Abstract
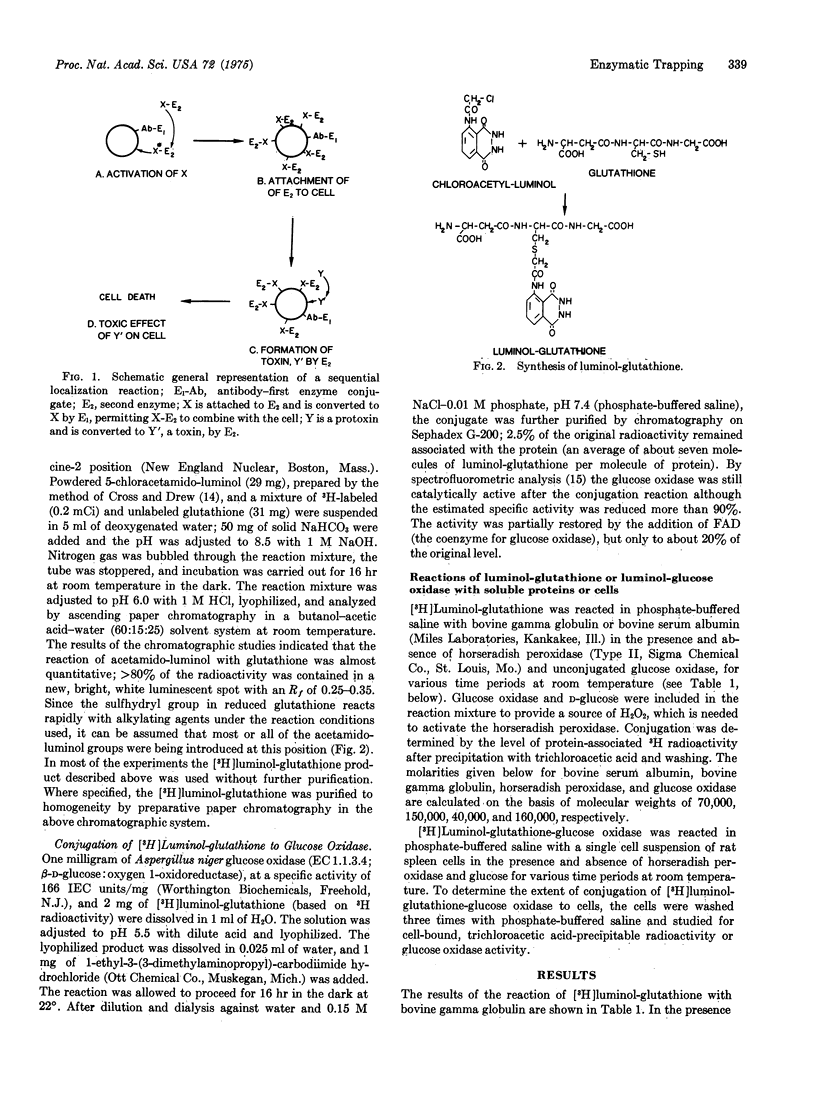
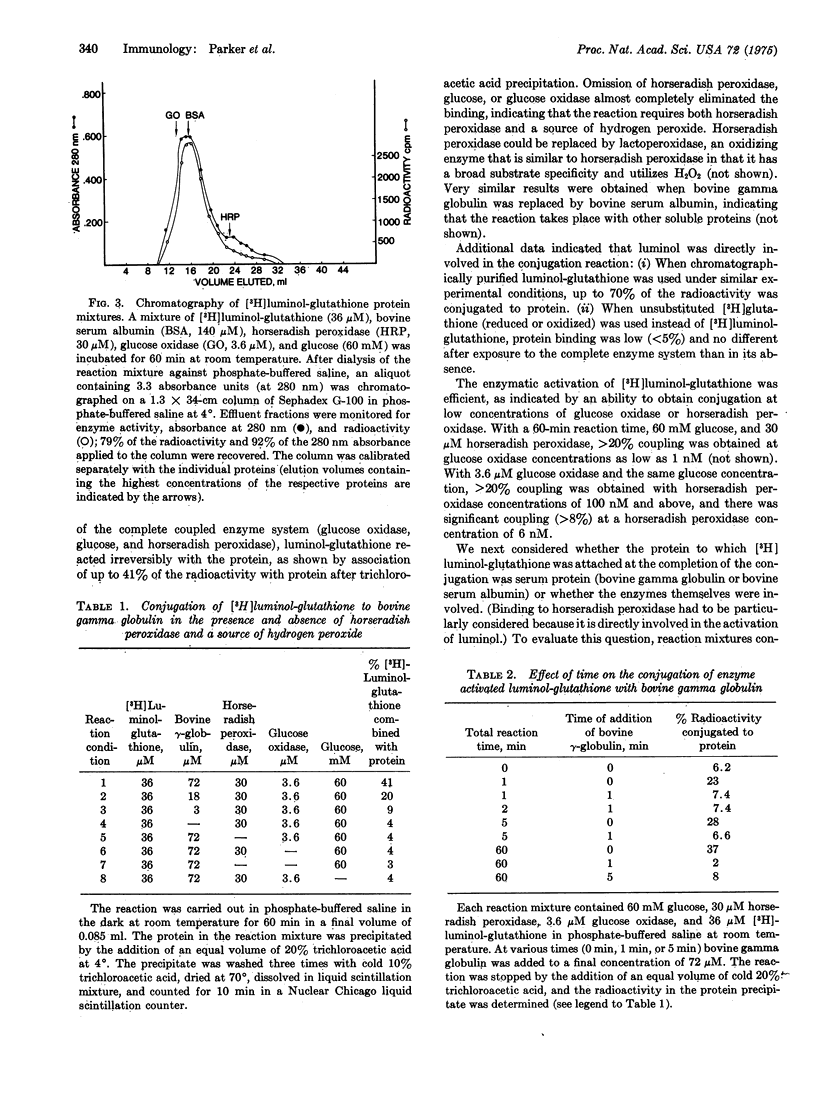
Glutathione and glucose oxidase (EC 1.1.3.4) conjugates containing covalently bound luminol were prepared as prototypes for peptides and proteins with latent, enzyme-activatable chemical reactivity. In the presence of small quantities of activated horseradish peroxidase, conjugated luminol molecules were oxidized to unstable free radicals which reacted rapidly with soluble proteins and cells. These observations are of interest in regard to possible sequential localization reactions in which a few molecules of cell-bound antibody-horseradish peroxidase would be used to catalytically alter and trap many molecules of a second (luminol-substituted) enzyme, toxin, or hapten in the same area, as might be desirable in promoting selective cell destruction.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cormier M. J., Prichard P. M. An investigation of the mechanifm of the luminescent peroxidation of luminol by stopped flow techniques. J Biol Chem. 1968 Sep 25;243(18):4706–4714. [PubMed] [Google Scholar]
- Ghose T., Norvell S. T., Guclu A., Cameron D., Bodurtha A., MacDonald A. S. Immunochemotherapy of cancer with chlorambucil-carrying antibody. Br Med J. 1972 Aug 26;3(5825):495–499. doi: 10.1136/bmj.3.5825.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KESTON A. S., BRANDT R. THE FLUOROMETRIC ANALYSIS OF ULTRAMICRO QUANTITIES OF HYDROGEN PEROXIDE. Anal Biochem. 1965 Apr;11:1–5. doi: 10.1016/0003-2697(65)90034-5. [DOI] [PubMed] [Google Scholar]
- Knowles D. M., 2nd, Sulivan T. J., 3rd, Parker C. W., Williams R. C., Jr In vitro antibody-enzyme conjugates with specific bactericidal activity. J Clin Invest. 1973 Jun;52(6):1443–1452. doi: 10.1172/JCI107318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine R. A., Hakomori S. Incorporation of exogenous glycosphingolipids in plasma membranes of cultured hamster cells and concurrent change of growth behavior. Biochem Biophys Res Commun. 1973 Oct 1;54(3):1039–1045. doi: 10.1016/0006-291x(73)90798-5. [DOI] [PubMed] [Google Scholar]
- Moolten F. L., Cooperband S. R. Selective destruction of target cells by diphtheria toxin conjugated to antibody directed against antigens on the cells. Science. 1970 Jul 3;169(3940):68–70. doi: 10.1126/science.169.3940.68. [DOI] [PubMed] [Google Scholar]
- Parker C. W. The immunotherapy of cancer. Pharmacol Rev. 1973 Jun;25(2):325–342. [PubMed] [Google Scholar]
- Philpott G. W., Bower R. J., Parker C. W. Selective iodination and cytotoxicity of tumor cells with an antibody-enzyme conjugate. Surgery. 1973 Jul;74(1):51–58. [PubMed] [Google Scholar]
- Philpott G. W., Bower R. J., Parker K. L., Shearer W. T., Parker C. W. Affinity cytotoxicity of tumor cells with antibody-glucose oxidase conjugates, peroxidase, and arsphenamine. Cancer Res. 1974 Sep;34(9):2159–2164. [PubMed] [Google Scholar]
- Philpott G. W., Shearer W. T., Bower R. J., Parker C. W. Selective cytotoxicity of hapten-substituted cells with an antibody-enzyme conjugate. J Immunol. 1973 Sep;111(3):921–929. [PubMed] [Google Scholar]
- Sullivan T. J., Parker K. L., Parker C. W. Specific killing of parasites by antibody-enzyme conjugates. Res Commun Chem Pathol Pharmacol. 1973 Sep;6(2):709–717. [PubMed] [Google Scholar]
- Wong H. S., Tolpin E. I., Lipscomb W. N. Boron hydride derivatives for neutron capture therapy. Antibody approach. J Med Chem. 1974 Aug;17(8):785–791. doi: 10.1021/jm00254a001. [DOI] [PubMed] [Google Scholar]


