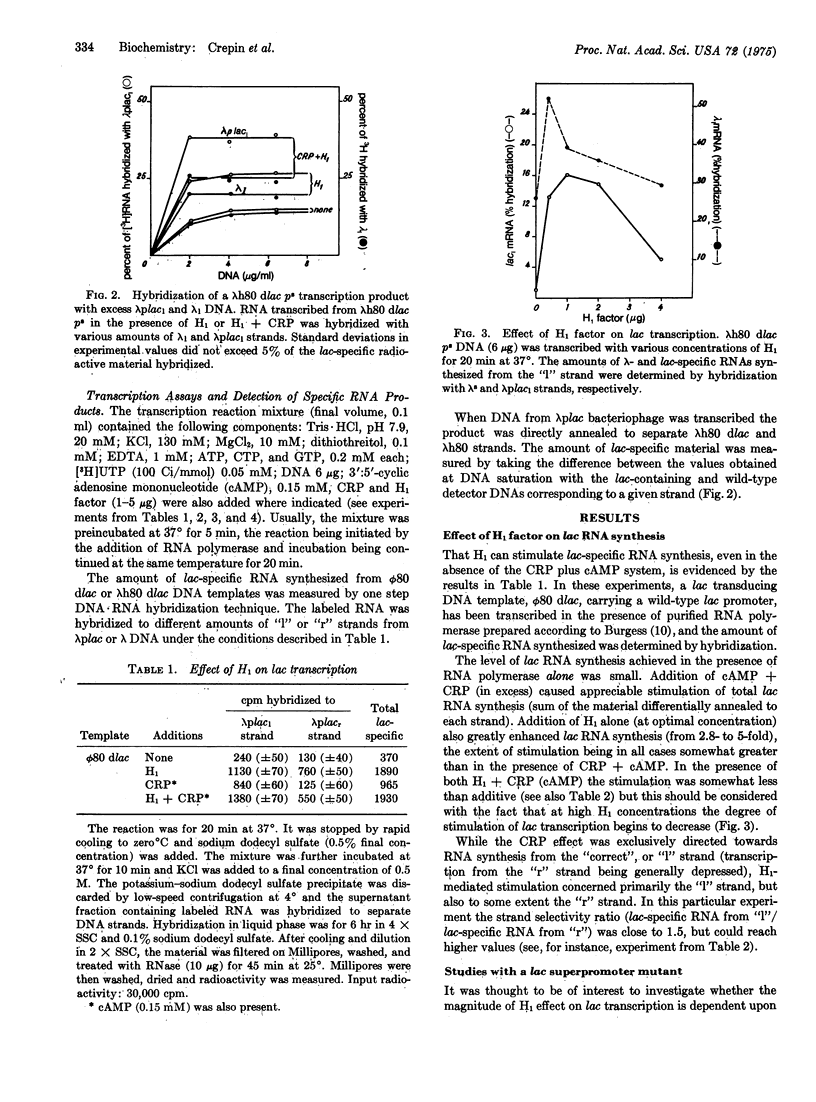
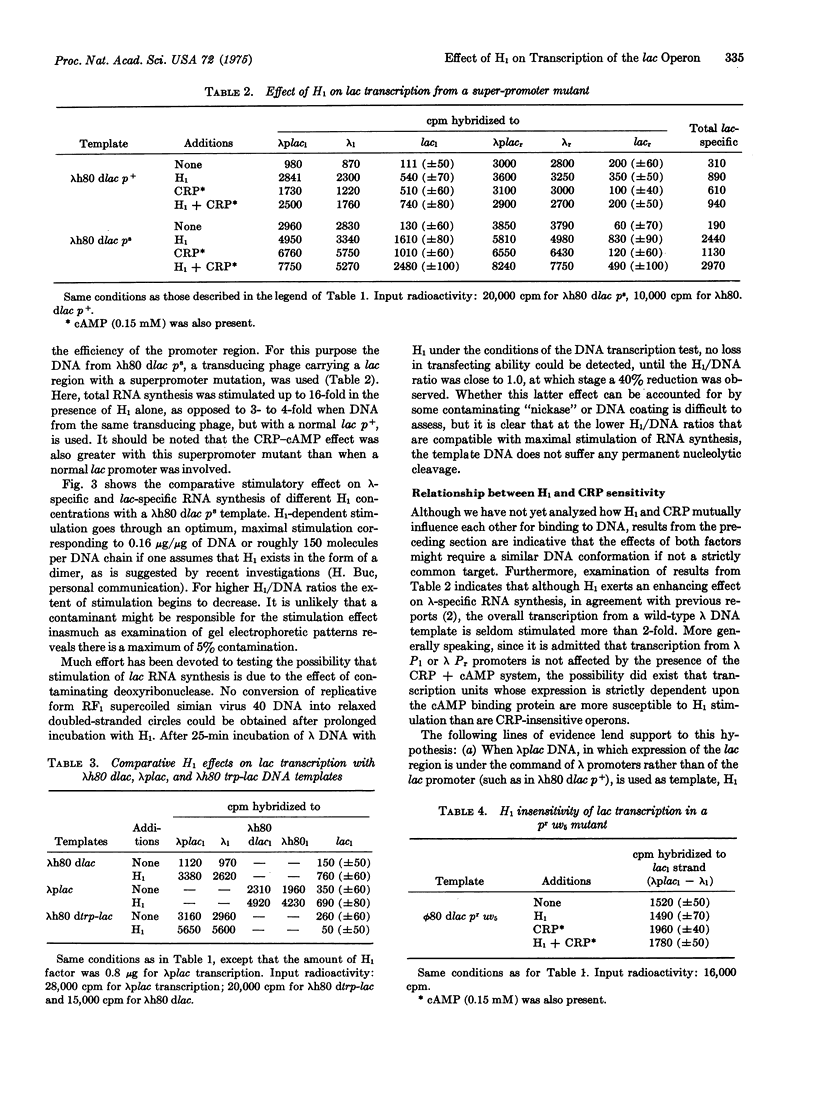
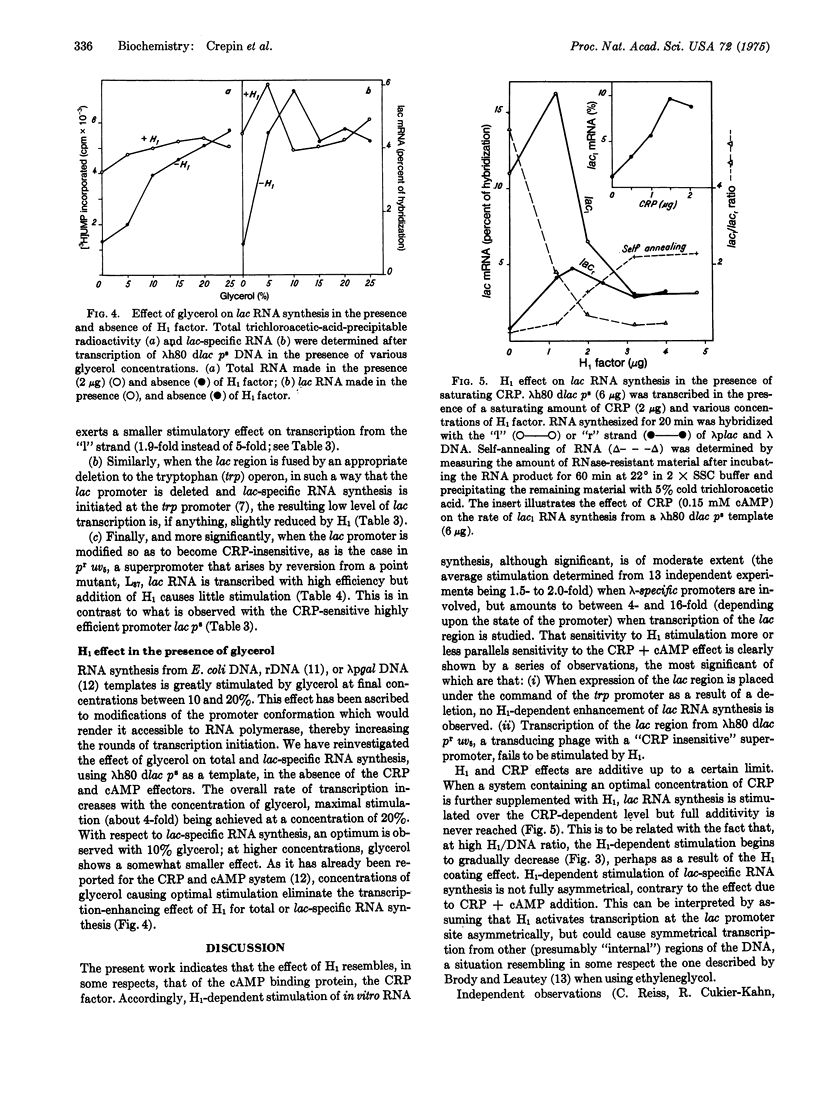
Abstract
H1 protein, a heat-stable low-molecular-weight DNA-binding factor previously described by Cukier-Kahn et al. [Proc. Nat. Acad. Sci USA (1972) 69, 3643-3647] markedly stimulates in vitro synthesis of lac-specific RNA directed by bacteriophage lambdah80 dlac or phi80 dlac DNA templates in the presence of purified E. coli RNA polymerase holoenzyme. The extent of stimulation obtained by addition of H1 alone is usually greater than that observed with the cAMP receptor protein-cAMP combination. H1 effect varies quite appreciably (from 4- to 16-fold) with the functional state of the promoter, being much larger with lambdah80 dlac p-s, a transducing DNA carrying a superpromoter mutation, than with lambdah80 dlac p+. H1 and cAMP receptor protein effects are nearly additive, although interpretation of the data obtained at high H1 concentration is complicated by the appearance of some inhibitory property. While the cAMP-receptor-protein-mediated synthesis is asymmetrical ("I" strand almost exclusively copied), the degree of asymmetry observed with H1 is less pronounced, suggesting asymmetrical copying from the lac promoter and symmetric transcription from other regions of the DNA. Synthesis of lac-specific RNA from lambdah80 dtrp/lac or phi80 dlac p-r uv5 templates, in which lac promoters are insensitive to cAMP receptor protein, either as a result of lac fusion to the trp operon or mutation in the lac promoter, is totally H1-insensitive. Glycerol (10-15% w/w) can fully substitute for H1 in stimulating lac RNA synthesis in a fashion analogous to that reported for the cAMP receptor protein-cAMP system. The possibility that H1 acts by causing conformational modifications at the promoter level in a way that increases its functional state, and that this effect is more pronounced with operons sensitive to cAMP receptor protein, is discussed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brody E. N., Leautey J. Transcription in vitro using mixtures of ethylene glycol and water. Eur J Biochem. 1973 Jul 16;36(2):347–361. doi: 10.1111/j.1432-1033.1973.tb02919.x. [DOI] [PubMed] [Google Scholar]
- Burgess R. R. A new method for the large scale purification of Escherichia coli deoxyribonucleic acid-dependent ribonucleic acid polymerase. J Biol Chem. 1969 Nov 25;244(22):6160–6167. [PubMed] [Google Scholar]
- Cukier-Kahn R., Jacquet M., Gros F. Two heat-resistant, low molecular weight proteins from Escherichia coli that stimulate DNA-directed RNA synthesis. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3643–3647. doi: 10.1073/pnas.69.12.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Crombrugghe B., Chen B., Anderson W., Nissley P., Gottesman M., Pastan I., Perlman R. Lac DNA, RNA polymerase and cyclic AMP receptor protein, cyclic AMP, lac repressor and inducer are the essential elements for controlled lac transcription. Nat New Biol. 1971 Jun 2;231(22):139–142. doi: 10.1038/newbio231139a0. [DOI] [PubMed] [Google Scholar]
- Emmer M., deCrombrugghe B., Pastan I., Perlman R. Cyclic AMP receptor protein of E. coli: its role in the synthesis of inducible enzymes. Proc Natl Acad Sci U S A. 1970 Jun;66(2):480–487. doi: 10.1073/pnas.66.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eron L., Block R. Mechanism of initiation and repression of in vitro transcription of the lac operon of Escherichia coli. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1828–1832. doi: 10.1073/pnas.68.8.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S., Echols H. Purification and properties of D protein: a transcription factor of Escherichia coli. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3660–3664. doi: 10.1073/pnas.69.12.3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquet M., Cukier-Kahn R., Pla J., Gros F. A thermostable protein factor acting on in vitro DNA transcription. Biochem Biophys Res Commun. 1971 Dec 17;45(6):1597–1607. doi: 10.1016/0006-291x(71)90204-x. [DOI] [PubMed] [Google Scholar]
- Nakanishi S., Adhya S., Gottesman M., Pastan I. Activation of transcription at specific promoters by glycerol. J Biol Chem. 1974 Jul 10;249(13):4050–4056. [PubMed] [Google Scholar]
- Nisseley S. P., Anderson W. B., Gottesman M. E., Perlman R. L., Pastan I. In vitro transcription of the gal operon requires cyclic adenosine monophosphate and cyclic adenosine monophosphate receptor protein. J Biol Chem. 1971 Aug 10;246(15):4671–4678. [PubMed] [Google Scholar]
- Travers A., Baillie D. L., Pedersen S. Effect of DNA conformation on ribosomal RNA synthesis in vitro. Nat New Biol. 1973 Jun 6;243(127):161–163. doi: 10.1038/newbio243161a0. [DOI] [PubMed] [Google Scholar]
- Travers A., Cukier-Kahn R. Effect of H1 protein on in vitro ribosomal RNA synthesis. FEBS Lett. 1974 Jul 1;43(1):86–88. doi: 10.1016/0014-5793(74)81111-7. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Zubay G., Morse D. E., Schrenk W. J., Miller J. H. Detection and isolation of the repressor protein for the tryptophan operon of Escherichia coli. Proc Natl Acad Sci U S A. 1972 May;69(5):1100–1103. doi: 10.1073/pnas.69.5.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubay G., Schwartz D., Beckwith J. Mechanism of activation of catabolite-sensitive genes: a positive control system. Proc Natl Acad Sci U S A. 1970 May;66(1):104–110. doi: 10.1073/pnas.66.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]




