Abstract
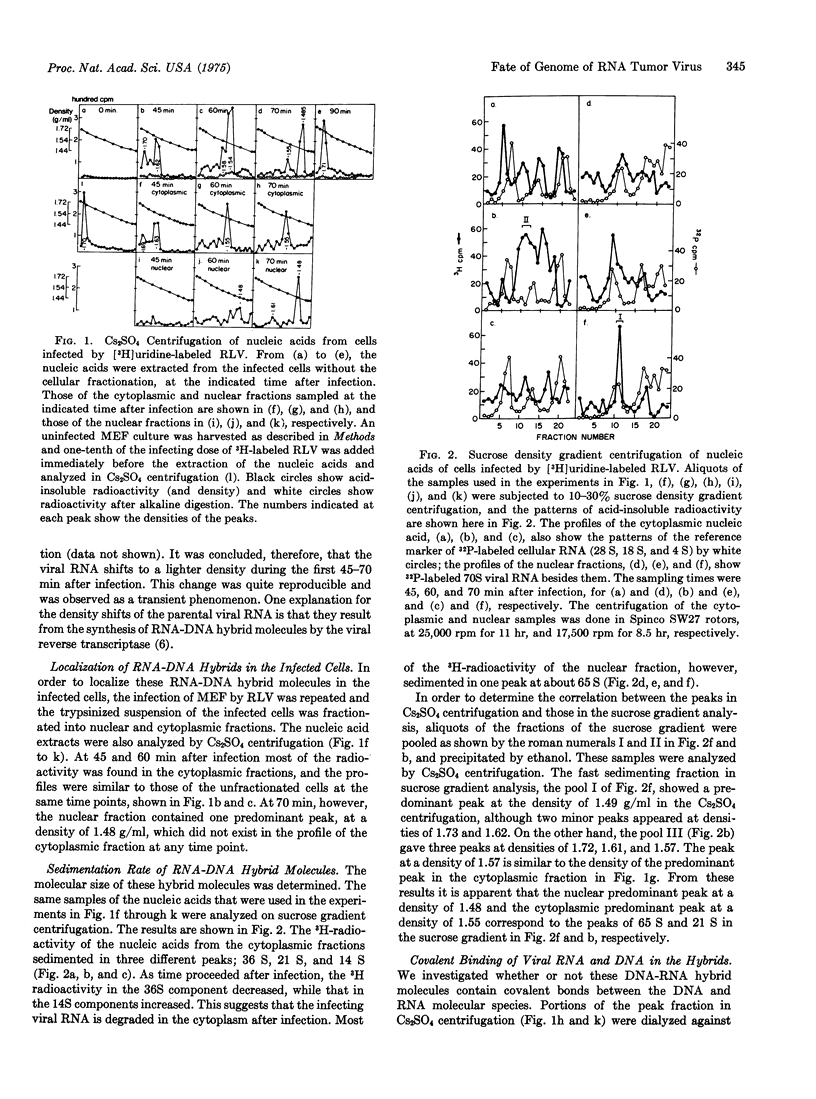
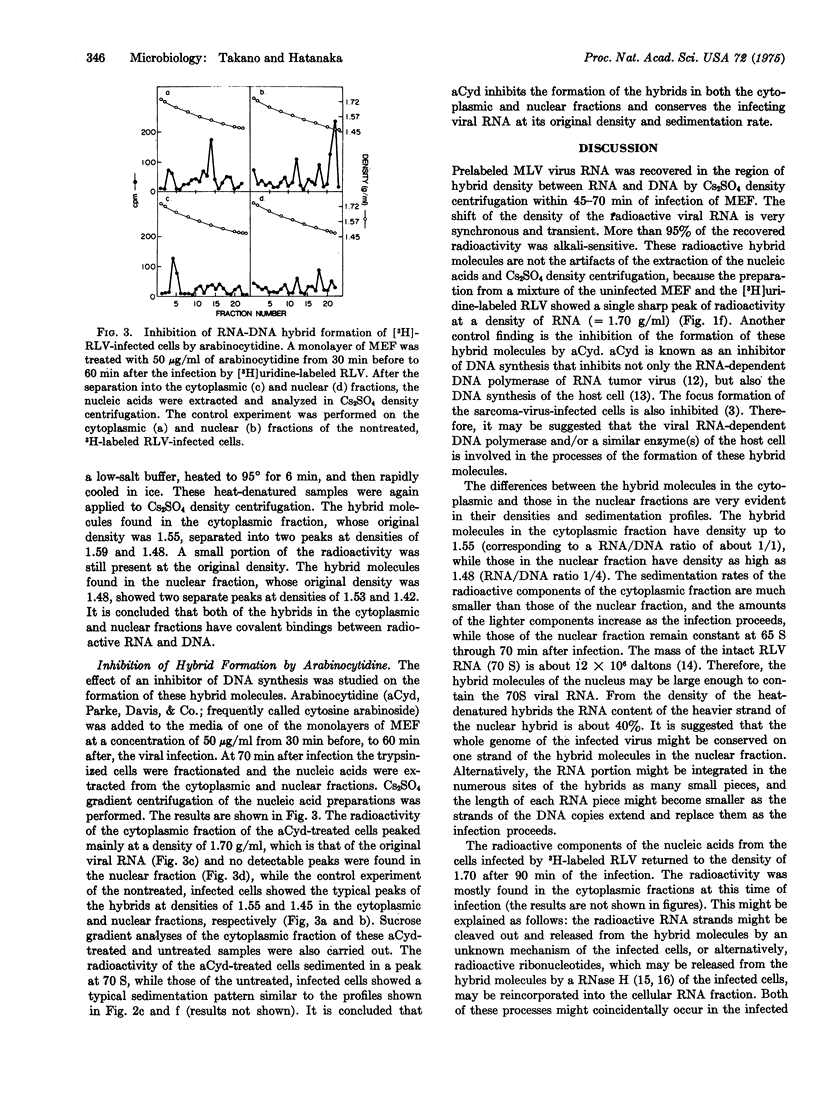
[3H]Uridine-labeled Rauscher leukemia virus was used to infect mouse embryo fibroblasts. After the infected cells were separated into nuclear and cytoplasmic fractions nucleic acid was extracted by sodium dodecyl sulfate-phenol-chloroform treatment and analyzed by Cs2SO4 and sucrose density gradient centrifugation. Between 45 and 70 min after infection a transient and synchronized shift of the acid-insoluble radioactive peak toward the RNA-DNA hybrid region occurred in both the nuclear and cytoplasmic fractions. The density of the cytoplasmic hybrid shifted to 1.56 g/ml (RNA equals about 50%), while the sedimentation rate decreased from 36 S to 14 S; however, the density of the nuclear hybrid shifted to 1.58-1.48 g/ml (RNA equals 57-17%, respectively), while its sedimentation rate remained about 65 S. The hybrids in both the nuclear and the cytoplasmic fractions still showed hybrid density after heat denaturation. The processes of the early stages of RNA tumor virus infection are discussed with regard to the functions of viral RNA-dependent DNA polymerase (reverse transcriptase) and a possible integration of viral genetic information into the host chromosome.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BADER J. P. TRANSFORMATION OF ROUS SARCOMA VIRUS: A REQUIREMENT OF DNA SYNTHESIS. Science. 1965 Aug 13;149(3685):757–758. doi: 10.1126/science.149.3685.757. [DOI] [PubMed] [Google Scholar]
- Bader J. P. Metabolic requirements for infection by Rous sarcoma virus. I. The transient requirement for DNA synthesis. Virology. 1966 Jul;29(3):444–451. doi: 10.1016/0042-6822(66)90220-0. [DOI] [PubMed] [Google Scholar]
- Baltimore D., Smoler D. F. Association of an endoribonuclease with the avian myeloblastosis virus deoxyribonucleic acid polymerase. J Biol Chem. 1972 Nov 25;247(22):7282–7287. [PubMed] [Google Scholar]
- Bishop D. H., Ruprecht R., Simpson R. W., Spiegelman S. Deoxyribonucleic acid polymerase of Rous sarcoma virus: reaction conditions and analysis of the reaction product nucleic acids. J Virol. 1971 Nov;8(5):730–741. doi: 10.1128/jvi.8.5.730-741.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manly K. F., Smoler D. F., Bromfeld E., Baltimore D. Forms of deoxyribonucleic acid produced by virions of the ribonucleic acid tumor viruses. J Virol. 1971 Jan;7(1):106–111. doi: 10.1128/jvi.7.1.106-111.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham P. D., Baluda M. A. Integrated state of oncornavirus DNA in normal chicken cells and in cells transformed by avian myeloblastosis virus. J Virol. 1973 Oct;12(4):721–732. doi: 10.1128/jvi.12.4.721-732.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mölling K., Bolognesi D. P., Bauer H., Büsen W., Plassmann H. W., Hausen P. Association of viral reverse transcriptase with an enzyme degrading the RNA moiety of RNA-DNA hybrids. Nat New Biol. 1971 Dec 22;234(51):240–243. doi: 10.1038/newbio234240a0. [DOI] [PubMed] [Google Scholar]
- Robinson W. S., Pitkanen A., Rubin H. The nucleic acid of the Bryan strain of Rous sarcoma virus: purification of the virus and isolation of the nucleic acid. Proc Natl Acad Sci U S A. 1965 Jul;54(1):137–144. doi: 10.1073/pnas.54.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokutanda M., Rokutanda H., Green M., Fujinaga K., Ray R. K., Gurgo C. Formation of viral RNA-DNA hybrid molecules by the DNA polymerase of sarcoma-leukaemia viruses. Nature. 1970 Sep 5;227(5262):1026–1028. doi: 10.1038/2271026a0. [DOI] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Stephens R., Traul K., Lowry G., Zelljadt I., Mayyasi S. Differential morphology of the RD virus from the human rhabdomyosarcoma, RD-114B cell line demonstrated by negative staining electron microscopy. Nat New Biol. 1972 Dec 13;240(102):212–214. doi: 10.1038/newbio240212a0. [DOI] [PubMed] [Google Scholar]
- Sveda M. M., Fields B. N., Soeiro R. Host restriction of friend leukemia virus; fate of input virion RNA. Cell. 1974 Aug;2(4):271–277. doi: 10.1016/0092-8674(74)90021-x. [DOI] [PubMed] [Google Scholar]
- TEMIN H. M. THE PARTICIPATION OF DNA IN ROUS SARCOMA VIRUS PRODUCTION. Virology. 1964 Aug;23:486–494. doi: 10.1016/0042-6822(64)90232-6. [DOI] [PubMed] [Google Scholar]
- Temin H. M., Baltimore D. RNA-directed DNA synthesis and RNA tumor viruses. Adv Virus Res. 1972;17:129–186. doi: 10.1016/s0065-3527(08)60749-6. [DOI] [PubMed] [Google Scholar]
- Tuominen F. W., Kenney F. T. Inhibition of RNA-directed DNA polymerase from Rauscher leukemia virus by the 5'-triphosphate of cytosine arabinoside. Biochem Biophys Res Commun. 1972 Sep 26;48(6):1469–1475. doi: 10.1016/0006-291x(72)90879-0. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Bishop J. M., Vogt P. K. Appearance of virus-specific DNA in mammalian cells following transformation by Rous sarcoma virus. J Mol Biol. 1973 Mar 15;74(4):613–626. doi: 10.1016/0022-2836(73)90052-1. [DOI] [PubMed] [Google Scholar]
- Wright B. S., O'Brien P. A., Shibley G. P., Mayyasi S. A., Lasfargues J. C. Infection of an established mouse bone marrow cell line (JLS-V9) with Rauscher and Moloney murine leukemia viruses. Cancer Res. 1967 Sep;27(9):1672–1677. [PubMed] [Google Scholar]


