Abstract
The blue pigment of cornflower, protocyanin, has been investigated for a long time, but its precise structure was not entirely explained until recently. The molecular structure of the pigment was recently shown to be a metal complex of six molecules each of anthocyanin and flavone glycoside, with one ferric iron, one magnesium and two calcium ions by X-ray crystallographic analysis. The studies provided the answer to the question posed in the early part of the last century, “why is the cornflower blue and rose red when both flowers contain the same anthocyanin?” This work was achieved on the basis of the results of long years of the studies made by many researchers. In this review, the author focuses on the investigations of the blue metal complex pigments involved in the bluing of flowers, commelinin from Commelina commusis, protocyanin from Centaurea cyanus, protodelphin from Salvia patens and hydrangea blue pigment.
Keywords: Flower color, anthocyanin, blue pigment, metal complex, protocyanin, commelinin
Introduction
Flower colors in the range from red to blue are mostly produced by anthocyanins.1),2) In 1913, R. Willstätter found that the blue cornflower contains the same anthocyanin that is also present in the red rose.3) This finding presented an enigma about flower color variation, and ever since many investigations have been carried out on anthocyanin pigments with special reference to flower color. The blue pigment of the corn-flower, named protocyanin, has been investigated for a long time, but its precise structure had remained unclear, as the pigment is a high molecular-weight complex compound and probably because of difficulty in purification of the pigment from nature. Recently, we revealed the molecular structure of protocyanin and demonstrated that the blue color is developed by a tetra-metal (Fe3+, Mg2+, 2Ca2+) complex pigment comprising anthocyanins, flavone glycosides and metals. In this review, the studies on blue flower pigments, especially on blue metal complex pigments, are reviewed.
Historical aspects
R. Willstätter first isolated an anthocyanin, named cyanin, as a red oxonium salt, from the blue cornflower, Centaurea cyanus, and determined the chemical structure in 1913.3) In 1915, he found the same pigment in red rose and ascribed the color variation to pH, since anthocyanin changes its color according to the pH of the solution, red in acidic and blue in alkaline solution.4) K. Shibata and Y. Shibata, a plant physiologist and a chemist, questioned this hypothesis because flower petals are slightly acidic, and proposed the metal complex theory in 1919,5) according to which the blue color is produced by a complex of anthocyanin and metal ions such as magnesium and calcium. The metal complex theory was based on the fact that they obtained a blue complex of anthocyanin and metal ions on reduction of flavonol derivatives to anthocyanins with magnesium and acid. Furthermore, they observed color changes of the natural anthocyanin solutions from red to blue or violet when the salts of alkaline earths and heavy metals were added to the solutions. However, the theory was immediately strongly refuted by A. E. Everest, one of Willstätter’s collaborators, describing the evidence as unsatisfactory.6) Later, R. Robinson and G. M. Robinson proposed the copigment theory in 1931,7),8) according to which the presence of copigments such as tannins and flavonols in cell sap containing anthocyanins intensifies and modifies the color.
Anthocyanin pigments were generally extracted with a solvent containing hydrochloric acid and isolated as red oxonium salts, chlorides, because the pigments are stable in acidic condition, while unstable in neutral solvents. However, the only possible solution of this problem was the isolation of the native pigments without any color changes. A new turn in the research on flower color variation was the isolation of blue pigments from blue flowers using only neutral solvents, protocyanin from the blue cornflower Centaurea cyanus by E. Bayer (1958)9) and commelinin from the blue flowers of Commelina communis, by K. Hayashi (1957).10)
Commelinin
K. Hayashi (1957),10) K. Hayashi, Y. Abe and S. Mitsui (1958),11) and S. Mitsui, K. Hayashi and S. Hattori (1959)12) isolated a blue anthocyanin pigment as blue prismatic needles using only neutral solvents, ethanol and water, from the blue flowers of Commelina communis, and named it commelinin. The pigment crystals dissolve in water very easily with a deep blue color and the pigment is non-dialysable through a semi-permeable membrane. The UV-Vis absorption spectrum showed characteristically two peaks in the visible region, λmax 273, 316, 591 and 643 nm in water. On electrophoresis, the blue spot of the pigment moved rapidly towards the anode. They demonstrated that commelinin is a high molecular-weight compound, which is composed of an anthocyanin (thought to be awobanin at that time13)), a flavonoid-like substance and Mg and K in the ratio of 4:4:1:2 (Mg 0.42%). Mg in the pigment molecule remained even after treatment either with 1% hydrochloric acid, or EDTA, or cation exchangers, and no perceptible color change was caused by the treatments. On the basis of some analytical results obtained, they concluded that commelinin is a metallo-anthocyanin, in which 4 molecules of anthocyanin assemble together under mediation of 1 atom of Mg to form a co-ordination compound. The blue chelation compound formed was further linked weakly to a flavonoid-like substance, which might contribute to the stability of the blue color.
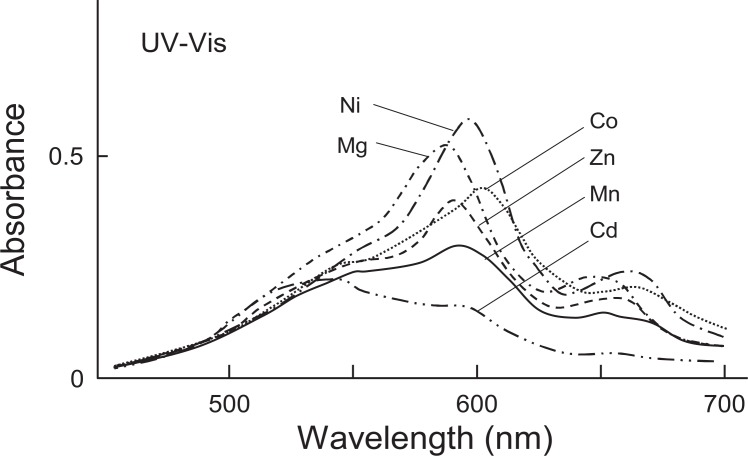
K. Takeda, S. Mitsui and K. Hayashi (1966)14) determined the structure of the flavonoid-like compound to be 6-C-glucosylgenkwanin 4′-O-glucoside (= swertisin 4′-O-glucoside15)) and named it flavocommelin (Fig. 1). Bayer16) expressed his view that the presence of Mg in commelinin must be due to impurities, because a divalent metal such as Mg in general does not form any stable complex compounds. In order to clarify this matter, further purification of commelinin with Sephadex column chromatography and repeated recrystallization was carried out.17) The results of quantitative analyses on the purest specimen showed that the ratio of awobanin:flavocommelin:Mg:K was 2:2:1:1(Mg 0.70%). The values of two components of the purified pigment, anthocyanin and flavone glucoside, were almost the same as those of the previous experiments,12) whereas the value for Mg was nearly twice as high as that obtained before. For further evidence for the presence of Mg as an essential component in the compound, we tried to synthesize the blue complex pigment using awobanin,18) flavocommelin, which were isolated as crystals, and Mg2+, and succeeded in reconstruction of commelinin.19) The reconstructed pigment was finally obtained as crystals. The UV-Vis and IR absorption spectra showed the identity of the reconstructed and natural pigments (Fig. 2). On electrophoresis, both pigments were characterized by rapid migration of the spots toward the anode. The molecular ratio of awobanin, flavocommelin and Mg2+ contained in the reconstructed pigment was practically equal to 2:2:1 (Mg 0.83%), a value which corresponds with that found for natural commelinin. Furthermore, it was found that Mg2+ in commelinin could be replaced with some other bivalent metals, that is, Mn2+, Co2+, Ni2+, Zn2+ and Cd2+.20) Using awobanin, flavocommelin and the five different metals, Mn-, Co-, Ni-, Zn- and Cd-commelinins were prepared and obtained as crystals. The IR spectra of the five compounds were identical with that of commelinin. The UV-Vis spectra of all the metal-replaced commelinins exhibited the two conspicuous peaks in the visible region, which were characteristic of commelinin. However, the positions of the two peaks were slightly different according to the kind of metals substituted (Fig. 3). The mole ratios of the components, awobanin, flavocommelin and the metal in the metal-substituted commelinins were 2:2:1. These facts indicated that the metals play an important part in the formation of commelinin and the metal-substituted commelinin molecules.
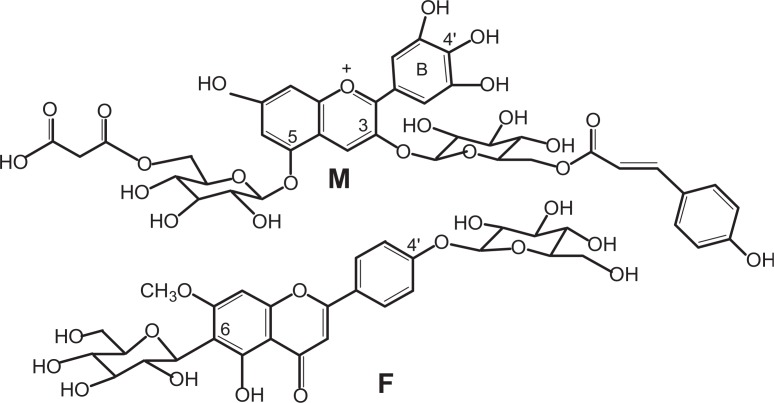
Fig. 1.
The structures of anthocyanin and flavone glycoside in commelinin. M: malonylawobanin; F: flavocommelin.
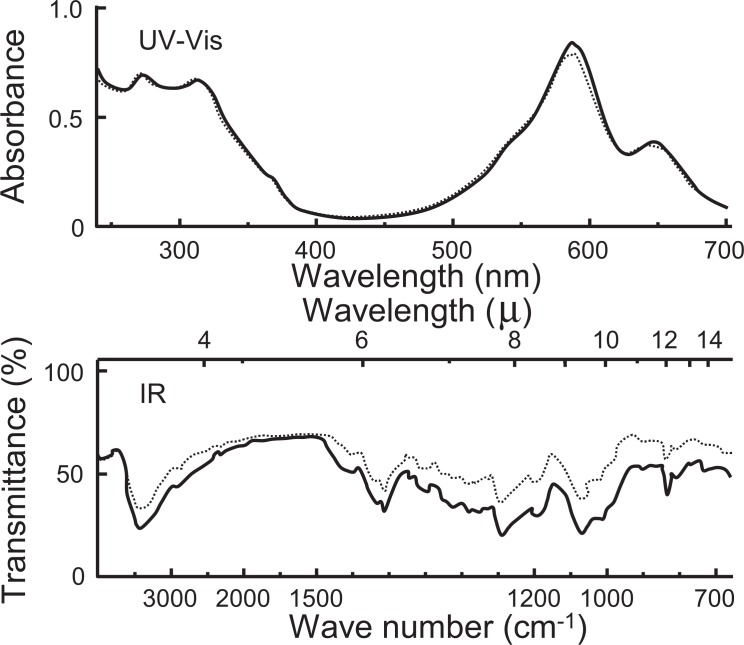
Fig. 2.
UV-Vis and IR spectra of natural and reconstructed commelinin (UV-Vis, 300 mg/l in 0.05 M acetate buffer of pH 4.80; IR, KBr). --------- natural; ——— reconstructed commelinin.
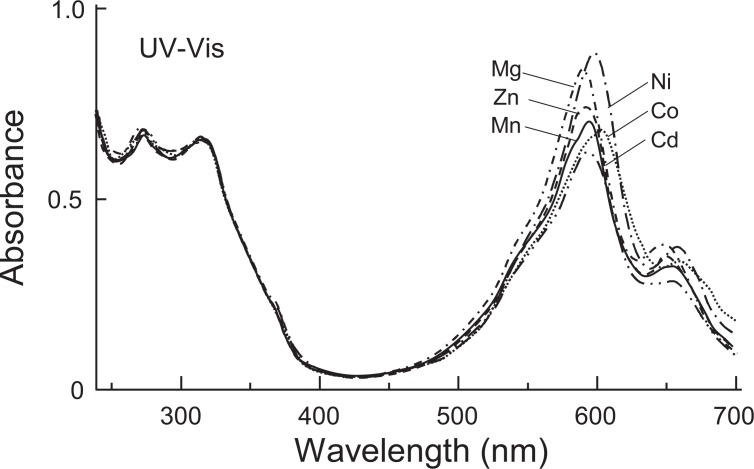
Fig. 3.
UV-Vis spectra of metal substituted commelinins (300 mg/l in 0.05 M acetate buffer of pH 4.80). Mn-commelinin: λmax 273, 315, 595, 653 nm; Co-commelinin: λmax 274, 314, 603, 661 nm; Ni-commelinin: λmax 274, 314, 599, 658 nm; Zn-commelinin: λmax 274, 315, 593, 650 nm; Cd-commelinin: λmax 273, 316, 593, 652 nm; Mg-commelinin and natural commelinin: λmax 274, 314, 591, 647 nm.
In contrast, T. Goto, T. Hoshino and S. Takase (1979) 21) reported the formation of commelinin from a mixture of awobanin22) and flavocommelin without the addition of Mg2+. Commelinin thus prepared showed UV, IR, and CD spectra superimposable on those of natural commelinin. The Mg2+ content of the synthetic pigment was 0.013%. Thus, they concluded that evidently Mg2+ is not an essential component to produce the blue color of commelinin. Mg2+ in commelinin and the divalent metals in the metal substituted commelinins were supposed to be contained as salts, since commelinin has negative charge(s). They proposed a model structure of the molecular complex of awobanin and flavocommelin, in which the aromatic rings of awobanin and flavocommelin face each other and are surrounded by the four glucose moieties. Furthermore, they reported that the p-coumaroyl group of awobanin (delphinidin 3-p-coumaroylglucoside-5-glucoside) has an important role in the stability and bluing effect of anthocyanin-copigment complex.23)
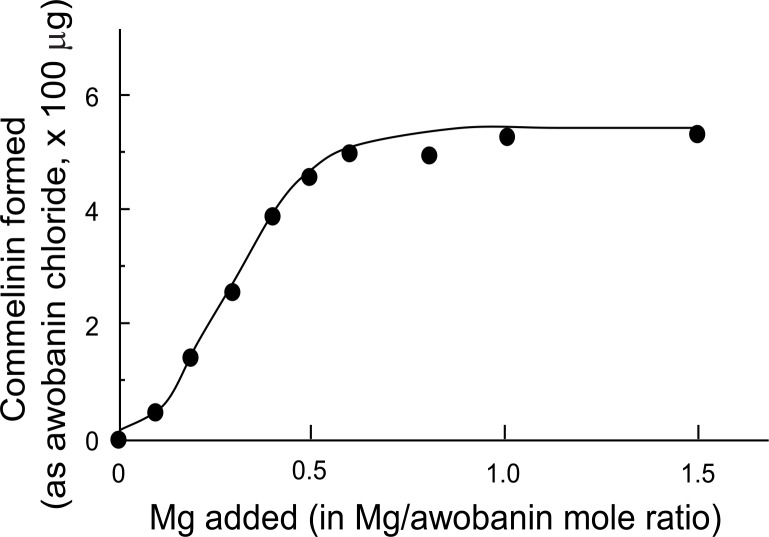
K. Takeda, F. Narashima and S. Nonaka (1980) attempted the synthesis of commelinin-like metal complexes using flavocommelin, metals (with Mg2+, Mn2+, Co2+, Ni2+, Zn2+, Cd2+) and some anthocyanins having structures similar to awobanin, that is, delphinidin 3,5-diglucoside (delphin); delphinidin 3-[4-O-(p-coumaroyl)rhamnosylglucoside]-5-glucoside (violanin22),24)); cyanidin 3-(6-O-p-coumaroylglucoside)-5-glucoside (shisonin18),22)); and cyanidin 3,5-diglucoside (cyanin).25) Only shisonin formed a commelinin-like blue metal complex with flavocommelin and Mg2+. The results showed that the p-coumaroyl glucose residues of awobanin and of shisonin play an important role in the formation of commelinin, the metal-substituted commelinins and the analogous complexes obtained with shisonin respectively. The ineffectiveness of violanin, similar to awobanin except for a different p-coumaroyl sugar chain, indicated that the length and nature of the sugar chain are critical factors for the formation of commelinin and similar complex pigments. Furthermore, in the synthesis of commelinin from its components, awobanin, flavocommelin and Mg2+, the yield of commelinin was shown to be proportional to the amount of Mg2+ added and commelinin was not obtained in the absence of Mg2+ 26) (Fig. 4). The stabilities of commelinin and other metal complexes, Mn, Co, Ni, Zn and Cd-commelinins, in acidic solutions (pH 2.4–5.2), were different from one another according to the metal present (Fig. 5). Ni- and Mg-commelinins were most stable, whereas Cd-commelinin was very unstable. These facts also indicated that Mg2+ plays a part in the formation of the stable blue complex commelinin.
Fig. 4.
The effect of Mg on the formation of the blue complex molecule of commelinin. Values are taken from the averages of five separate experiments.
Fig. 5.
Absorption spectra of Mg-, Ni-, Cd-, Zn-, Mn- and Co-commelinins, 100 mg/l in 0.05 M citrate phosphate buffer, pH 2.6 (light path length of 3 mm).
T. Goto et al. (1983) found that the anthocyanin in the blue petals of Commelina communis is malonylated.27) The structure was determined to be delphinidin 3-O-(6-O-p-coumaroylglucoside)-5-O-(6-O-malonylglucoside) and named malonylawobanin (Fig. 1). Commelinin was known as a complex pigment bearing a negative charge, because commelinin migrates to the anode on electrophoresis at pH 6.0.11) However, the components of commelinin, awobanin, flavocommelin and Mg2+, have no negative charges. This finding gave the answer to the mysterious behavior of commelinin on electrophoresis. Accordingly, the anthocyanin in commelinin reported in past should be described as malonylawobanin. The present author confirmed that the anthocyanin samples used as awobanin in our reconstruction experiments were also malonylated, and thus were malonylawobanin (data unpublished). H. Tamura, T. Kondo and T. Goto (1986) synthesized commelinin using malonylawobanin, flavocommelin and Mg2+.28) Besides, they synthesized a commelinin-like pigment using flavocommelin, Mg2+ and awobanin (demalonated anthocyanin). On electrophoresis, commelinin moved fast, but the pigment from awobanin showed a spot with little mobility. On the other hand, commelinin-like pigments synthesized using a mixture of malonylawobanin and awobanin (demalonated) gave seven spots on electrophoresis. The spot (spot 1) that moved fastest contained only malonylawobanin as the anthocyanin component, whereas the spot (spot 7) that moved slowest contained only awobanin (demalonated). Other spots, spots 2∼6, contained malonylawobanin and awobanin in the ratios of 5.0:1.0 (spot 2), 4.0:1.9 (spot 3), 3.0:2.9 (spot 4), 2.1:3.8 (spot 5), 1.3:4.7 (spot 6), respectively. The observed molecular weight by analytical centrifugation was 9,300. The content of Mg2+ in commelinin was 0.47%. They concluded that commelinin is composed of six molecules each of malonylawobanin and flavocommelin, and 2Mg2+.
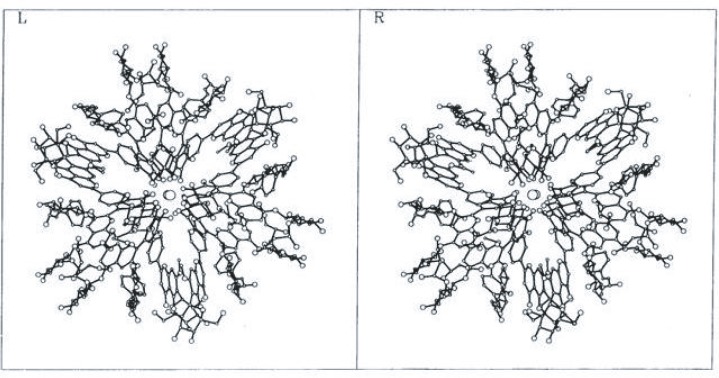
Finally, Kondo et al. (1992) determined the structure of commelinin by X-ray crystallography using the crystals of Cd-commelinin29), 30) (Fig. 6). Two Cd ions (Mg ions in commelinin) are located at the center of the molecule, on a crystallographic three-fold axis. Both malonylawobanin (M) and flavocommelin (F) are self-associated with each other as MM and FF. The three self-associated Ms are placed around the three fold axis alternatively with self-associated Fs. The B-ring of malonylawobanin is of the 4′-keto-quinoidal anion form with the oxygen atoms chelated to the cadmium ions.
Fig. 6.
The stereo structure of Mg-commelinin molecule. Two Mg ions are located at the center of the molecule. [Courtesy of Prof. A. Nakagawa, Osaka University, from J. Cryst. Soc. Japan 35, 332 (1993),30) with permission.]
Protocyanin
E. Bayer (1958)9) and Bayer et al. (1966)16) isolated a blue pigment from the blue corn-flower, named protocyanin and showed that it was a metal complex of high molecular weight (MW ca. 6,200). The pigment contained Fe3+ and Al3+ as essential metals, cyanin (19.2%) and a polysaccharide (ca. 80%), the main component of which was galacturonic acid. They proposed a structure of protocyanin, in which the metal ions, Fe3+ and Al3+, are coordinated to two anthocyanin molecules and polygalacturonic acid.
K. Hayashi, N. Saito and S. Mitsui (1961), 31) and N. Saito, S. Mitsui and K. Hayashi (1961)32) isolated protocyanin as crystals and demonstrated that the pigment was a high molecular-weight compound having the molecular weight of about 20,000, the principal part of which was built up of cyanin (8 moles), Mg (2 atoms), Fe (1 atom), and K (24 atoms). Besides, the pigment was bound to a certain peptide, carbohydrate, and a pale yellow flavonoid-like substance, which might contribute chiefly to the maintenance of the blue color of protocyanin. Mg and Fe remained in the blue pigment even after 48 hours’ dialysis, whereas K was removable without color change. Furthermore, N. Saito and K. Hayashi (1965) analyzed the pigment after treatment with ionexchangers and showed that the two metals, Mg and Fe, were essential for the occurrence of the blue color of protocyanin.33)
S. Asen and L. Jurd (1967) obtained a blue crystalline pigment from the blue cornflower, and reported that the pigment was an iron complex of 4 molecules of cyanin and 3 molecules of a bisflavone glucoside.34) They named the pigment cyanocentaurin, since it differed markedly from Bayer’s protocyanin. Later, Asen and R. M. Horowitz (1974) identified the flavone in the complex as apigenin 7-O-glucuronide-4′-O-glucoside.35) Y. Osawa (1982) compared a specimen of cyanocentaurin with that of Hayashi’s protocyanin by molecular sieving, ultracentrifugation, electrophoresis and spectrophotometry, and found no difference between the two pigments.36)
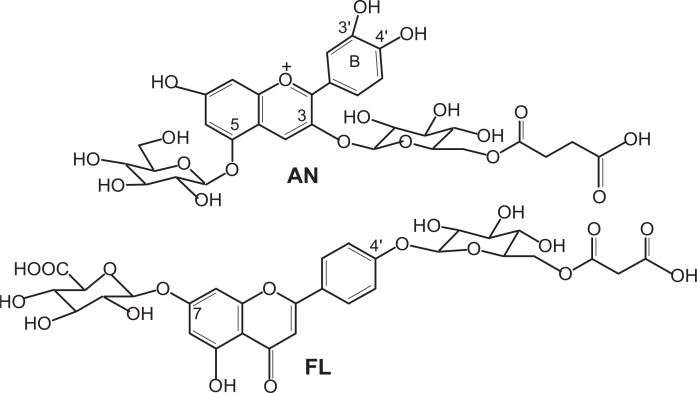
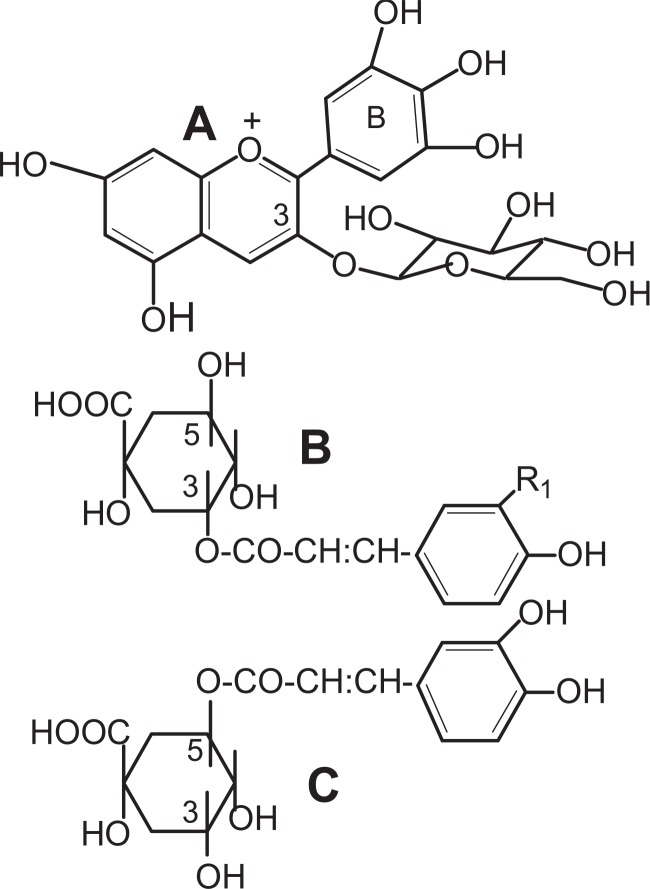
The anthocyanin in protocyanin had long been thought to be cyanidin 3-O-glucoside-5-O-glucoside (cyanin), but it was determined to be cyanidin 3-O-(6-O-succinylglucoside)-5-O-glucoside, centaurocyanin (AN)37),38) (Fig. 7). Moreover, the flavone was now identified as apigenin 7-O-glucuronide-4′-O-(6-O-malonylglucoside) (FL)38) (Fig. 7).
Fig. 7.
The structures of anthocyanin (AN) and flavone glycoside (FL) in protocyanin.
To clarify the nature of protocyanin, a reconstruction experiment was an important step as in the case of commelinin.19),20) Immediately after the success of the reconstruction of commelinin from the components, we attempted to reconstruct protocyanin from the metal ions, Fe and Mg, and the organic components, the anthocyanin and the flavone glycoside, which were prepared from purified protocyanin. However, such a stable blue complex pigment was not obtained.
Meanwhile, Kondo et al. (1994) reported reconstruction of protocyanin using the anthocyanin, the flavone glycoside, Fe2+ and Mg2+ .39) The reconstructed pigment showed the same UV-Vis and CD spectra as the natural protocyanin and moved the same distance to the anode on electrophoresis. The ratio of anthocyanin (AN):flavone glycoside (FL):Fe:Mg in the pigment was 6∼8:6∼8:1:1. They measured the molecular weight of pro-tocyanin by using electrospray ionization mass spectrometry in the negative-ion mode and observed the molecular ion at m/z 8508. The exact composition was reported to be [AN6FL6Fe3+Mg2+] (C366H384O228FeMg, MW = 8511). The iron ion in protocyanin reconstructed using Fe2+ was shown to be contained as Fe3+ by ESR and Mössbauer spectroscopies. Reconstruction using Fe3+ gave the same product. For further elucidation of the mechanism of the blue color development of protocyanin, Kondo et al. (1998) prepared metal ion replaced protocyanins, in which Mg2+ was replaced wth Mn2+, Zn2+ and Cd2+, and Fe3+ was replaced with Al3+, Ga3+, In3+ and Co3+.40) They analyzed the metal-replaced protocyanins and reconstructed protocyanin by 1H NMR spectra, UV-Vis and CD spectra, and magnetic circular dichroism spectra, and showed that protocyanin is composed of six molecules each of flavone and anthocyanin, complexed with Mg2+ and Fe3+ ions. They concluded that the blue color of protocyanin is developed by ligand-to-metal charge transfer interaction between anthocyanin and ferric ions, rather than arising from the formation of a simple anhydrobase anion of the chromophore, and a model structure of the pigment was presented.
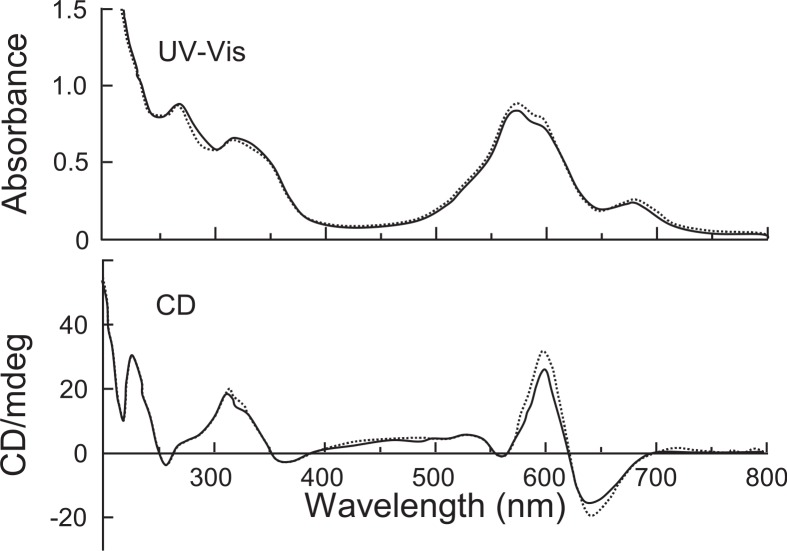
Recently, our reconstruction experiments using highly purified anthocyanin (AN), flavone glycoside (FL) and metals, Fe2+ and Mg2+, showed the presence of another factor essential for the formation of protocyanin.41) The unknown factor was revealed to be Ca2+. In fact, protocyanin was not reconstructed without Ca2+, that is, protocyanin was not obtained from the mixtures containing anthocyanin, flavone, and only Fe2+ and Mg2+ as metals. However, the yield of protocyanin increased as the amount of Ca2+ added to the reaction mixtures rose. At the approximate 3 mole ratio of Ca2+, the amount of protocyanin formed reached a maximum, and addition of an excess amount of Ca2+ decreased the yield of the blue complex pigment (Table I). These results indicated that Ca2+ is essential for the formation of protocyanin. As for the mole ratios of Fe2+ and Mg2+ added to the reaction mixtures, ratios of 0.1 for Fe2+ and 2 for Mg2+ were effective for the formation of the blue complex. After purification, reconstructed protocyanin was isolated as crystals for the first time. The reconstructed protocyanin was identical to purified protocyanin from nature as to the UV-Vis absorption spectra (λmax 267, 317, 574 and 676 nm) and CD spectra (λ vis-ext 559, 600 and 639 nm) (Fig. 8). The mole ratios of the components, anthocyanin, flavone, Fe, Mg and Ca were approximately 6:6:1:1∼2:3 in the reconstructed protocyanin and 6:6:1:2:3 in natural protocyanin, respectively.
Table I.
Formation of the blue complex pigment with anthocyanin (AN), flavone glycoside (FL), Fe2+, Mg2+ in mole ratio of 1:1:0.1:2 and Ca2+ in various mole ratios
| Mole ratio of Ca used | Blue pigment separable on Sephadex column | Amounts of the complex pigment formed (Absorbance at 574 nma) |
|---|---|---|
| 0 | trace | 0.03 |
| 1 | + | 0.74 |
| 2 | + | 0.97 |
| 3 | + | 1.30 |
| 5 | + | 1.18 |
| 6 | + | 0.69 |
Light path length of 3 mm.
Fig. 8.
UV-Vis and CD spectra of reconstructed and natural protocyanin in 0.05 M acetate buffer of pH 4.80 (light path length of 1 mm).------ natural; —— reconstructed.
Ca2+ in protocyanin could be substituted with some other bivalent metals such as Sr2+, Ba2+, Zn2+ and Cd2+. Especially Ba2+ and Sr2+, which both also belong to the alkaline earth metal group, formed stable blue complex pigments which showed a practically identical pattern of absorption spectra with protocyanin. The pigments in which Ca2+ was replaced with Ba2+ and Sr2+, were more stable than that with Ca2+. Secondly, Mg2+ in protocyanin could be substituted with Mn2+, Co2+, Ni2+, Zn2+, and Cd2+, as in the case of commelinin.20) Substitution of Fe2+ in protocyanin with Al3+ (AlCl3) as well as the above other bivalent metals was also attempted. However, a blue pigment similar to protocyanin was not obtained without Fe2+. A blue complex pigment formed using Fe3+ showed an absorption spectrum identical to that of the reconstructed protocyanin with Fe2+, as reported by Kondo et al.39)
Protocyanin is a high molecular-weight and complex pigment,32),33) and application of NMR techniques to this pigment was not practical, as the compound contains Fe3+ as an essential metal.40) Success in crystallization of reconstructed protocyanin enabled us to try X-ray crystallographic analysis for elucidation of the total structure of protocyanin. For X-ray structure determination, protocyanin and metal-substituted protocyanins, FeMgBa-, FeCdBa- and FeMnBa-protocyanins were reconstructed and isolated as crystals.42) The UV-Vis absorption spectra of reconstructed and metal-substituted protocyanins prepared were similar to that of natural protocyanin. Data sets for the crystals were collected at the Photon Factory, KEK and at Spring-8. The protocyanin crystal belongs to space group P212121 with unit cell dimensions of a=29.7 Å, b=49.2 Å and c=78.3 Å, and two protocyanin molecules are contained in an asymmetric unit. On the other hand, all the metal substituted protocyanin crystals belong to space group P6322 with cell dimensions of a=b=32.3 Å and c=28.5 Å, and contain one sixth of the protocyanin molecule, one AN and one FL, in an asymmetric unit.
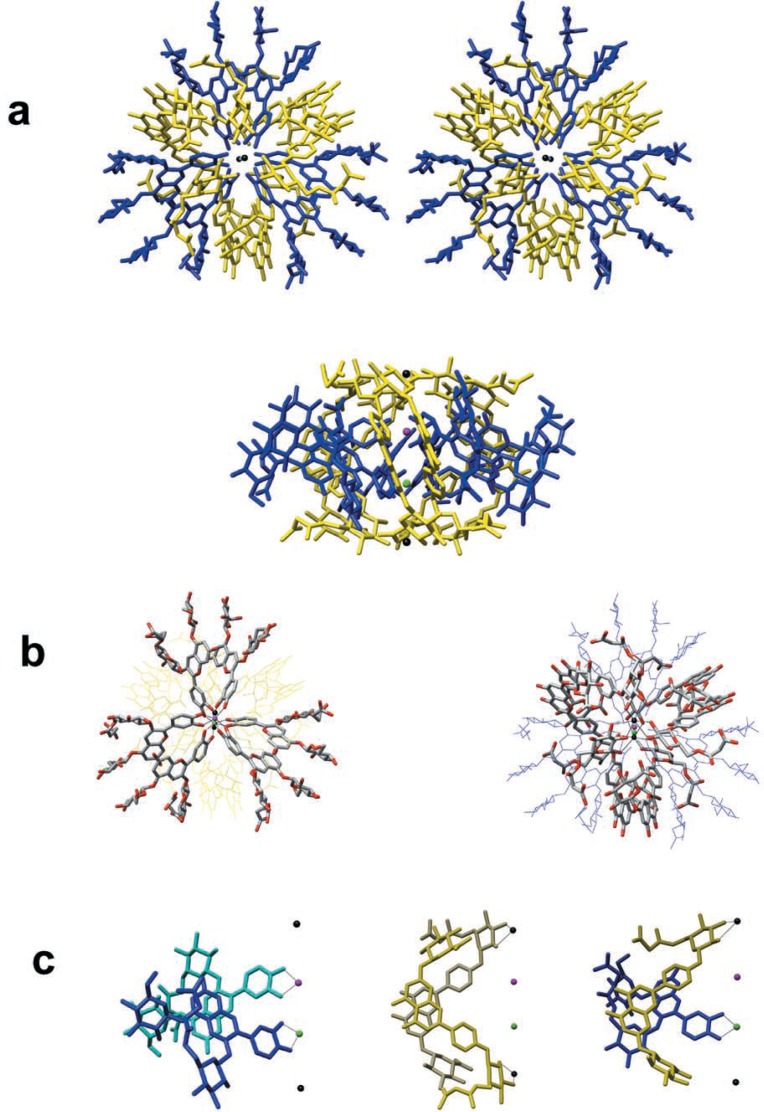
The crystal structure of the reconstructed protocyanin was determined at a resolution of 1.05 Å. The molecule has pseudo three-fold symmetry and four metal ions, Fe3+, Mg2+ and two Ca2+, align along the pseudo three-fold axis (Fig. 9a). The two sites of the inner nuclei have the same amount of electron density which appears to be the average of those of Fe3+ and Mg2+. This suggested that the sites are each occupied by Fe3+ and Mg2+ due to the random orientation of the molecule in the direction of the pseudo three-fold axis. X-ray structures of metal-substituted protocyanins, FeMgBa-, FeMnBa- and FeCdBa-protocyanins clarified the metal ions at this position. In FeMgBa-protocyanin, the amount of the electron density of the inner nuclei was almost the same as that in protocyanin (FeMgCa). On the other hand, those in FeMnBa- and FeCdBa-protocyanins were the average of Fe3+ and the substituted ions, respectively. In addition, the distances between the metal ions and the coordinating oxygen atoms of positions 3′ and 4′ of cyanidin B-ring vary according to the radii of the substituted metal ions. However, the positions of the B-ring of the cyanidin nuclei were almost the same as each other. However, the distances between the two metal ions increased along the three fold axis according to the radii of the replaced metal ions. These results indicated that the inner two metal ions are heterogeneous. This was also indicated by the anomalous dispersion measurement using FeMnBa-protocyanin.
Fig. 9.
Crystal structure of the protocyanin molecule. Blue, anthocyanin (AN); yellow, flavone glycoside (FL); red ball, Fe3+ ion; green ball, Mg2+ ion; black balls, Ca2+ ions. a, Stereo view along the pseudo three-fold axis (top) and a side view (bottom). b, Left: A diagonal view from above, emphasizing the arrangement of the six ANs which bind to Fe3+ and Mg2+ ions. The bicolour frames, grey and red, ANs. Right: A diagonal view from above, emphasizing the arrangement of the six FLs which bind to Ca2+ ions. The bicolour frames, grey and red, FLs. c, Left: A side view of stacking AN and AN. One AN binds to an Fe3+ ion, while the other binds to a Mg2+ ion. Blue, the front side AN; cyan, back side AN. Center: A side view of stacking FL and FL, which bind to Ca2+ ions. Yellow, the front side FL, khaki, the back side FL. Right: A side view of stacking FL and AN, which bind to Ca2+ and Mg2+ ions respectively.
In the protocyanin molecule, both anthocyanin (AN) and flavone (FL) are self-associated with each other as AN-AN and FL-FL in pairs. The Fe3+ and Mg2+ are each coordinated to a different AN fragment of the associated AN-AN pair (Fig. 9b, left; c left). In a similar manner, a Fe3+ ion binds with three ANs, one from each of the three AN-AN pairs, and a Mg2+ ion also binds with three ANs. Furthermore, the outer two Ca2+ are each coordinated with a separate FL fragment of the associated FL-FL pair (Fig. 9b, right; c, centre). Two Ca2+ each bind to three FLs of the three FL-FL pairs. Stacking of AN and FL, that is, copigmentation, is also present in the protocyanin molecule (Fig. 9c, right). The C-C and C-O bond lengths in the B-ring indicated that the B-ring of AN is in 4′-keto-quinoidal form.
Blue colors are developed mostly by delphinidin-type anthocyanins. In protocyanin, however, the blue color is developed by a cyanidin-type anthocyanin. The chelate formation of Fe3+ and Mg2+ with 4′-keto-quinoidal base of AN apparently plays an important role for the bluing in protocyanin. Furthermore, two Ca2+ coordinate with FLs to form the components, which bring about copigmentation as well as stabilization of the molecule. Thus, the development of the blue color in protocyanin is based on a tetra-nuclear metal complex pigment, a new type of supramolecular pigment.
Protodelphin
Another metallo-anthocyanin, protodelphin, was isolated from the blue flowers of Salvia patens by K. Takeda et al. (1994).43) The absorption spectrum of its aqueous solution showed maxima at 260, 317, 590 and 648 nm, and the pattern of the spectrum was similar to that of commelinin.12),19) The blue complex was stable in neutral or acidic conditions. Protodelphin was shown to be composed of malonylawobanin, apigenin 7,4′-O,O-diglucoside,44) in the ratio of 1:1, and Mg2+. This was confirmed by the reconstruction of protodelphin from the three components. The absorption spectrum of the reconstructed protodelphin was identical with that of the natural pigment. The yield of protodelphin was to a certain extent proportional to the amount of Mg2+ added to the reaction mixture, and the stable blue complex pigment was not obtained without Mg2+. Similar blue complexes were formed by the use of Mn2+, Co2+, Ni2+, Zn2+ and Cd2+ instead of Mg2+, as in the case of commelinin.20) T. Kondo, K. Oyama, and K. Yoshida (2001) further studied the structure of protodelphin by electron spray ionization mass spectrometry, CD and NMR spectra, and examined the formation of metal complexes using the synthetic apigenin 7,4′-diglucosides derived from D- or L-glucose.45) They concluded that the chirality of the sugar moiety is responsible for the chiral molecular recognition on the formation of a metalloanthocyanin, and that the complex consists of six anthocyanin molecules coordinated to two Mg2+ with a minus helical arrangement of six flavone glycoside molecules intercalated.
Hydrangea
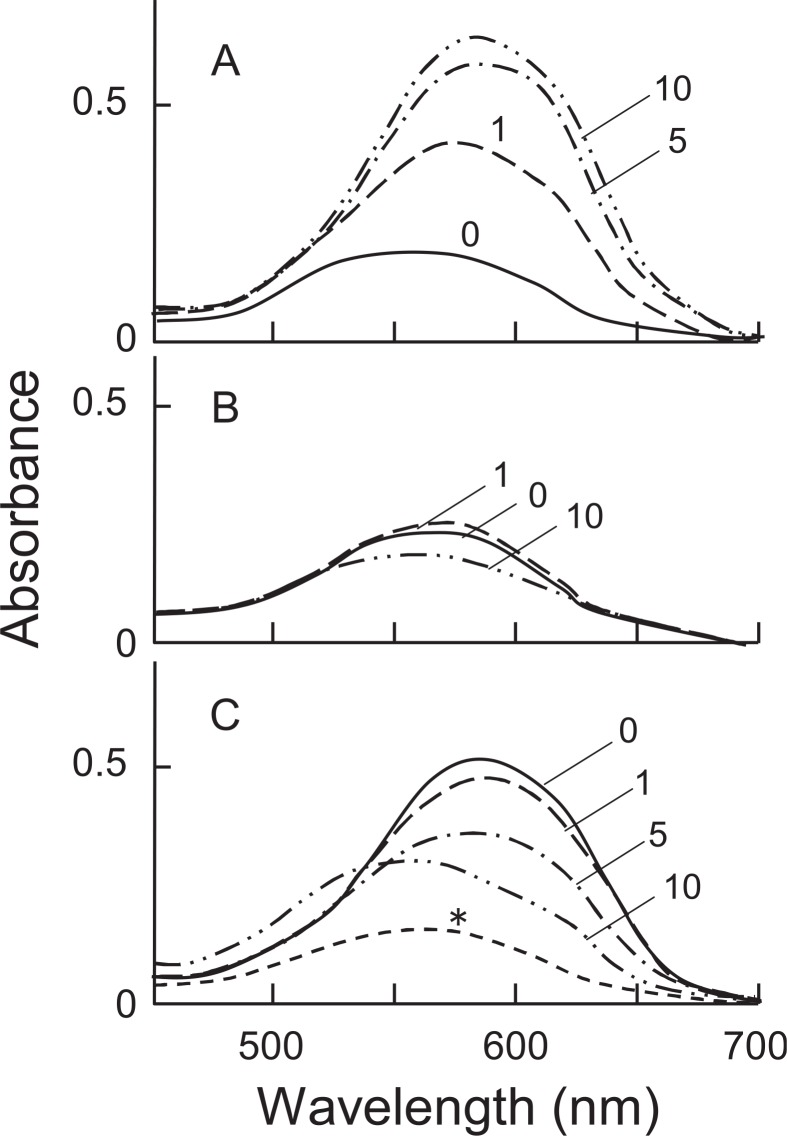
The participation of Al3+ in the bluing of hydrangea sepals is well known.46),47) However, the nature of the blue pigment remained obscure. We found the presence of copigments which show a bluing effect on the hydrangea anthocyanin.48) The anthocyanin in red and blue sepals of hydrangea was confirmed to be delphinidin 3-glucoside,49) and the copigments were identified as 3-caffeoylquinic acid (3-Caf) and 3-p-coumaroylquinic acid (3-pC) (Fig. 10). 5-Caffeoylquinic acid (chlorogenic acid, 5-Caf), which was also found in the blue sepals, however, did not show such a bluing effect though it acted as a copigment. Blue and red sepals of various hydrangea cultivars were quantitatively analyzed for Al, anthocyanin and copigments.50),51) All the blue sepals examined contained both Al and copigments, 3-Caf and 3-pC, in considerable amounts, while red sepals contained 5-Caf in large amounts rather than 3-Caf and 3-pC. In in vitro experiments, using the copigments, Al3+ and delphinidin 3-glucoside, it was shown that 3-Caf and 3-pC formed a blue complex with Al3+ and the anthocyanin (Fig. 11A). Absorption spectra of the blue complex, measured at pH 3.7, were practically identical with those of the blue solutions (pH 3.5–4.1) obtained from blue hydrangea sepals. In contrast, 5-Caf gave only a red-purple color (Fig. 11B) and the presence of a large amount of 5-Caf inhibited the formation of the blue complex (Fig. 11C). Color augmentation occurred with 3-Caf (3-pC), Al3+ and cyanidin 3-glucoside, but not with pelargonidin or malvidin 3-glucosides.52) Neither 3-Caf (3-pC) nor Al3+ independently produced blue color when mixed with the anthocyanin. The results showed that the blue color of hydrangea sepals is due to the blue complex of delphinidin 3-glucoside-Al3+-3-Caf or -3-pC, in which aluminium conjugates with the ortho-dihydroxy group of the anthocyanin B-ring, and the carboxyl and α-hydroxyl groups of the quinic acid moiety. Recently, Yoshida et al. (2003) measured the vacuolar pH of the red and blue protoplasts prepared from hydrangea sepals, using a combination of micro-spectrophotometry and a proton-selective micro-electrode, and showed that the pH values of blue (λvis-max: 589 nm) and red cells (λvis-max: 537 nm) were 4.1 and 3.3, respectively.53) The vacuolar pH in cells of blue hydrangea sepals was higher than that in cells of red sepals.
Fig. 10.
The structure of anthocyanin and copigments. A: delphinidin 3-glucoside; B: 3-caffeoylquinic acid (R=OH), 3-p-coumaroylquinic acid (R=H); C: 5-caffeoylquinic acid (Chlorogenic acid).
Fig. 11.
- Added with 3-Caf. The numbers show the mole ratio of 3-Caf to Del-3G.
- Added with 5-Caf. The numbers show the mole ratio of 5-Caf to Del-3G.
- Added with 3-Caf (8 × 10−4 M) and 5-Caf. The numbers show the mole ratio of 5-Caf to 3-Caf. *: Del-3G (10−4 M) + Al (10−4 M).
Conclusion
In this article, the studies on the metal complex pigments involved in the true blue flower color were reviewed. Since Willstätter’s findings in 1913 that the blue cornflower contains an anthocyanin, cyanin, and the same anthocyanin is contained in the red rose, many studies on flower color variations have been carried out, especially concerning the bluing of anthocyanin color in flowers. The blue pigment of the corn-flower, named protocyanin, was finally revealed by X-ray structure analysis to be a tetra-metal (Fe3+, Mg2+, 2Ca2+) nuclear complex of twelve molecules of anthocyanin and flavone glycoside, involving copigmentation and intermolecular hydrophobic association. The elucidation of the molecular structures of protocyanin and commelinin demonstrated that the true blue color in those flowers is developed by the metal complex pigments. The blue color of the blue flowers of Salvia patens and the blue sepals of Hydrangea macrophylla is also developed by metal complex pigments. Participation of the bivalent metal ions, Mg2+and Ca2+, as well as trivalent ions, Fe3+ and Al3+, in the formation of the blue color in those flowers is now clear, though studies showing the presence of metals in complex pigments had not generally been accepted for a long time.16),21) These studies established proof for the metal complex theory proposed by K. Shibata et al.5) Besides anthocyanins and metal ions, complex flower pigments contain copigments8) such as flavone glycosides and phenolic acid derivatives, which may contribute to produce the stable complexes.
On the basis of these studies, further studies on blue metal complex pigments in nature, especially molecular biological as well as biochemical approaches to this subject, would now be expected.
Acknowledgments
The author wishes to thank to Dr. C. Grayer (University of Reading) and Dr. R. J. Grayer (Royal Botanic Garden Kew) for critical reading of the manuscript. He is grateful to Dr. M. Shiono (Department of Physics, Kyushu University) and Dr. N. Matsugaki (Photon Factory, High Energy Accelerator Research organization) who have collaborated in X-ray crystallography. He acknowledges Dr. T. Iwashina and Dr. T. Konishi of the laboratory of Tsukuba Botanical Garden for giving him an opportunity to carry out part of the work at their laboratory as an invited researcher. The author’s investigations described here were accomplished with the efforts of many collaborators whose names are cited in the references.
References
- 1.Strack, D., and Wray, V. (1994) InThe Flavonoids, Advances in Research since 1986 (ed. Harborne J. B.). Chapman and Hall, London, pp. 1–22. [Google Scholar]
- 2.Brouillard, R. and Dangles, O. (1994) InThe Flavonoids, Advances in Research since 1986 (ed. Harborne J. B.). Chapman and Hall, London, pp. 565–588. [Google Scholar]
- 3.Willstätter, R., and Everest, A. E. (1913) Über den Farbstoff der Kornblume. Justus Liebigs Ann. Chem. 401, 189–232. [Google Scholar]
- 4.Willstätter, R., and Mallison, H. (1915) Über Variationen der Blütenfarben. Justus Liebigs Ann. Chem. 408, 147–162. [Google Scholar]
- 5.Shibata, K., Shibata, Y., and Kasiwagi, I. (1919) Studies on anthocyanins: Color variation in anthocyanins. J. Amer. Chem. Soc. 41, 208–220. [Google Scholar]
- 6.Everest, A. E., and Hall, A. J. (1921) Anthocyanins and anthocyanidins. Observations on (a) anthocyan colours in flowers, and (b) the formation of anthocyans in plants. Proc. Roy. Soc. London 92B, 150–162. [Google Scholar]
- 7.Robinson, G. M., and Robinson, R. (1931) A survey of anthocyanins. 1. Biochem. J. 25, 1687–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robinson, R., and Robinson, G. M. (1939) The colloid chemistry of leaf and flower pigments and the precursors of the anthocyanins. J. Amer. Chem. Soc. 61, 1605–1606. [Google Scholar]
- 9.Bayer, E. (1958) Über den blauen Farbstoff der Kornblume, I. Chem. Ber. 91, 1115–1122. [Google Scholar]
- 10.Hayashi, K. (1957) Fortschritte der Anthocyanforschung in Japan mit besonderer Berücksichtigung der papierchromatographischen Methoden. Pharmazie 12, 245–249. [PubMed] [Google Scholar]
- 11.Hayashi, K., Abe, Y., and Mitsui, S. (1958) Blue anthocyanin from the flowers of Commelina, the crystallization and some properties thereof. Proc. Jpn. Acad. 34, 373–378. [Google Scholar]
- 12.Mitsui, S., Hayashi, K., and Hattori, S. (1959) Further studies on commelinin, a crystalline blue metallo-anthocyanin from the flowers of Commelina. Proc. Jpn. Acad. 35, 169–174. [Google Scholar]
- 13.Kuroda, C. (1936) The constitution of awobanin and awobanol, the colouring matter of awobana and its co-pigment. Bull. Chem. Soc. Japan 11, 265–271. [Google Scholar]
- 14.Takeda, K., Mitsui, S., and Hayashi, K. (1966) Structure of a new flavonoid in the blue complex molecule of commelinin. Bot. Mag. Tokyo 79, 578–587. [Google Scholar]
- 15.Komatsu, M., Tomimori, T., Takeda, K., and Hayashi, K. (1968) Experiments showing the identity of swertisin and flavocommelitin. Chem. Pharm. Bull. 16, 1413–1415. [Google Scholar]
- 16.Bayer, E., Egeter, H., Fink, A., Nether, K., and Wegmann, K. (1966) Komplexbildung und Blütenfarben. Angew. Chem. 78, 834–841. [Google Scholar]
- 17.Hayashi, K., and Takeda, K. (1970) Further purification and component analysis of commelinin showing the presence of magnesium in this blue complex molecule. Proc. Jpn. Acad. 46, 535–540. [Google Scholar]
- 18.Takeda, K., and Hayashi, K. (1964) Oxidative degradation of acylated anthocyanins showing the presence of organic acid-sugar linkage in the 3-position of anthocyanidins; experiments on ensatin, awobanin and shisonin. Proc. Jpn. Acad. 40, 510–515. [Google Scholar]
- 19.Takeda, K., and Hayashi, K. (1977) Reconstruction of commelinin from its components, awobanin, flavocommelin and magnesium. Proc. Jpn. Acad., Ser. B 53, 1–5. [Google Scholar]
- 20.Takeda, K. (1977) Further experiments of synthesizing crystalline blue metallo-anthocyanins using various kinds of bivalent metals. Proc. Jpn. Acad., Ser. B 53, 257–261. [Google Scholar]
- 21.Goto, T., Hoshino, T., and Takase, S. (1979) A proposed structure of commelinin, a sky-blue anthocyanin complex obtained from the flower petals of Commelina. Tetrahedron Lett., 2905–2908. [Google Scholar]
- 22.Goto, T., Takase, S., and Kondo, T. (1978) PMR spectra of natural acylated anthocyanins: Determination of stereostructure of awobanin, shisonin, and violanin. Tetrahedron Lett., 2413–2416. [Google Scholar]
- 23.Hoshino, T., Matsumoto, U., and Goto, T. (1980) The stabilizing effect of the acyl group on the co-pigmentation of acylated anthocyanins with C-glucosylflavones. Phytochemistry 19, 663–667. [Google Scholar]
- 24.Takeda, K., and Hayashi, K. (1963) Further evidence for the new structure of violanin as revealed by degradation with hydrogen peroxide. Proc. Jpn. Acad. 39, 484–488. [Google Scholar]
- 25.Takeda, K., Narashima, F., and Nonaka, S. (1980) Participation of p-coumaroyl glucose residue of awobanin in synthesis of the complex molecule of commelinin. Phytochemistry 19, 2175–2177. [Google Scholar]
- 26.Takeda, K., Fujii, T., and Iida, M. (1984) Magnesium in the blue pigment complex commelinin. Phytochemistry 23, 879–881. [Google Scholar]
- 27.Goto, T., Kondo, T., Tamura, H., and Takase, S. (1983) Structure of malonylawobanin, the real anthocyanin present in blue-colored flower petals of Commelina communis. Tetrahedron Lett. 24, 4863–4866. [Google Scholar]
- 28.Tamura, H., Kondo, T., and Goto, T. (1986) The composition of commelinin, a highly associated metalloanthocyanin present in the blue flower petals of Commelina communis. Terahedron Lett. 27, 1801–1804. [Google Scholar]
- 29.Kondo, T., Yoshida, K., Nakagawa, A., Kawai, T., Tamura, H., and Goto, T. (1992) Structural basis of blue-colour development in flower petals from Commelina communis. Nature 358, 515–518. [Google Scholar]
- 30.Nakagawa, A. (1993) X-ray structure determination of commelinin from Commelina communis and its blue-color development. J. Cryst. Soc. Japan 35, 327–333. [Google Scholar]
- 31.Hayashi, K., Saito, N., and Mitsui, S. (1961) On the metallic components in newly crystallized specimen of Bayer’s protocyanin, a blue metallo-anthocyanin from the cornflower. Proc. Jpn. Acad. 37, 393–397. [Google Scholar]
- 32.Saito, N., Mitsui, S., and Hayashi, K. (1961) Further analysis of organic and inorganic components in crystalline protocyanin. Proc. Jpn. Acad. 37, 485–490. [Google Scholar]
- 33.Saito, N., and Hayashi, K. (1965) Contribution to the structure studies of protocyanin, a blue metallo-anthocyanin from the cornflower, with special regard to the spectral change before and after treatment with ion-exchangers. Sci. Rep. Tokyo Kyoiku Daigaku, Sec. B 12, 39–54. [Google Scholar]
- 34.Asen, S., and Jurd, L. (1967) The constitution of a crystalline, blue cornflower pigment. Phytochemistry 6, 577–584. [Google Scholar]
- 35.Asen, S. and Horowitz, R. M. (1974) Apigenin 4′-O-β -D-glucoside 7-O-β -D-glucuronide: The copigment in the blue pigment of Centaure cyanus. Phytochemistry 13, 1219–1223. [Google Scholar]
- 36.Osawa, Y. (1982) InAnthocyanins as Food Colors (ed. Markakis P.). Academic Press, New York, pp. 41–68. [Google Scholar]
- 37.Takeda, K., and Tominaga, S. (1983) The anthocyanin in blue flowers of Centaurea cyanus. Bot. Mag. Tokyo 96, 359–363. [Google Scholar]
- 38.Tamura, H., Kondo, T., Kato, Y., Goto, T. (1983) Structures of a succinyl anthocyanin and a malonyl flavone, two constituents of the complex blue pigment of cornflower Centaurea cyanus. Tetrahedron Lett. 24, 5749–5752. [Google Scholar]
- 39.Kondo, T., Ueda, M., Tamura, H., Yoshida, K., Isobe, M., and Goto, T. (1994) Composition of protocyanin, a self-assembled supramolecular pigment from the blue cornflower, Centaurea cyanus. Angew. Chem. Int. Ed. Engl. 33, 978–979. [Google Scholar]
- 40.Kondo, T., Ueda, M., Isobe, M., and Goto, T. (1998) A new molecular mechanism of blue color development with protocyanin, a supramolecular pigment from cornflower, Centaurea cyanus. Tetrahedron Lett. 39, 8307–8310. [Google Scholar]
- 41.Takeda, K., Osakabe, A., Saito, S., Furuyama, D., Tomita, A., Kojima, Y., Yamadera, M., and Sakuta, M. (2005) Components of protocyanin, a blue pigment from the blue flowers of Centaurea cyanus. Phytochemistry 66, 1607–1613. [DOI] [PubMed] [Google Scholar]
- 42.Shiono, M., Matsugaki, N., and Takeda, K. (2005) Structure of the blue cornflower pigment. Nature 436, 791. [DOI] [PubMed] [Google Scholar]
- 43.Takeda, K., Yanagisawa, M., Kifune, T., Kinoshita, T., and Timberlake, C. F. (1994) A blue pigment complex in flowers of Salvia patens. Phytochemistry 35, 1167–1169. [Google Scholar]
- 44.Veitch, N. C., Grayer, R. J., Irwin, J. L., and Takeda, K. (1998) Flavonoid cellobiosides from Salvia uliginosa. Phytochemistry 48, 389–393. [Google Scholar]
- 45.Kondo, T., Oyama, K., and Yoshida, K. (2001) Chiral molecular recognition on formation of a metalloanthocyanin: A supramolecular metal complex pigment from blue flowers of Salvia patens. Angew. Chem. Int. Ed. 40, 894–897. [DOI] [PubMed] [Google Scholar]
- 46.Allen, R. C. (1943) Influence of aluminum on the flower color of Hydrangea macrophylla DC. Contrbs. Boyce Thompson Inst. 13, 221–242. [Google Scholar]
- 47.Asen, S., and Siegelman, H. W. (1957) Effect of aluminum on absorption specta of the anthocyanin and flavonols from sepals of Hydrangea macrophylla var. Merveille. Proc. Amer. Soc. Hort. Sci. 70, 478–481. [Google Scholar]
- 48.Takeda, K., Kubota, R., and Yagioka, C. (1985) Copigments in the bluing of sepal colour of Hydrangea macrophylla. Phytochemistry 24, 1207–1209. [Google Scholar]
- 49.Robinson, G. M. (1939) Notes on variable colors of flower petals. J. Amer. Chem. Soc. 61, 1606–1607. [Google Scholar]
- 50.Takeda, K., Kariuda, M., and Itoi, H. (1985) Blueing of sepal colour of Hydrangea macrophylla. Phytochemistry 24, 2251–2254. [Google Scholar]
- 51.Takeda, K., Kato, Y., and Iwata, K. (1990) Blueing of the flower colour of Hydrangea macrophylla. Proc. 15th Internat. Conf. Group Polyphenols 15, 25–28. [Google Scholar]
- 52.Takeda, K., Yamashita, T., Takahashi, A., and Timberlake, C. F. (1990) Stable blue complexes of anthocyanin-aluminium-3-p-coumaroyl- or 3-caffeoyl-quinic acid involved in the blueing of hydrangea flower. Phytochemistry 29, 1089–1091. [Google Scholar]
- 53.Yoshida, K., Toyama-Kato, Y., Kameda, K., and Kondo, T. (2003) Sepal color variation of Hydrangea macrophylla and vacuolar pH measured with a proton-selective micro-electrode. Plant Cell Physiol. 44, 262–268. [DOI] [PubMed] [Google Scholar]