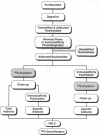
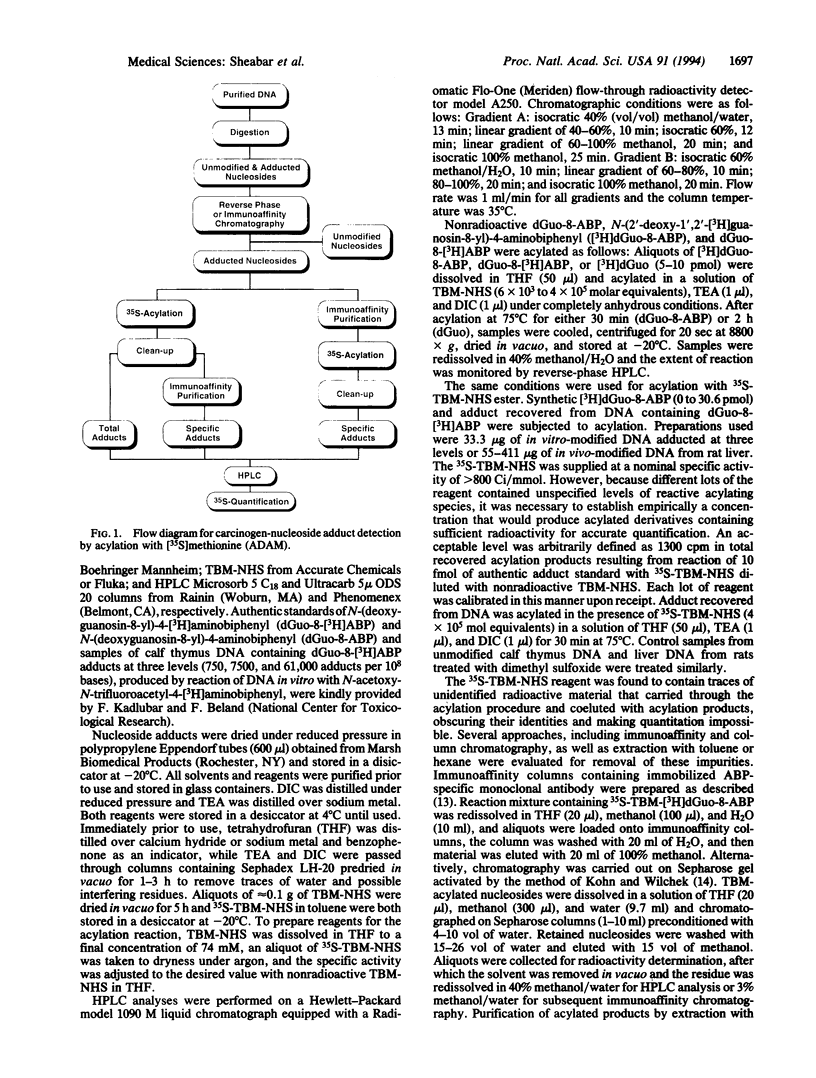
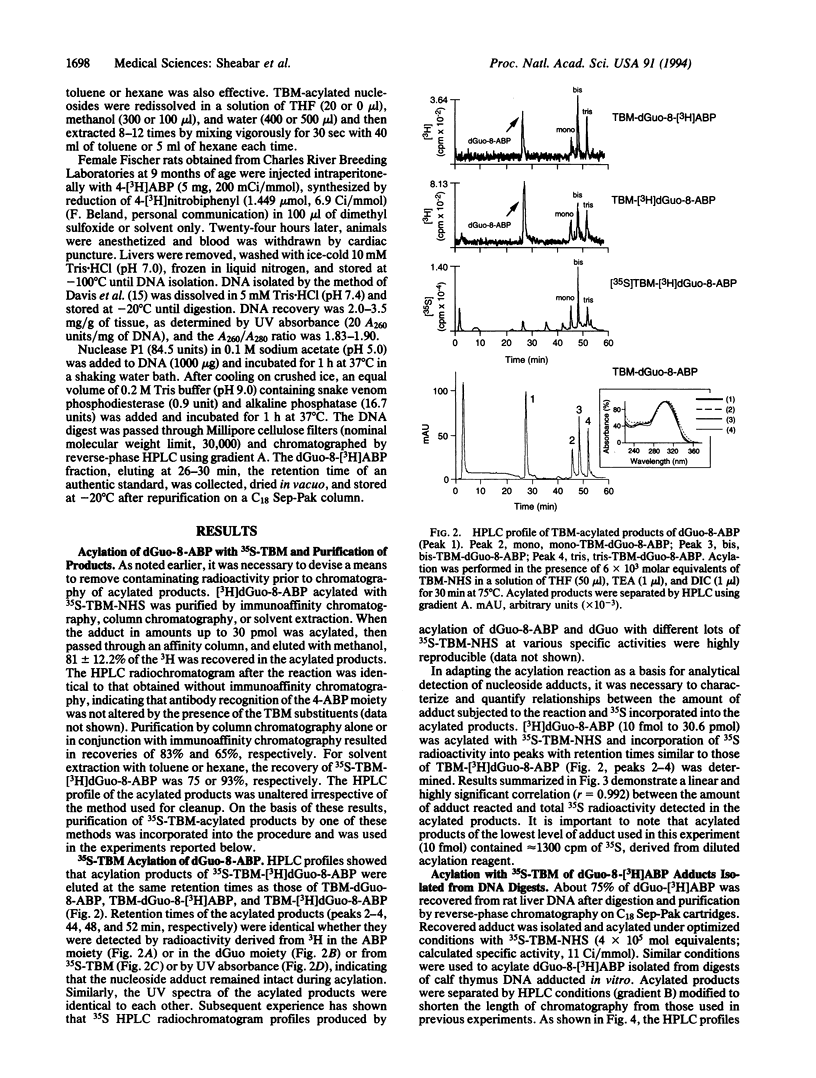
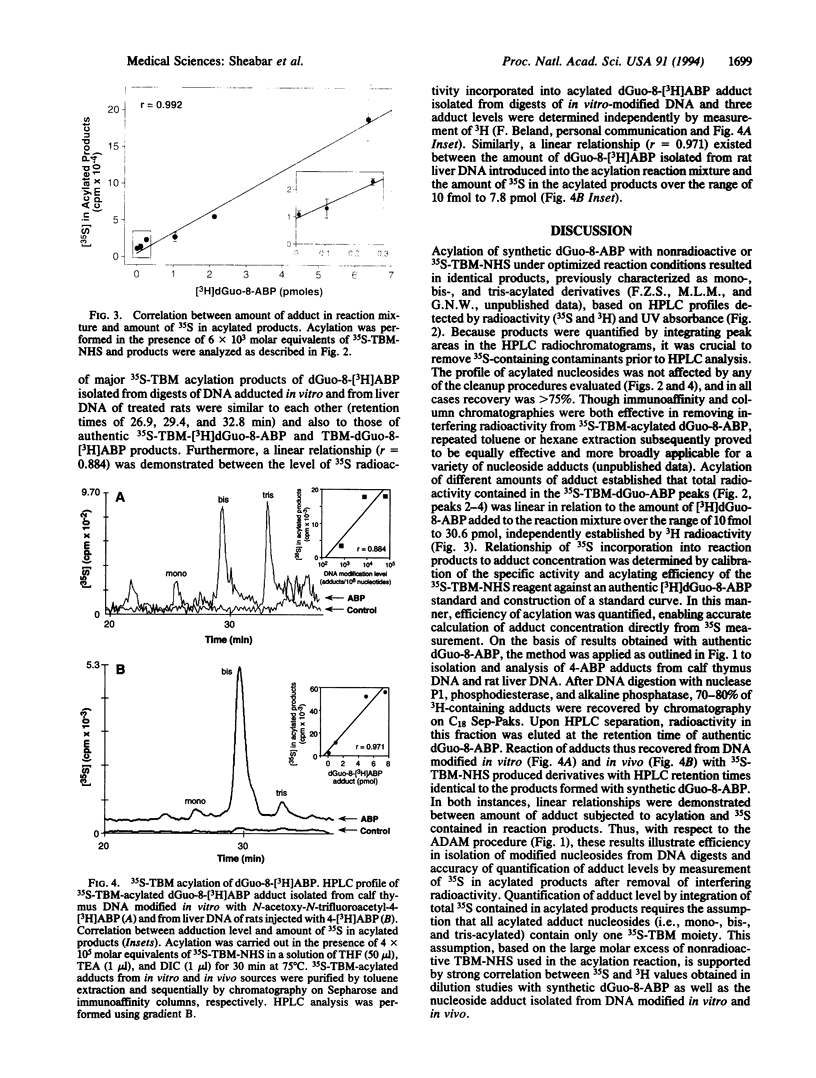
Abstract
Reaction of synthetic N-(2'-deoxyguanosin-8-yl)-4-aminobiphenyl (dGuo-8-ABP) with t-butoxycarbonyl-L-[35S]methionine, N-hydroxysuccinimidyl ester (35S-labeled TBM-NHS), under optimized conditions produced mono-, bis-, and tris-TBM-acylated nucleosides that were separable by HPLC. Reaction of different amounts of N-(2'-deoxy-1',2'-[3H]guanosin-8-yl)-4-aminobiphenyl ([3H]dGuo-8-ABP) with 35S-labeled TBM-NHS established that total 35S content of acylated products was linearly related to adduct concentration (r = 0.992) over the range of 10 fmol to 30.6 pmol. Additionally, the N-(deoxyguanosin-8-yl)-4-[3H]aminobiphenyl (dGuo-8-[3H]ABP) adduct was isolated from calf thymus DNA adducted in vitro and from rat liver DNA adducted in vivo and similarly reacted with 35S-labeled TBM-NHS. Acylation products of dGuo-8-ABP from all three sources showed HPLC retention times identical to those of authentic TBM-dGuo-8-ABP, and 35S incorporation into acylated products was linearly related to amount of adduct reacted. These results indicate that the procedure, to which we have referred as adduct detection by acylation with methionine (ADAM), has potential applicability as an analytical procedure for detection and quantification of DNA adducts in human tissues in the molecular epidemiology of cancer.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beach A. C., Gupta R. C. Human biomonitoring and the 32P-postlabeling assay. Carcinogenesis. 1992 Jul;13(7):1053–1074. doi: 10.1093/carcin/13.7.1053. [DOI] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennell T. R., Juhl U., Miller E. C., Miller J. A. Identification and quantitation of hepatic DNA adducts formed in B6C3F1 mice from 1'-hydroxy-2',3'-dehydroestragole: comparison of the adducts detected with the 1'-3H-labelled carcinogen and by 32P-postlabelling. Carcinogenesis. 1986 Nov;7(11):1881–1887. doi: 10.1093/carcin/7.11.1881. [DOI] [PubMed] [Google Scholar]
- Gorelick N. J. Application of HPLC in the 32P-postlabeling assay. Mutat Res. 1993 Jul;288(1):5–18. doi: 10.1016/0027-5107(93)90203-r. [DOI] [PubMed] [Google Scholar]
- Groopman J. D., Skipper P. L., Donahue P. R., Trudel L. J., Wildschutte M., Kadlubar F. F., Tannenbaum S. R. Monoclonal antibodies and rabbit antisera recognizing 4-aminobiphenyl--DNA adducts and application to immunoaffinity chromatography. Carcinogenesis. 1992 Jun;13(6):917–922. doi: 10.1093/carcin/13.6.917. [DOI] [PubMed] [Google Scholar]
- Kaderlik R. K., Lin D. X., Lang N. P., Kadlubar F. F. Advantages and limitations of laboratory methods for measurement of carcinogen-DNA adducts for epidemiological studies. Toxicol Lett. 1992 Dec;64-65 Spec No:469–475. doi: 10.1016/0378-4274(92)90221-5. [DOI] [PubMed] [Google Scholar]
- Kato S., Petruzzelli S., Bowman E. D., Turteltaub K. W., Blomeke B., Weston A., Shields P. G. 7-Alkyldeoxyguanosine adduct detection by two-step HPLC and the 32P-postlabeling assay. Carcinogenesis. 1993 Apr;14(4):545–550. doi: 10.1093/carcin/14.4.545. [DOI] [PubMed] [Google Scholar]
- Poirier M. C. Antisera specific for carcinogen-DNA adducts and carcinogen-modified DNA: applications for detection of xenobiotics in biological samples. Mutat Res. 1993 Jul;288(1):31–38. doi: 10.1016/0027-5107(93)90205-t. [DOI] [PubMed] [Google Scholar]
- Randerath K., Randerath E., Danna T. F., van Golen L., Putman K. L. A new sensitive 32P-postlabeling assay based on the specific enzymatic conversion of bulky DNA lesions to radiolabeled dinucleotides and nucleoside 5'-monophosphates. Carcinogenesis. 1989 Jul;10(7):1231–1239. doi: 10.1093/carcin/10.7.1231. [DOI] [PubMed] [Google Scholar]
- Reddy M. V., Gupta R. C., Randerath E., Randerath K. 32P-postlabeling test for covalent DNA binding of chemicals in vivo: application to a variety of aromatic carcinogens and methylating agents. Carcinogenesis. 1984 Feb;5(2):231–243. doi: 10.1093/carcin/5.2.231. [DOI] [PubMed] [Google Scholar]
- Ross R. K., Yuan J. M., Yu M. C., Wogan G. N., Qian G. S., Tu J. T., Groopman J. D., Gao Y. T., Henderson B. E. Urinary aflatoxin biomarkers and risk of hepatocellular carcinoma. Lancet. 1992 Apr 18;339(8799):943–946. doi: 10.1016/0140-6736(92)91528-g. [DOI] [PubMed] [Google Scholar]
- Shields P. G., Povey A. C., Wilson V. L., Weston A., Harris C. C. Combined high-performance liquid chromatography/32P-postlabeling assay of N7-methyldeoxyguanosine. Cancer Res. 1990 Oct 15;50(20):6580–6584. [PubMed] [Google Scholar]
- Vahakangas K., Haugen A., Harris C. C. An applied synchronous fluorescence spectrophotometric assay to study benzo[a]pyrene-diolepoxide-DNA adducts. Carcinogenesis. 1985 Aug;6(8):1109–1115. doi: 10.1093/carcin/6.8.1109. [DOI] [PubMed] [Google Scholar]
- Watson W. P. Post-radiolabelling for detecting DNA damage. Mutagenesis. 1987 Sep;2(5):319–331. doi: 10.1093/mutage/2.5.319. [DOI] [PubMed] [Google Scholar]
- Weston A. Physical methods for the detection of carcinogen-DNA adducts in humans. Mutat Res. 1993 Jul;288(1):19–29. doi: 10.1016/0027-5107(93)90204-s. [DOI] [PubMed] [Google Scholar]
- Wogan G. N. Molecular epidemiology in cancer risk assessment and prevention: recent progress and avenues for future research. Environ Health Perspect. 1992 Nov;98:167–178. doi: 10.1289/ehp.9298167. [DOI] [PMC free article] [PubMed] [Google Scholar]