Abstract
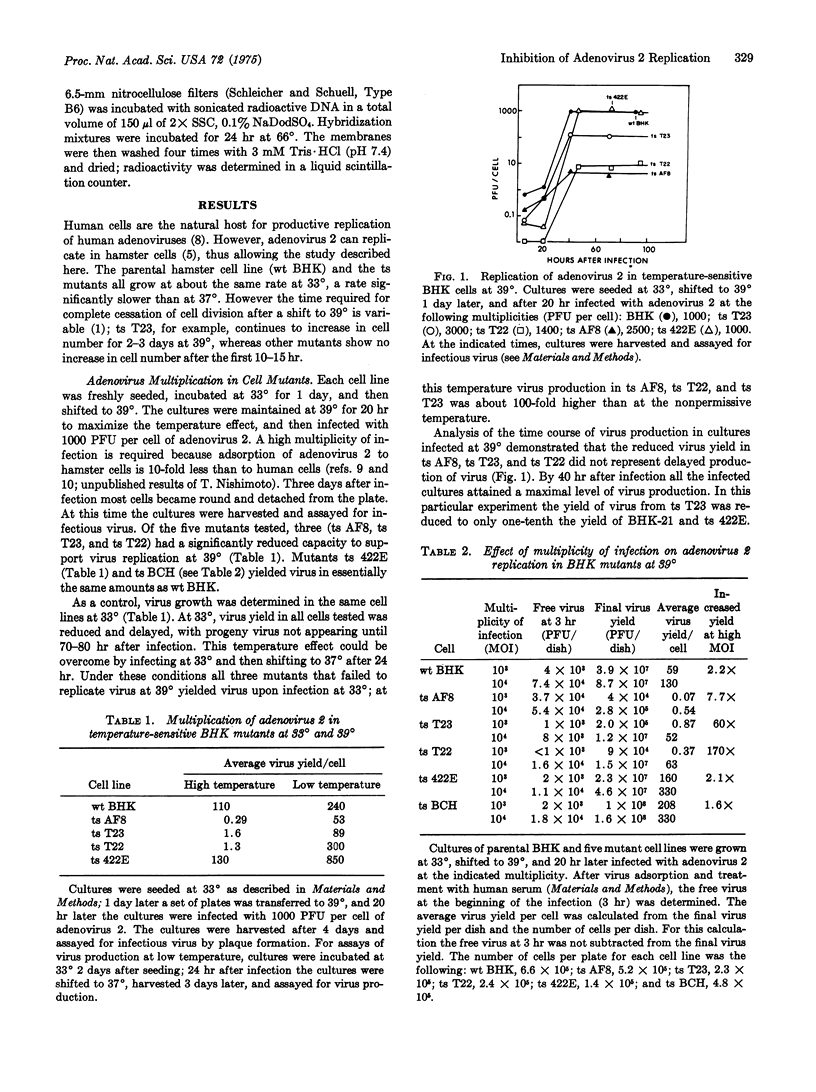
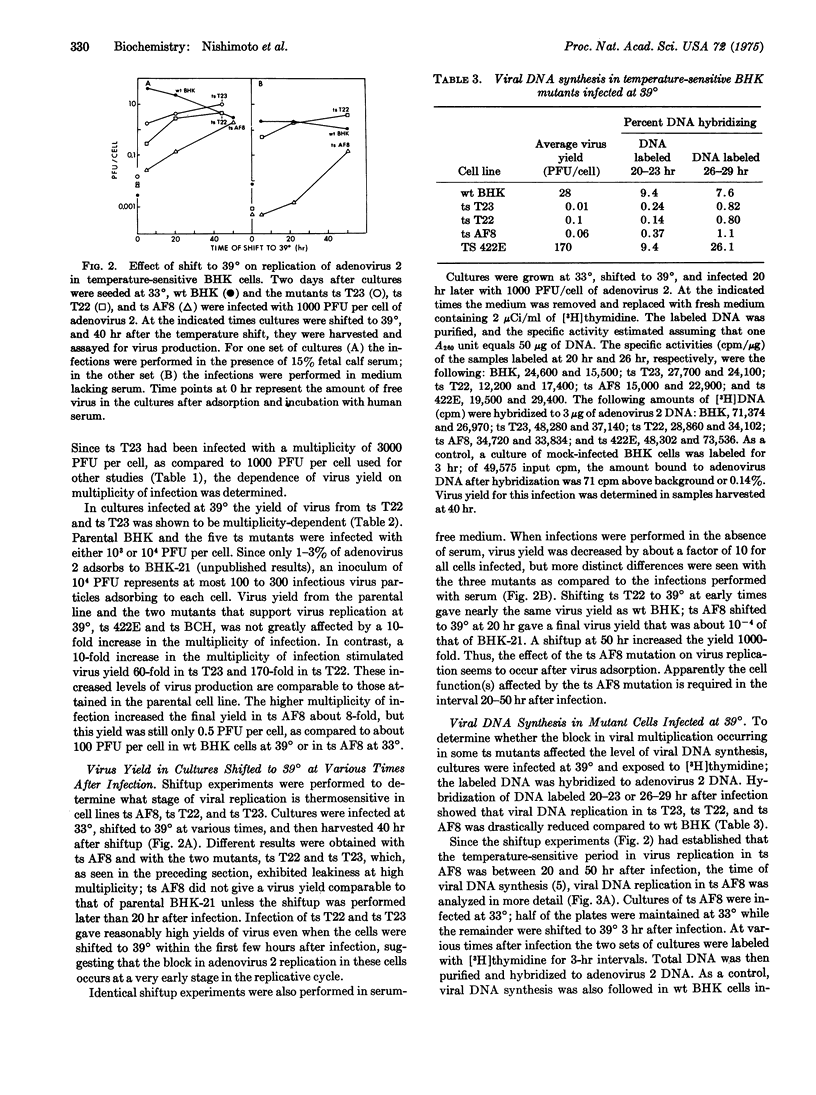
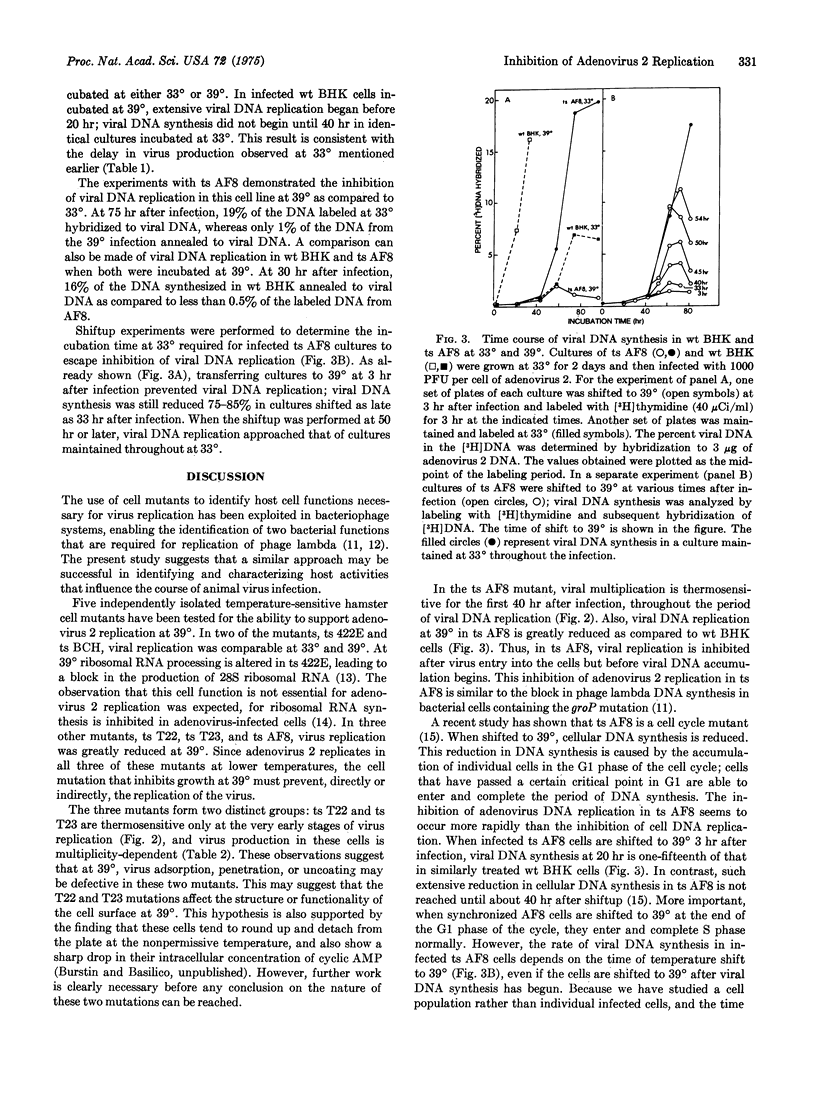
Five temperature-sensitive growth mutants of the hamster cell line BHK-21 were tested for the ability to support adenovirus 2 multiplication at 39 degrees and 33 degrees. Wild-type BHK-21 and mutants ts 422E and ts BCH yielded comparable amounts of virus at 33 degrees and 39 degrees, whereas in three other mutants, ts T22, ts T23, and ts AF8, virus production at 39 degrees was reduced to about 1% of that at 33 degrees. Virus yield in the three mutants was not reduced because of a delay in virus production; for all cells tested maximal virus yield at 39 degrees was obtained by 40-50 hr after infection. Normal yields of infectious virus were not obtained from ts AF8 even with a very high multiplicity of infection. In contrast, the virus yield from ts T22 and ts T23 was multiplicity-dependent. Shiftup experiments demonstrated that in ts AF8, a cell cycle mutant which at 39 degrees becomes arrested in G1, virus multiplication was thermosensitive for the first 40 hr of infection. In ts T22 and ts T23, the thermosensitivity was only for the first 3-4 hr of the infection. In all three mutants viral DNA synthesis was reduced by at least 95% at the higher temperature. The cell function specified by the ts AF8 mutation seems to be required for the early period of adenovirus 2 replication, after virus entry into the cell but before the onset of viral DNA replication.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burstin S. J., Meiss H. K., Basilico C. A temperature-sensitive cell cycle mutant of the BHK cell line. J Cell Physiol. 1974 Dec;84(3):397–408. doi: 10.1002/jcp.1040840308. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerfler W. Integration of the deoxyribonucleic acid of adenovirus type 12 into the deoxyribonucleic acid of baby hamster kidney cells. J Virol. 1970 Nov;6(5):652–666. doi: 10.1128/jvi.6.5.652-666.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerfler W. Nonproductive infection of baby hamster kidney cells (BHK21) with adenovirus type 12. Virology. 1969 Aug;38(4):587–606. doi: 10.1016/0042-6822(69)90179-2. [DOI] [PubMed] [Google Scholar]
- GREEN M., PINA M. BIOCHEMICAL STUDIES ON ADENOVIRUS MULTIPLICATION, VI. PROPERTIES OF HIGHLY PURIFIED TUMORIGENIC HUMAN ADENOVIRUSES AND THEIR DNA. Proc Natl Acad Sci U S A. 1964 Jun;51:1251–1259. doi: 10.1073/pnas.51.6.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN M., PINA M. Biochemical studies on adenovirus multiplication. IV. Isolation, purification, and chemical analysis of adenovirus. Virology. 1963 May;20:199–207. doi: 10.1016/0042-6822(63)90157-0. [DOI] [PubMed] [Google Scholar]
- Green M., Piña M., Kimes R. C. Biochemical studies on adenovirus multiplication. XII. Plaquing efficiencies of purified human adenoviruses. Virology. 1967 Mar;31(3):562–565. doi: 10.1016/0042-6822(67)90241-3. [DOI] [PubMed] [Google Scholar]
- Hodge L. D., Scharff M. D. Effect of adenovirus on host cell DNA synthesis in synchronized cells. Virology. 1969 Apr;37(4):554–564. doi: 10.1016/0042-6822(69)90273-6. [DOI] [PubMed] [Google Scholar]
- Meiss H. K., Basilico C. Temperature sensitive mutants of BHK 21 cells. Nat New Biol. 1972 Sep 20;239(90):66–68. doi: 10.1038/newbio239066a0. [DOI] [PubMed] [Google Scholar]
- Piña M., Green M. Biochemical studies on adenovirus multiplication. XIV. Macromolecule and enzyme synthesis in cells replicating oncogenic and nononcogenic human adenovirus. Virology. 1969 Aug;38(4):573–586. doi: 10.1016/0042-6822(69)90178-0. [DOI] [PubMed] [Google Scholar]
- Raskas H. J., Thomas D. C., Green M. Biochemical studies on adenovirus multiplication. XVII. Ribosome synthesis in uninfected and infected KB cells. Virology. 1970 Apr;40(4):893–902. doi: 10.1016/0042-6822(70)90135-2. [DOI] [PubMed] [Google Scholar]
- Rouse H. C., Strohl W. A., Schlesinger R. W. Properties of cells derived from adenovirus-induced hamster tumors by long-term in vitro cultivation. I. Clonal stability of three biological characteristics. Virology. 1966 Apr;28(4):633–644. doi: 10.1016/0042-6822(66)90248-0. [DOI] [PubMed] [Google Scholar]
- Schlesinger R. W. Adenoviruses: the nature of the virion and of controlling factors in productive or abortive infection and tumorigenesis. Adv Virus Res. 1969;14:1–61. doi: 10.1016/s0065-3527(08)60556-4. [DOI] [PubMed] [Google Scholar]
- Toniolo D., Meiss H. K., Basilico C. A temperature-sensitive mutation affecting 28S ribosomal RNA production in mammalian cells. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1273–1277. doi: 10.1073/pnas.70.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]


