 4.9465E-05 | 0.367752036 | 0.010429262 | 3.343359036 | 0.239889706 | Homo sapiens tubulin tyrosine ligase (TTL), mRNA [NM_153712] |
| TTL | chr2:113002674-113002733 | 5.408645901 | 0.000358103 | 2.956077359 | 0.005525021 | 0.546546661 | 0.044370259 | 1.848052212 | 0.001789355 | Homo sapiens tubulin tyrosine ligase (TTL), mRNA [NM_153712] |
| TTLL1 | chr22:41772389-41772330 | 2.455246928 | 0.004617839 | 1.608578367 | 0.082121147 | 0.655159507 | 0.044975404 | 1.001655349 | 0.995238055 | Homo sapiens tubulin tyrosine ligase-like family, member 1 (TTLL1), transcript variant 2, mRNA [NM_001008572] |
| TTLL10 | chr1:1160312-1160371 | n/a | n/a | n/a | n/a | 1.326906974 | n/a | 2.978726245 | n/a | Homo sapiens tubulin tyrosine ligase-like family, member 10 (TTLL10), mRNA [NM_153254] |
| TTLL11 | chr9:121831419-121831360 | 0.698405365 | 0.182417357 | 0.630991229 | 0.048646634 | 0.903474202 | 0.729798999 | 0.647220855 | 0.120318237 | Homo sapiens tubulin tyrosine ligase-like family, member 11 (TTLL11), mRNA [NM_194252] |
| TTLL12 | chr22:41887399-41887340 | 2.138427795 | 0.0014636 | 1.858673953 | 0.00429454 | 0.869177794 | 0.386698421 | 2.855889328 | 0.000450266 | Homo sapiens tubulin tyrosine ligase-like family, member 12 (TTLL12), mRNA [NM_015140] |
| TTLL2 | chr6:167725671-167725730 | n/a | n/a | n/a | n/a | n/a | n/a | 1.628620287 | n/a | Homo sapiens tubulin tyrosine ligase-like family, member 2 (TTLL2), mRNA [NM_031949] |
| TTLL3 | chr3:9843876-9843935 | 0.344169157 | 0.001825435 | 0.292954647 | 0.000801308 | 0.851193782 | 0.304955949 | 0.485375458 | 0.008726558 | Homo sapiens tubulin tyrosine ligase-like family, member 3, mRNA (cDNA clone IMAGE:3841498), complete cds. [BC009479] |
| TTLL3 | chr3:9851825-9851884 | 1.065101337 | 0.676523871 | 0.774999999 | 0.101099692 | 0.727630294 | 0.071154571 | 2.437055254 | 0.000714055 | Homo sapiens tubulin tyrosine ligase-like family, member 3 (TTLL3), transcript variant 2, mRNA [NM_015644] |
| TTLL3 | chr3:9852427-9852486 | 0.731172239 | 0.19043441 | 0.805874282 | 0.309120618 | 1.102167505 | 0.638033707 | 2.514457619 | 0.006736965 | Homo sapiens tubulin tyrosine ligase-like family, member 3 (TTLL3), transcript variant 2, mRNA [NM_015644] |
| TTLL4 | chr2:219443837-219443896 | 2.550471097 | 0.000415031 | 3.571844465 | 2.43491E-05 | 1.400464592 | 0.009065142 | 3.094473776 | 0.006425222 | Homo sapiens tubulin tyrosine ligase-like family, member 4 (TTLL4), mRNA [NM_014640] |
| TTLL5 | chr14:75399792-75399851 | 1.155370394 | 0.596550761 | 1.824202391 | 0.001201405 | 1.57888968 | 0.139034148 | 2.380077506 | 9.96478E-08 | Homo sapiens tubulin tyrosine ligase-like family, member 5 (TTLL5), mRNA [NM_015072] |
| TTLL6 | chr17:44195237-44195178 | 0.43520045 | 0.150939071 | 0.827230613 | 0.77291541 | 1.90080367 | 0.235451172 | 0.342220962 | 0.299756463 | Homo sapiens tubulin tyrosine ligase-like family, member 6 (TTLL6), mRNA [NM_173623] |
| TTLL6 | chr17:44217418-44217359 | 0.608140153 | n/a | 0.746786542 | n/a | 1.22798427 | n/a | 0.611001865 | 0.653278394 | Homo sapiens tubulin tyrosine ligase-like family, member 6 (TTLL6), mRNA [NM_173623] |
| TTLL7 | chr1:84068058-84060815 | 1.648672303 | 0.354687912 | 0.519839499 | 0.288668436 | 0.315307959 | 0.112306095 | 0.080480022 | 3.35687E-05 | Homo sapiens tubulin tyrosine ligase-like family, member 7 (TTLL7), mRNA [NM_024686] |
| TTLL7 | chr1:84081836-84068180 | 1.310065691 | 0.611118581 | 0.964126748 | 0.964425663 | 0.735937712 | 0.727708411 | 0.027896848 | 3.02846E-07 | Homo sapiens tubulin tyrosine ligase-like family, member 7 (TTLL7), mRNA [NM_024686] |
| TUBA1 | chr2:219940634-219940575 | 0.572675993 | 0.087416476 | 1.104193813 | 0.805321055 | 1.928130087 | 0.164332075 | 2.126422654 | 0.008900223 | Homo sapiens tubulin, alpha 1 (testis specific) (TUBA1), mRNA [NM_006000] |
| TUBA1 | chr2:219940711-219940652 | 0.687745013 | 0.202200336 | 1.269904259 | 0.540851238 | 1.846475417 | 0.183063653 | 1.951896463 | 0.017287701 | Homo sapiens tubulin, alpha 1 (testis specific) (TUBA1), mRNA [NM_006000] |
| TUBA1 | chr2:219940759-219940700 | 0.750639634 | 0.102679812 | 1.081207133 | 0.734460844 | 1.440381088 | 0.137420149 | 1.152073381 | 0.54717231 | Homo sapiens tubulin, alpha 1 (testis specific) (TUBA1), mRNA [NM_006000] |
| TUBA1 | chr2:219941227-219941168 | 0.849512285 | 0.379802777 | 1.298166666 | 0.33820833 | 1.528131717 | 0.162818869 | 1.760373391 | 0.020135594 | Homo sapiens tubulin, alpha 1 (testis specific) (TUBA1), mRNA [NM_006000] |
| TUBA2 | chr13:18649197-18649138 | 1.119600211 | 0.626472071 | 1.816294981 | 0.047888997 | 1.622271024 | 0.162113969 | 2.514239847 | 0.010244898 | Homo sapiens tubulin, alpha 2 (TUBA2), transcript variant 2, mRNA [NM_079836] |
| TUBA3 | chr12:47865739-47865680 | 0.939133616 | 0.821975246 | 1.160677517 | 0.585287238 | 1.235902428 | 0.460677371 | 0.845469497 | 0.506095413 | Homo sapiens tubulin, alpha 3 (TUBA3), mRNA [NM_006009] |
| TUBA6 | chr12:47949701-47949900 | 1.13867431 | 0.670390308 | 1.458724134 | 0.175768655 | 1.281072314 | 0.425448758 | 0.896282829 | 0.679542622 | Homo sapiens tubulin, alpha 6 (TUBA6), mRNA [NM_032704] |
| TUBA6 | chr12:47952552-47952611 | 1.439720094 | 0.184643278 | 1.671964313 | 0.051572976 | 1.161312063 | 0.593785586 | 1.146649828 | 0.511143061 | Homo sapiens tubulin, alpha 6 (TUBA6), mRNA [NM_032704] |
| TUBA6 | chr12:47953065-47953124 | 1.096186884 | 0.659628265 | 1.364777204 | 0.187923114 | 1.245022381 | 0.426930697 | 1.263565105 | 0.298454674 | Homo sapiens tubulin, alpha 6 (TUBA6), mRNA [NM_032704] |
| TUBA6 | chr12:47953297-47953356 | 2.519503536 | 0.004459479 | 2.463328773 | 0.00149327 | 0.977704035 | 0.931823772 | 2.773231793 | 0.000728057 | Homo sapiens tubulin, alpha 6 (TUBA6), mRNA [NM_032704] |
| TUBA8 | chr22:16988374-16988433 | 1.004108425 | 0.989047759 | 0.953525457 | 0.80109154 | 0.949623998 | 0.856962984 | 1.167233958 | 0.393680863 | Homo sapiens tubulin, alpha 8 (TUBA8), mRNA [NM_018943] |
| TUBAL3 | chr10:5425268-5425209 | 0.204902439 | 0.255851845 | 0.994791897 | 0.996853074 | 4.854953908 | 0.030404166 | 1.092128748 | 0.947104434 | Homo sapiens tubulin, alpha-like 3 (TUBAL3), mRNA [NM_024803] |
| TUBB | chr6:30799847-30799906 | 1.584278885 | 0.00566468 | 1.372285719 | 0.184164808 | 0.866189489 | 0.517774048 | 1.524299646 | 0.04644169 | Homo sapiens tubulin, beta (TUBB), mRNA [NM_178014] |
| TUBB | chr6:30799884-30799943 | 1.492109899 | 0.079513857 | 1.244767024 | 0.453531673 | 0.834232804 | 0.493657273 | 1.234137979 | 0.376482678 | Homo sapiens tubulin, beta (TUBB), mRNA [NM_178014] |
| TUBB | chr6:30800501-30800560 | 1.584623411 | 0.107036108 | 1.554573084 | 0.12797646 | 0.981036297 | 0.947810448 | 1.767278199 | 0.074347725 | Homo sapiens tubulin, beta (TUBB), mRNA [NM_178014] |
| TUBB1 | chr20:57033076-57033135 | 1.283709237 | n/a | n/a | n/a | n/a | n/a | 0.560774764 | n/a | Homo sapiens tubulin, beta 1 (TUBB1), mRNA [NM_030773] |
| TUBB1 | chr20:57034639-57034698 | n/a | n/a | n/a | n/a | 0.623141934 | n/a | 1.150878305 | n/a | Homo sapiens tubulin, beta 1 (TUBB1), mRNA [NM_030773] |
| TUBB2A | chr6:3099093-3099034 | 0.515399738 | 0.128243869 | 0.223915793 | 0.001080418 | 0.434450731 | 0.106193622 | 0.225151386 | 0.000305294 | Homo sapiens tubulin, beta 2A (TUBB2A), mRNA [NM_001069] |
| TUBB2A | chr6:3099458-3099399 | 0.953497535 | 0.865848881 | 0.569640111 | 0.099851658 | 0.597421692 | 0.168494941 | 0.428657284 | 0.006462263 | Homo sapiens tubulin, beta 2A (TUBB2A), mRNA [NM_001069] |
| TUBB2B | chr6:3169809-3169750 | 0.275439519 | 0.163388644 | 0.286915192 | 0.040654721 | 1.041663132 | 0.964822468 | 0.019668205 | 1.90633E-07 | Homo sapiens tubulin, beta 2B (TUBB2B), mRNA [NM_178012] |
| TUBB2C | chr9:137413483-137413542 | 1.756253855 | 0.028564242 | 1.582950165 | 0.036152087 | 0.901321959 | 0.686052399 | 2.291435797 | 0.004324287 | Homo sapiens tubulin, beta 2C (TUBB2C), mRNA [NM_006088] |
| TUBB2C | chr9:137413938-137413996 | 1.254805703 | 0.383709045 | 1.297507044 | 0.227619348 | 1.034030241 | 0.901015552 | 2.08304547 | 0.014414019 | Homo sapiens tubulin, beta 2C (TUBB2C), mRNA [NM_006088] |
| TUBB3 | chr16:88529436-88529495 | 1.400627824 | 0.01905224 | 1.051885025 | 0.818812703 | 0.751009659 | 0.236299682 | 1.236941281 | 0.121543798 | Homo sapiens tubulin, beta 3 (TUBB3), mRNA [NM_006086] |
| TUBB3 | chr16:88529938-88529997 | 3.034430862 | 0.008413314 | 1.200762639 | 0.641393838 | 0.395712637 | 0.056793328 | 1.056767975 | 0.804078784 | Homo sapiens tubulin, beta 3 (TUBB3), mRNA [NM_006086] |
| TUBB4 | chr19:6445704-6445645 | 1.53070267 | 0.03149165 | 1.34635951 | 0.050495638 | 0.879569584 | 0.42965393 | 0.66604622 | 0.095386797 | Homo sapiens tubulin, beta 4 (TUBB4), mRNA [NM_006087] |
| TUBB4 | chr19:6446948-6446889 | 0.863357155 | 0.673930756 | 0.374835762 | 0.032436531 | 0.434160717 | 0.068039613 | 0.168094436 | 0.001095858 | Homo sapiens tubulin, beta 4 (TUBB4), mRNA [NM_006087] |
| TUBB6 | chr18:12315997-12316056 | 1.0407388 | 0.734339924 | 0.787111865 | 0.266446156 | 0.756301067 | 0.182486723 | 0.686841947 | 0.109123548 | Homo sapiens tubulin, beta 6 (TUBB6), mRNA [NM_032525] |
| TUBB6 | chr18:12316476-12316535 | 0.178899863 | 0.152612007 | 0.033417784 | 0.000983938 | 0.18679603 | 0.200910155 | 0.115261559 | 0.000208772 | Homo sapiens tubulin, beta 6 (TUBB6), mRNA [NM_032525] |
| TUBB8 | chr10:83404-83345 | 1.324871811 | 0.14689598 | 1.033948749 | 0.870229955 | 0.780414181 | 0.341973178 | 1.059355311 | 0.706490087 | Homo sapiens tubulin, beta 8 (TUBB8), mRNA [NM_177987] |
| TUBD1 | chr17:55292536-55292477 | 0.813032587 | 0.606588421 | 1.079556477 | 0.80028366 | 1.327814524 | 0.380245482 | 0.75772502 | 0.315577512 | Homo sapiens tubulin, delta 1 (TUBD1), mRNA [NM_016261] |
| TUBE1 | chr6:112502698-112502639 | 5.282486849 | 0.000264413 | 2.52173674 | 0.005352034 | 0.477376814 | 0.016883159 | 0.789883777 | 0.127356069 | Homo sapiens tubulin, epsilon 1 (TUBE1), mRNA [NM_016262] |
| TUBG1 | chr17:38018548-38018607 | 1.981144469 | 0.058338359 | 2.052718766 | 0.000182858 | 1.036127752 | 0.903060784 | 1.300489191 | 0.368611715 | Homo sapiens tubulin, gamma 1 (TUBG1), mRNA [NM_001070] |
| TUBG2 | chr17:38069000-38071031 | 1.677095651 | 0.074754047 | 1.651259413 | 0.000681646 | 0.984594654 | 0.948279178 | 1.161587557 | 0.597912576 | Homo sapiens tubulin, gamma 2 (TUBG2), mRNA [NM_016437] |
| TUBGCP2 | chr10:134986328-134986269 | 1.218542935 | 0.204636766 | 1.353312879 | 0.010136853 | 1.110599257 | 0.519396123 | 1.069323142 | 0.739996284 | Homo sapiens tubulin, gamma complex associated protein 2 (TUBGCP2), mRNA [NM_006659] |
| TUBGCP3 | chr13:112188148-112188089 | 1.873083313 | 0.011437334 | 1.775375299 | 0.023289891 | 0.947835735 | 0.835335934 | 2.393651106 | 0.002363706 | Homo sapiens tubulin, gamma complex associated protein 3 (TUBGCP3), mRNA [NM_006322] |
| TUBGCP3 | chr13:112188401-112188342 | 2.13005795 | 0.043459515 | 1.948168191 | 0.009516649 | 0.91460807 | 0.793708237 | 2.478424836 | 0.000169652 | Homo sapiens tubulin, gamma complex associated protein 3 (TUBGCP3), mRNA [NM_006322] |
| TUBGCP3 | chr13:112206955-112206434 | 4.316434459 | 0.002124934 | 4.104401538 | 1.76066E-06 | 0.950877762 | 0.859372307 | 1.843666311 | 0.048125256 | Homo sapiens tubulin, gamma complex associated protein 3 (TUBGCP3), mRNA [NM_006322] |
| TUBGCP5 | chr15:20420386-20421393 | 2.190443907 | 0.016181992 | 2.373349141 | 0.011563644 | 1.083501446 | 0.557521077 | 1.677043363 | 0.144830062 | Homo sapiens tubulin, gamma complex associated protein 5 (TUBGCP5), mRNA [NM_052903] |
| TUBGCP6 | chr22:48959229-48959170 | 1.094052854 | 0.361173466 | 0.717879194 | 0.02461539 | 0.656165003 | 0.003882231 | 1.220462294 | 0.199609257 | Homo sapiens tubulin, gamma complex associated protein 6 (TUBGCP6), transcript variant 2, mRNA [NM_001008658] |
Discussion
In an effort to understand the causal events leading to the development of aneuploidy in tumorigenesis, we have undertaken a comparative analysis of centrosomes and gene expression profiles in cultured normal foreskin fibriblasts, diploid and aneuploid tumor cell lines with and without multipolar mitoses. Most published reports of centrosome analysis in primary tumors and tumor cell lines address the number and morphology of these structures in interphase nuclei as visualized by immunocytochemistry with antibodies against γ-tubulin or PCNT. In addition to an examination of their presence and abundance in interphase nuclei, we also wanted to determine the manifestations of such aberrations in dividing cells and attempt to make a correlation between these two phases of the cell cycle (Figure 2). Our analysis included a categorization of interphase and metaphase cells into three distinct classes. Those we labeled as Type 2 and Type 3 corresponded to cells with an increase in centrosome number. According to Theodore Boveri, such cells might be expected to undergo aberrant chromosome segregation. This is in fact what we observed in the pancreatic cell lines, p53HCT116 and Trp53−/− MEFs. Whether the resulting daughter cells would be viable is an issue that can only truly be addressed by identifying the aberrant cells and following them through cell division.
The situation, however, was quite different in the CRC cancer cell lines. Defects in DNA mismatch repair pathway (MMR−/−) results in the accumulation of point mutations or small lesions throughout the genome, eventually occurring in tumor suppressor genes or oncogenes. Such genomic alteration abrogates the need for changes in chromosome copy number during tumorigenesis [Lengauer et al. 1998]. Consistent with this mechanism of mutagenesis, we found that these cells contain normal numbers of centrosomes, all of uniform size, shape and protein composition as determined by immunocytochemistry (ICC) against multiple centrosome-associated structural proteins and kinases. Their capacity to nucleate α-tubulin containing microtubules was similar to control fibroblast cells [Ghadimi et al. 2000] and each centrosome was found to contain two perpendicularly oriented centrioles.
The loss of TP53 function can induce true centrosome amplification in otherwise wildtype (Trp53−/− MEFSs) and MMR deficient cells (p53HCT116). The TP53 deficient diploid CRC cell line DLD1, which contains only 1–2 normal centrosomes, provides evidence that there may be other factors that can counteract this predisposition. Our observations in the p53HCT116 cell line suggest that although TP53 loss can induce the amplification of functional centrosomes and multipolar mitoses in MMR−/− cells, it did not result in an increase in chromosome instability (data not shown).
The aneuploid colorectal cancers contain an intact DNA mismatch repair pathway (MMR+), but generally have loss of TP53 function. As such, the accumulation of genomic alterations primarily occurs through their inability to correctly respond to certain types of DNA lesions. This includes a failure to arrest the cell cycle and to activate the repair pathways necessary for proper repair of the damaged DNA. Such cells are also capable of altering the number of intact oncogenes and tumor suppressor genes via the gain and loss of chromosomes or chromosomal regions [Eshleman et al. 1998]. One potential mechanism for altering chromosome copy number is interference with the chromosome segregation machinery. Consistent with this idea was the presence of multiple γ-tubulin structures in the aneuploid colorectal cells that presumably represented functional centrosomes. We have now demonstrated that these extra structures contain a number of microtubule organizing center (MTOC) proteins considered to be the hallmark of functional centrosomes (i.e., γ-tubulin and PCNT) [Doxsey et al. 1994; Moritz et al. 1995]. The co-localization pattern of these proteins, as well as the cell-cycle dependent co-localization and redistribution of two kinases known to be involved in centrosome segregation and migration (i.e., PLK1 and AURKA), were identical in aneuploid and diploid CRC cell lines and in normal cells.
Further examination, however, revealed that these supernumerary structures did not behave like centrosomes. The presence of multipolar mitosis in the Brca1−/− and Tp53−/− MEFs, p53HCT116 and pancreatic tumor cell lines, all of which contained multiple γ-tubulin structures, was in sharp contrast to observations in the aneuploid CRC cell lines. The inability of the structures in the latter lines to nucleate α-tubulin containing microtubules, as revealed in our nucleation assays, provides one explanation. This is somewhat surprising given evidence that γ-tubulin is responsible for the nucleation activity of centrosomes [Mogensen et al. 1997].
Our dual immunocytochemistry / EM analysis revealed the presence of only 1–2 pairs of centrioles in most of the aneuploid CRC cells despite the presence of multiple γ-tubulin structures. Even in SW837 where we observed multiple centrioles in an exceedingly low percentage of cells (Fig. 6I–L), we did not observe more than two clusters of centrioles per cell. Thus, there appears to be a clear correlation between nucleation ability and the presence of centrioles in the tumor cell lines analyzed here. This is in contrast to observations in early mouse development and certain Drosophila cell lines, where centrosomes stripped of their centrioles have been shown to have nucleation activity [Debec et al. 1995]. It is also possible that nucleation from these structures occurs but is severely impaired, hence the inability of it to be detected in our assay.
Perhaps modification of γ-tubulin is essential to its ability for nucleation [Khodjakov and Rieder 2001]. Our co-localization studies indicate that two proteins (PLK1 and AURKA) whose modification of γ-tubulin is critical for progression through the cell cycle are associated with, and therefore in a position to modify, γ-tubulin in these non-functional structures [Feng et al. 1999]. Our analysis here of CRC cell lines, as well as of primary colon tumors (data not shown), confirms the findings of previous studies demonstrating elevated levels of these proteins in primary human colorectal cancers [Bischoff et al. 1998; Takahashi et al. 2000]. While the protein concentrations and localization may be sufficient for the modification of γ-tubulin in the aneuploid cells, their enzymatic activity was not assessed. Analysis of Xenopus extracts has identified Xmap215 as a protein which is critical for the nucleation of mitotic spindles [Popov et al. 2002]. Perhaps a failure of the human orthologous protein (CKAP5), which we observed to be significantly increased at the transcriptional level, to localize to the supernumerary, centriole-lacking structures is responsible for their inability to nucleate spindle formation.
Further molecular interrogation of the differentially expressed genes identified through our analysis will be necessary to determine if they are in any way related to the functional capacity of γ-tubulin containing structures to nucleate mitotic spindles and form multipolar mitoses capable of causing chromosome mis-segregation. Overexpression of CSPP1, as occurs in the aneuploid CRC cell lines, has been shown to result in a cell cycle block at M and early G1 phases, with mitosis-arrested cells containing aberrant spindles [Patzke et al. 2005]. CETN2 localizes to spindles [Salisbury 1995] and more recently has been found associated with the nuclear pore complex [Resendes et al. 2008] were it regulates mRNA and protein export into the cytoplasm. It has also been demonstrated to stimulate the early stages of nucleotide excision repair [Nishi et al. 2005] through its interaction with xeroderma pigmentosum group C protein (XPC1) [Popescu et al. 2003]. One of the γ-tubulin complex proteins, TUBGCP6, was expressed at lower levels in the aneuploid CRC cell lines. Because of its association with the γ-tubulin complex, a decrease in expression might explain the failure of the supernumerary structures in the CRC aneuploid cell lines to nucleate microtubules [Murphy et al. 2001].
Our results suggest the potential of antibodies against γ-tubulin or PCNT alone to be misleading, particularly when drawing inferences about the functionality of these structures. We have identified three distinct categories of tumor cells based on their centrosome number and functionality. The first contain a normal complement of functional centrosomes. This is exemplified by the near diploid colorectal tumors. The second consists of cells with supernumerary centrosomes that have nucleating capacity, thereby resulting in active chromosome mis-segregation via multipolar mitoses. These would include the pancreatic tumor cell lines, Brca1 and Tp53-deficient MEFs, p53HCT116 and Brca1-deficient tumors [Weaver et al. 2002; Xu et al. 1999]. The degree of chromosomal instability in these cells may, however, depend on other factors such as the rate of mis-segregation and the fate of the daughter cells. The final series of cell lines, which includes the aneuploid colorectal cancer cell lines, have an abnormal distribution of centrosome-associated proteins, possibly through fragmentation of the pericentriolar matrix. Since the supernumerary structures were not capable of nucleating microtubules, and because multipolar mitoses were never observed, one might speculate that the consequence is an alteration of the functional capacity of "legitimate" centrosomes. As such, mis-segregation would result from a failure of sister chromatid separation rather than the active aberrant partitioning of chromosomes via attachment to the supernumerary centrosomes. Perhaps not surprisingly, these cell lines were no more karyotypically unstable than those cell lines containing a normal pair of centrosomes (data not shown).
In conclusion, the presence of γ-tubulin, pericentrin, and the modifying kinases PLK and AURKA are insufficient for the generation of a functional centrosome with the capacity to nucleate microtubules. Such supernumerary structures are therefore incapable of inducing the formation of multipolar mitoses, however we cannot exclude a possible role in chromosome mis-segregation through disruption of the normal bipolar spindle. The assessment of centrosome function with respect to chromosome segregation must therefore take into consideration the presence of centrioles and the capacity to nucleate microtubules.
Acknowledgements
The authors would like to thank Kunio Nagashima for help with the EM analysis, Cristina Montagna for her insightful suggestions, Andre Nussenzweig and Curtis Harris for generously providing cell lines, and David Spector for critical reading of the manuscript. Supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
References
- Alieva IB, Uzbekov RE. The centrosome is a polyfunctional multiprotein cell complex. Biochemistry (Mosc) 2008;73(6):626–643. doi: 10.1134/s0006297908060023. [DOI] [PubMed] [Google Scholar]
- Bischoff JR, Anderson L, Zhu Y, Mossie K, Ng L, Souza B, Schryver B, Flanagan P, Clairvoyant F, Ginther C, et al. A homologue of Drosophila aurora kinase is oncogenic and amplified in human colorectal cancers. Embo J. 1998;17(11):3052–3065. doi: 10.1093/emboj/17.11.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveri T. The origin of malignant tumors. Baltimore: Williams & Wilkins; 1929. [Google Scholar]
- Bowerman B. Cell division: timing the machine. Nature. 2004;430(7002):840–842. doi: 10.1038/430840a. [DOI] [PubMed] [Google Scholar]
- Brinkley BR. Managing the centrosome numbers game: from chaos to stability in cancer cell division. Trends Cell Biol. 2001;11(1):18–21. doi: 10.1016/s0962-8924(00)01872-9. [DOI] [PubMed] [Google Scholar]
- Brinkley BR, Goepfert TM. Supernumerary centrosomes and cancer: Boveri's hypothesis resurrected. Cell Motil Cytoskeleton. 1998;41(4):281–288. doi: 10.1002/(SICI)1097-0169(1998)41:4<281::AID-CM1>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown JP, Sedivy JM, Kinzler KW, Vogelstein B. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282(5393):1497–1501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- Camps J, Grade M, Nguyen QT, Hormann P, Becker S, Hummon AB, Rodriguez V, Chandrasekharappa S, Chen Y, Difilippantonio MJ, et al. Chromosomal breakpoints in primary colon cancer cluster at sites of structural variants in the genome. Cancer Res. 2008;68(5):1284–1295. doi: 10.1158/0008-5472.CAN-07-2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camps J, Nguyen QT, Padilla-Nash HM, Knutsen T, McNeil NE, Wangsa D, Hummon AB, Grade M, Ried T, Difilippantonio MJ. Integrative Genomics Reveals Mechanisms of Copy Number Alterations Responsible for Transcriptional Deregulation in Colorectal Cancer. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debec A, Detraves C, Montmory C, Geraud G, Wright M. Polar organization of gamma-tubulin in acentriolar mitotic spindles of Drosophila melanogaster cells. J Cell Sci. 1995;108(Pt 7):2645–2653. doi: 10.1242/jcs.108.7.2645. [DOI] [PubMed] [Google Scholar]
- Doxsey SJ, Stein P, Evans L, Calarco PD, Kirschner M. Pericentrin, a highly conserved centrosome protein involved in microtubule organization. Cell. 1994;76(4):639–650. doi: 10.1016/0092-8674(94)90504-5. [DOI] [PubMed] [Google Scholar]
- Dufva M. Introduction to microarray technology. Methods Mol Biol. 2009;529:1–22. doi: 10.1007/978-1-59745-538-1_1. [DOI] [PubMed] [Google Scholar]
- Erikson E, Haystead TA, Qian YW, Maller JL. A feedback loop in the polo-like kinase activation pathway. J Biol Chem. 2004;279(31):32219–32224. doi: 10.1074/jbc.M403840200. [DOI] [PubMed] [Google Scholar]
- Eshleman JR, Casey G, Kochera ME, Sedwick WD, Swinler SE, Veigl ML, Willson JK, Schwartz S, Markowitz SD. Chromosome number and structure both are markedly stable in RER colorectal cancers and are not destabilized by mutation of p53. Oncogene. 1998;17(6):719–725. doi: 10.1038/sj.onc.1201986. [DOI] [PubMed] [Google Scholar]
- Feng Y, Hodge DR, Palmieri G, Chase DL, Longo DL, Ferris DK. Association of polo-like kinase with alpha-, beta- and gamma-tubulins in a stable complex. Biochem J. 1999;339(Pt 2):435–442. [PMC free article] [PubMed] [Google Scholar]
- Fukasawa K, Choi T, Kuriyama R, Rulong S, Vande Woude GF. Abnormal centrosome amplification in the absence of p53. Science. 1996;271(5256):1744–1747. doi: 10.1126/science.271.5256.1744. [DOI] [PubMed] [Google Scholar]
- Ghadimi BM, Sackett DL, Difilippantonio MJ, Schrock E, Neumann T, Jauho A, Auer G, Ried T. Centrosome amplification and instability occurs exclusively in aneuploid, but not in diploid colorectal cancer cell lines, and correlates with numerical chromosomal aberrations. Genes Chromosomes Cancer. 2000;27(2):183–190. [PMC free article] [PubMed] [Google Scholar]
- Glover DM, Leibowitz MH, McLean DA, Parry H. Mutations in aurora prevent centrosome separation leading to the formation of monopolar spindles. Cell. 1995;81(1):95–105. doi: 10.1016/0092-8674(95)90374-7. [DOI] [PubMed] [Google Scholar]
- Gonda MA, Aaronson SA, Ellmore N, Zeve VH, Nagashima K. Ultrastructural studies of surface features of human normal and tumor cells in tissue culture by scanning and transmission electron microscopy. J Natl Cancer Inst. 1976;56(2):245–263. doi: 10.1093/jnci/56.2.245. [DOI] [PubMed] [Google Scholar]
- Hamanaka R, Smith MR, O'Connor PM, Maloid S, Mihalic K, Spivak JL, Longo DL, Ferris DK. Polo-like kinase is a cell cycle-regulated kinase activated during mitosis. J Biol Chem. 1995;270(36):21086–21091. doi: 10.1074/jbc.270.36.21086. [DOI] [PubMed] [Google Scholar]
- Hinchcliffe EH, Li C, Thompson EA, Maller JL, Sluder G. Requirement of Cdk2-cyclin E activity for repeated centrosome reproduction in Xenopus egg extracts [see comments] Science. 1999;283(5403):851–854. doi: 10.1126/science.283.5403.851. [DOI] [PubMed] [Google Scholar]
- Jordan A, Hadfield JA, Lawrence NJ, McGown AT. Tubulin as a target for anticancer drugs: agents which interact with the mitotic spindle. Med Res Rev. 1998;18(4):259–296. doi: 10.1002/(sici)1098-1128(199807)18:4<259::aid-med3>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Kallioniemi A, Kallioniemi OP, Sudar D, Rutovitz D, Gray JW, Waldman F, Pinkel D. Comparative genomic hybridization for molecular cytogenetic analysis of solid tumors. Science. 1992;258:818–821. doi: 10.1126/science.1359641. [DOI] [PubMed] [Google Scholar]
- Khodjakov A, Rieder CL. Centrosomes enhance the fidelity of cytokinesis in vertebrates and are required for cell cycle progression. J Cell Biol. 2001;153(1):237–242. doi: 10.1083/jcb.153.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane HA, Nigg EA. Antibody microinjection reveals an essential role for human polo-like kinase 1 (Plk1) in the functional maturation of mitotic centrosomes. Journal of Cell Biology. 1996;135:1701–1713. doi: 10.1083/jcb.135.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers. Nature. 1998;396(6712):643–649. doi: 10.1038/25292. [DOI] [PubMed] [Google Scholar]
- Lingle WL, Lutz WH, Ingle JN, Maihle NJ, Salisbury JL. Centrosome hypertrophy in human breast tumors: Implications for genomic stability and cell polarity. Proc.Natl.Acad.Sci.USA. 1998;95:2950–2955. doi: 10.1073/pnas.95.6.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen MM, Mackie JB, Doxsey SJ, Stearns T, Tucker JB. Centrosomal deployment of gamma-tubulin and pericentrin: evidence for a microtubule-nucleating domain and a minus-end docking domain in certain mouse epithelial cells. Cell Motil Cytoskeleton. 1997;36(3):276–290. doi: 10.1002/(SICI)1097-0169(1997)36:3<276::AID-CM8>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Moritz M, Braunfeld MB, Sedat JW, Alberts B, Agard DA. Microtubule nucleation by gamma-tubulin-containing rings in the centrosome. Nature. 1995;378(6557):638–640. doi: 10.1038/378638a0. [DOI] [PubMed] [Google Scholar]
- Murphy SM, Preble AM, Patel UK, O'Connell KL, Dias DP, Moritz M, Agard D, Stults JT, Stearns T. GCP5 and GCP6: two new members of the human gamma-tubulin complex. Mol Biol Cell. 2001;12(11):3340–3352. doi: 10.1091/mbc.12.11.3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neef R, Klein UR, Kopajtich R, Barr FA. Cooperation between mitotic kinesins controls the late stages of cytokinesis. Curr Biol. 2006;16(3):301–307. doi: 10.1016/j.cub.2005.12.030. [DOI] [PubMed] [Google Scholar]
- Nishi R, Okuda Y, Watanabe E, Mori T, Iwai S, Masutani C, Sugasawa K, Hanaoka F. Centrin 2 stimulates nucleotide excision repair by interacting with xeroderma pigmentosum group C protein. Mol Cell Biol. 2005;25(13):5664–5674. doi: 10.1128/MCB.25.13.5664-5674.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patzke S, Hauge H, Sioud M, Finne EF, Sivertsen EA, Delabie J, Stokke T, Aasheim HC. Identification of a novel centrosome/microtubule-associated coiled-coil protein involved in cell-cycle progression and spindle organization. Oncogene. 2005;24(7):1159–1173. doi: 10.1038/sj.onc.1208267. [DOI] [PubMed] [Google Scholar]
- Petronczki M, Glotzer M, Kraut N, Peters JM. Polo-like kinase 1 triggers the initiation of cytokinesis in human cells by promoting recruitment of the RhoGEF Ect2 to the central spindle. Dev Cell. 2007;12(5):713–725. doi: 10.1016/j.devcel.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Pettersson E, Lundeberg J, Ahmadian A. Generations of sequencing technologies. Genomics. 2009;93(2):105–111. doi: 10.1016/j.ygeno.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Pihan GA, Doxsey SJ. The mitotic machinery as a source of genetic instability in cancer. Semin Cancer Biol. 1999;9(4):289–302. doi: 10.1006/scbi.1999.0131. [DOI] [PubMed] [Google Scholar]
- Pihan GA, Purohit A, Wallace J, Knecht H, Woda B, Quesenberry P, Doxsey SJ. Centrosome defects and genetic instability in malignant tumors. Cancer Research. 1998;58:3974–3985. [PubMed] [Google Scholar]
- Popescu A, Miron S, Blouquit Y, Duchambon P, Christova P, Craescu CT. Xeroderma pigmentosum group C protein possesses a high affinity binding site to human centrin 2 and calmodulin. J Biol Chem. 2003;278(41):40252–40261. doi: 10.1074/jbc.M302546200. [DOI] [PubMed] [Google Scholar]
- Popov AV, Severin F, Karsenti E. XMAP215 is required for the microtubule-nucleating activity of centrosomes. Curr Biol. 2002;12(15):1326–1330. doi: 10.1016/s0960-9822(02)01033-3. [DOI] [PubMed] [Google Scholar]
- Qian YW, Erikson E, Li C, Maller JL. Activated polo-like kinase Plx1 is required at multiple points during mitosis in Xenopus laevis. Mol Cell Biol. 1998a;18(7):4262–4271. doi: 10.1128/mcb.18.7.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian YW, Erikson E, Maller JL. Purification and cloning of a protein kinase that phosphorylates and activates the polo-like kinase Plx1. Science. 1998b;282(5394):1701–1704. doi: 10.1126/science.282.5394.1701. [DOI] [PubMed] [Google Scholar]
- Resendes KK, Rasala BA, Forbes DJ. Centrin 2 localizes to the vertebrate nuclear pore and plays a role in mRNA and protein export. Mol Cell Biol. 2008;28(5):1755–1769. doi: 10.1128/MCB.01697-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ried T, Heselmeyer-Haddad K, Blegen H, Schrock E, Auer G. Genomic changes defining the genesis, progression, and malignancy potential in solid human tumors: a phenotype/genotype correlation. Genes Chromosom Cancer. 1999;25(3):195–204. doi: 10.1002/(sici)1098-2264(199907)25:3<195::aid-gcc1>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Rogers GC, Rogers SL, Schwimmer TA, Ems-McClung SC, Walczak CE, Vale RD, Scholey JM, Sharp DJ. Two mitotic kinesins cooperate to drive sister chromatid separation during anaphase. Nature. 2004;427(6972):364–370. doi: 10.1038/nature02256. [DOI] [PubMed] [Google Scholar]
- Salisbury JL. Centrin, centrosomes, and mitotic spindle poles. Curr Opin Cell Biol. 1995;7(1):39–45. doi: 10.1016/0955-0674(95)80043-3. [DOI] [PubMed] [Google Scholar]
- Sato N, Mizumoto K, Nakamura M, Maehara N, Minamishima YA, Nishio S, Nagai E, Tanaka M. Correlation between centrosome abnormalities and chromosomal instability in human pancreatic cancer cells. Cancer Genet Cytogenet. 2001;126(1):13–19. doi: 10.1016/s0165-4608(00)00384-8. [DOI] [PubMed] [Google Scholar]
- Schröck E, du Manoir S, Veldman T, Schoell B, Wienberg J, Ferguson-Smith MA, Ning Y, Ledbetter DH, Bar-Am I, Soenksen D, et al. Multicolor spectral karyotyping of human chromosomes. Science. 1996;273(5274):494–497. doi: 10.1126/science.273.5274.494. [DOI] [PubMed] [Google Scholar]
- Stearns T. Centrosome duplication. a centriolar pas de deux. Cell. 2001;105(4):417–420. doi: 10.1016/s0092-8674(01)00366-x. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Futamura M, Yoshimi N, Sano J, Katada M, Takagi Y, Kimura M, Yoshioka T, Okano Y, Saji S. Centrosomal kinases, HsAIRK1 and HsAIRK3, are overexpressed in primary colorectal cancers. Jpn J Cancer Res. 2000;91(10):1007–1014. doi: 10.1111/j.1349-7006.2000.tb00878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman T, Lengauer C, Kinzler KW, Vogelstein B. Uncoupling of S phase and mitosis induced by anticancer agents in cells lacking p21. Nature. 1996;381(6584):713–716. doi: 10.1038/381713a0. [DOI] [PubMed] [Google Scholar]
- Weaver Z, Montagna C, Xu X, Howard T, Gadina M, Brodie SG, Deng CX, Ried T. Mammary tumors in mice conditionally mutant for Brca1 exhibit gross genomic instability and centrosome amplification yet display a recurring distribution of genomic imbalances that is similar to human breast cancer. Oncogene. 2002;21(33):5097–5107. doi: 10.1038/sj.onc.1205636. [DOI] [PubMed] [Google Scholar]
- Xu X, Weaver Z, Linke SP, Li C, Gotay J, Wang XW, Harris CC, Ried T, Deng CX. Centrosome amplification and a defective G2-M cell cycle checkpoint induce genetic instability in BRCA1 exon 11 isoform-deficient cells. Mol Cell. 1999;3(3):389–395. doi: 10.1016/s1097-2765(00)80466-9. [DOI] [PubMed] [Google Scholar]
- Zhou H, Kuang J, Zhong L, Kuo W-L, Gray JW, Sahin A, Brinkely BR, Sen S. Tumor amplified kinase STK15/BTAK induces centrosome amplification, aneuploidy and transformation. Nature Genetics. 1998;20:189–193. doi: 10.1038/2496. [DOI] [PubMed] [Google Scholar]