Abstract
Subject PI demonstrated superior memory using a variant of a Method of Loci (MOL) technique to recite the first digits of the mathematical constant π to more than 216 decimal places. We report preliminary behavioral, functional magnetic resonance imaging (fMRI), and brain volumetric data from PI. fMRI data collected while PI recited the first 540 digits of π (i.e., during retrieval) revealed increased activity in medial frontal gyrus and dorsolateral prefrontal cortex. Encoding of a novel string of 100 random digits activated motor association areas, midline frontal regions, and visual association areas. Volumetric analyses indicated an increased volume of the right subgenual cingulate, a brain region implicated in emotion, mentalizing, and autonomic arousal. Wechsler Abbreviated Scale of Intelligence (WASI) testing indicated that PI is of average intelligence, and performance on mirror tracing, rotor pursuit, and the Silverman and Eals Location Memory Task revealed normal procedural and implicit memory. PI’s performance on the Wechsler Memory Scale (WMS-III) revealed average general memory abilities (50th percentile), but superior working memory abilities (99th percentile). Surprisingly, PI’s visual memory (WMS-III) for neutral faces and common events was remarkably poor (3rd percentile). PI’s self-report indicates that imagining affective situations and high emotional content is critical for successful recall. We speculate that PI’s reduced memory for neutral/non-emotional faces and common events, and the observed increase in volume of the right subgenual cingulate, may be related to extensive practice with memorizing highly emotional material.
Keywords: Memory, fMRI, Mnemonics, Method of loci, Brain, Emotion
INTRODUCTION
Despite popular fascination with individuals who perform unusual mnemonic feats, scientific investigations of the neural basis of human memory have focused primarily on examples of impaired, rather than superior, memory (Maguire, Valentine, Wilding, & Kapur, 2003; Tanaka, Michimata, Kaminaga, Honda, & Sadato, 2002; Wilding & Valentine, 1997). While individuals who can effortlessly recall large quantities of information may seem to possess generally superior memories (Ericsson & Chase, 1982), the available evidence indicates that superior memorists typically display superior memory only for relatively inconsequential material, such as strings of digits (Ericsson, 1985). Moreover, memory for digit recall can be dramatically improved in most people through mnemonic training (Chase & Ericsson, 1981; Ericsson 2003). Although some individuals show superior memory even in the apparent absence of an encoding strategy (Wilding & Valentine, 1997), the majority of the evidence suggests that a central component of a superior memory is the development of mnemonic skills through practice (Ericsson 2003; Wilding & Valentine, 1997).
One well-known mnemonic strategy is the Method of Loci (MOL) (Bellezza, 1981; Bower, 1970). MOL is a visuospatial mnemonic strategy in which items to be remembered are associated with specific objects or landmarks within a familiar physical space. This strategy has a long history: ancient orators used it to remember lengthy speeches (Yates, 1966). MOL combines the use of information organization, visualization, and association. One first identifies a vivid visual memory of a familiar path, as well as the objects and landmarks along that path. One then associates specific information from the material to be remembered with each object or landmark. Later, by revisualizing the objects and landmarks, one can enhance the recall of the associated information. Variations on this theme abound and are typically adapted to the preferences of individual memorists. The common element across these variations of MOL is the detailed visualization that is linked to recall. Behavioral findings suggest that MOL may dramatically improve memory performance (Bellezza & Reddy, 1978; Groninger, 1971; Ross & Lawrence, 1968).
Some functional neuroimaging studies have examined brain activations associated with the use of memory strategies (Bor, Duncan, Wiseman, & Owen, 2003; de Zubicaray, Zelaya, Andrew, Williams, & Bullmore, 2000; Maestu et al., 2003; Savage et al., 2001), but only more recently has this effort focused on MOL (Kondo et al., 2005). One neuroimaging study used functional magnetic resonance imaging (fMRI) to compare the memory-related brain activity in a group of 10 world-class memory performers with the activity in a matched control group (Maguire et al., 2003). Subjects were asked to memorize digits, faces, and images of snowflakes. Whereas no control subjects reported using a mnemonic strategy, all of the superior memorists reported using MOL. While processing digits, the expert group showed increased fMRI signal, relative to control subjects, in brain regions implicated in associative learning, including the posterior hippocampus, the medial parietal cortex, and the retrosplenial cortex. This study, however, could not discern whether group differences were related to expert use of a memory strategy or to naturally superior memory abilities in the expert group.
More recently, another study reported on the neural activity associated with use of the MOL strategy in initially naïve subjects (Kondo et al., 2005). The study, which complemented the previous one (Maguire et al., 2003), examined brain activity during encoding and retrieval of pictures of animate and inanimate objects, both before and after subjects were instructed in MOL. Researchers found that, relative to encoding before MOL, this strategy led to improved recall and was associated with enhanced activity during recall in the frontal cortex, fusiform gyrus, and lingual gyrus during encoding, as well as with enhanced parahippocampal, lingual, and fusiform gyrus activity. These findings suggest that successful use of a mnemonic strategy involves increased recruitment of attentional processes within frontal regions, as well as visualization processes within ventral visual regions. Previously naïve subjects therefore may attain improved memory performance when engaging these neural systems.
Individual cases can be an interesting bridge between healthy and abnormal psychological processes (Luria, 1968). We report behavioral, volumetric, and fMRI data from PI, who as a young adult demonstrated superior memory by reciting the first digits of the mathematical constant π to more than 216 decimal places with fewer than 24 mistakes. Previous experimental reports of π recitation focused on Mr. Rajan Mahadevan, who was once the holder of a Guinness Book record for flawlessly reciting π to over 30,000 decimals, and more recently on the synesthete (i.e., an individual in whom stimuli in one sensory domain evoke perceptions in multiple domains), ‘Arithmos’, who holds the European record for memorizing π to 22,500 decimal places (Azoulai, Hubbard, & Ramachandran, 2005; Ericsson, Delaney, Weaver, & Mahadevan, 2004; Thompson, Cowan, & Frieman, 1993).
We used fMRI to address two questions. First, we assessed which brain areas were used by PI to retrieve the first 540 digits of π. Second, we explored which regions were active while PI used MOL, or at least a self-developed variation thereof, to encode a new sequence of 100 random digits for later recall. Comparing the encoding of new material with the reciting of old information served a dual purpose: to explore the formation of new episodic memories compared to the retrieval of older semantic memories in a superior memorist, as well as to observe the effects of an increasing memory load. Our findings represent a preliminary glimpse into the neural circuits that are involved in this individual and suggest future directions for investigating both the neural substrates of superior memorists and the functioning of working memory and mnemonics.
METHODS
Subject
PI participated in this study at the age of 22 years. A healthy, unremarkable student of mechanical engineering, PI is right-handed with no prior diagnosis of either psychiatric or neurological illness. PI reports to have studied the classical MOL technique from various known sources and to have perfected it (hereafter PI-MOL). PI provided informed written consent and was paid ($60 as well as $20 per hour for fMRI scans and behavioral tests, respectively) for participation in this study.
Experiment 1
This study was designed to identify the brain regions PI used to recall and recite the digits of π. PI was scanned during 3 task conditions. In the first condition, PI recited the initial 540 digits of π, which had been memorized earlier. The second condition served as a contrast for the first condition and consisted of counting upward beginning from zero. Because recitation of π decimals was only by single digits, PI was instructed to recite separately each digit in double-digit numerals while counting (e.g., PI recited the number ‘10’ as ‘one, zero’). This task controlled for the recall and recitation of single digits from long-term memory, although its memory demands were slight compared to those required for the recall of the decimal digits of π. In the third condition, PI simply repeated the number ‘1’ over and over. This procedure controlled for the motor and timing demands required to recite single digits, while placing minimal demands on short- and long-term memory systems. This third condition served as a contrast for the second condition, and the second and third conditions taken together helped us to interpret better the contrast between the first two conditions.
PI recited the digits for 2 min without interruption, for each of the three conditions; each run of the experiment lasted for 6 min. The order of the 3 conditions was counterbalanced across runs. If unable to finish the sequence of 540 digits while reciting π in any of the 2-min blocks, PI was instructed to resume recitation where left off on the next 2-min block for condition 1. If finished the string of 540 digits within a previous block, PI was instructed to start the sequence again from the beginning.
Experiment 2
This study was designed to identify the brain regions that PI used to learn a novel sequence of random numbers. PI was scanned under 2 conditions. In the first condition, PI learned a sequence of 100 single digits obtained from a uniform random number generator. These numbers were presented in 10 rows of 10 numbers each that were positioned on a back-lit projection screen placed at the subject’s feet and viewed through the scanner’s head coil. PI reported that memorization of the sequence by the end of the third run of the experiment. Learning of the random sequence was confirmed immediately after the experiment, when PI recited the sequence without error. The second condition of Experiment 2 was the same as the second condition of Experiment 1.
PI recited the digits for 2 min without interruption, for each condition in each run of the experiment, which lasted 8 min in total. The order of the 2 conditions was counterbalanced across runs. If reciting the random sequence in any of the 2-min blocks did not finish, PI was instructed to resume recitation where left off on the next 2-min block for condition 1. If reciting the string of 100 digits within a previous block was complete, PI was instructed to start the sequence anew, from the beginning.
In each experiment, PI was also instructed to fix gaze on a cross-hair presented at the center of a projector screen viewed through a mirror mounted on the head coil. We instructed PI to subvocalize the digits through lightly closed teeth to minimize motion and susceptibility artifacts. Finally, PI was instructed to recite the digits in the control conditions (i.e., in the second and the third conditions of Experiment 1 and in the second condition of Experiment 2) at the same rate used for the numbers of the first condition in each experiment.
Auxiliary tests
Additional informal tests to examine visual memory were administered to ascertain stability of results.
Image acquisition
Images were acquired on a GE Signa 1.5 Tesla LX scanner (Milwaukee, WI) equipped with a quadrature head coil and echoplanar capability. The subject’s head was positioned in the magnet using the canthomeatal line.
Functional images
A T1-weighted sagittal localizing scan was used to position 14 axial images according to 14 axial sections of the Talairach coordinate system (Talairach & Tournoux, 1988). The slices were positioned with 5 slices below, 8 slices above, and 1 slice containing the anterior commissure-posterior commissure (AC-PC) line. Slice thickness was 7 mm, with a skip of 1.4 mm between slices to maintain correspondence with the Talairach coordinate system.
The functional images were obtained with a gradient echo, echo-planar imaging pulse sequence. Repetition Time was 1650 ms in Study 1 and 1750 ms in Study 2, and in both studies, Echo Time = 60 ms, flip angle = 60 degrees, single excitation per image, 20 × 20 cm field of view, and 64 × 64 matrix, providing a 3.1 × 3.1-mm in-plane resolution. During each of the 7 runs of Experiment 1, 219 echoplanar images were acquired in each slice, providing 511 images for each condition in each imaging plane. During each of 8 runs of Experiment 2, 274 echoplanar images were acquired in each slice, providing 1096 images for each condition in each imaging plane.
Anatomical images
High-resolution, T1-weighted anatomical scans for volumetric measurements were acquired using a sagittal spoiled gradient recall sequence (repetition time = 24 ms, echo time = 5 ms, flip = 45°, frequency encoding superior/inferior, no wrap, 256 × 192 matrix, field of view = 30 cm, 2 excitations, slice thickness = 1.2 mm, 124 contiguous slices).
Functional image processing
The images were visually inspected to ensure that the subject moved no more than 0.25 pixels in any direction during the study. SPM99 was used to remove from each pixel’s time course the correlations pertaining to first- and second-order motion in the x-, y-, and z-directions (Friston, Williams, Howard, Frackowiak, & Turner, 1996). Drift of baseline image intensity was removed using an 8th-order high-pass Butterworth filter with a frequency cutoff equal to ¾ of the task frequency. The time series were filtered once forward and once backward to ensure no change in phase of the signal in relation to the phase of the task. Low-intensity voxels outside of the brain were removed, and the images were spatially smoothed using a Gaussian filter with a full width at half maximum of 6.3 mm. fMRI signal differences between the conditions of each experiment were calculated as a t-statistic at each pixel of the subject’s scans. The resulting t-maps were then thresholded at p < 1.369E-09 to correct for multiple statistical comparisons. For Experiment 2, t-maps were constructed separately for the first 4 runs and last 4 runs of the experiment (corresponding with the times before and after the subject reported having committed the sequence to memory). fMRI signal differences between these 2 sections of the experiment were contrasted with one another to identify differences in regional brain activity between early and later learning. This contrast map was thresholded at a p < 3.272E-05.
Anatomical image processing
The methods for region definition of cortical, entricular, amygdala, hippocampus, and basal ganglia regions are described elsewhere (Peterson et al., 2003). Using statistical analyses in SAS v.9.1 (SAS Institute Inc., Cary, NC), we compared PI’s structural data with that of a sample drawn from our imaging database, which contains demographic information and detailed volumetric and morphological measures of precisely defined brain regions from 53 healthy subjects (18–35 years of age; 24 female, 29 male). Our imaging database also includes measures of whole brain volume (WBV) as well as of itemized volumetric information for the brainstem, dorsolateral prefrontal cortex (DLPFC), premotor region (PM), sensorimotor region (SM), parietooccipital area (Pariet), orbitofrontal cortex (OFC), subgenual region (SubGen), mid temporal area (Temp), inferior occipital cortex (InfOcc), amygdala (Amy), hippocampus (Hipp), thalamus (Thal), and cerebellum (Cereb) on both the left (L) and right (R) hemispheres (see Table 3). All volumes were independently entered as dependent variables in a linear regression. To correct for scaling effects, WBV, sex, and age (at the time of the MRI scan) were entered as independent variables. For each region, the parameter estimates were obtained from the linear regression to predict PI’s volume by using the following model:
TABLE 3.
Comparisons of PI’s neuroanatomical volumetric data to that of a matched sample (53 healthy subjects; 18–35 years of age; 24 female, 29 male) at upper and lower 95% prediction intervals based on two statistical models
| Anatomical region | PI’s raw measurement | Model’s prediction | Standard error | Moderate confidence intervals
|
Conservative confidence intervals
|
|||
|---|---|---|---|---|---|---|---|---|
| Lower limit | Upper limit | Lower limit | Upper limit | |||||
| L_DLPFC | 31760 | 31315 | 826.68 | 29651 | 32979 | 24469 | 38162 | |
| R_DLPFC | 33848 | 32286 | 661.02 | 30955 | 33616 | 26812 | 37760 | |
| L_PM | 32966 | 33035 | 902.40 | 31218 | 34851 | 25561 | 40508 | |
| R_PM | 36564 | 32950 | 883.52 | 31172 | 34728 | 25633 | 40267 | |
| L_SM | 38579 | 38897 | 684.27 | 37520 | 40275 | 33230 | 44564 | |
| R_SM | 41825 | 38878 | 690.01 | 37489 | 40267 | 33164 | 44593 | |
| L_Pariet | 80927 | 85035 | 1608 | 81795 | 88274 | 71977 | 98092 | |
| R_Pariet | 82863 | 89863 | 1713 | 86412 | 93313 | 75956 | 103770 | |
| L_OFC | 7972 | 8118 | 514.82 | 7082 | 9155 | 3855 | 12382 | |
| R_OFC | 10786 | 8601 | 475.90 | 7643 | 9558 | 4659 | 12542 | |
| L_SubGen | 20039 | 16611 | 611.04 | 15382 | 17841 | 11551 | 21672 | |
| ➝ | R_SubGen | 23108 | 17553 | 590.73 | 16363 | 18742 | 12660 | 22445 |
| L_Temp | 18476 | 20208 | 524.32 | 19153 | 21263 | 15866 | 24550 | |
| R_Temp | 22716 | 20266 | 480.80 | 19298 | 21233 | 16284 | 24248 | |
| L_InfOcc | 36367 | 32817 | 1110 | 30582 | 35052 | 23808 | 41826 | |
| R_InfOcc | 37004 | 32661 | 1292 | 30059 | 35262 | 22176 | 43146 | |
| L_Hipp | 3095 | 3043 | 111.66 | 2817 | 3268 | 2216 | 3869 | |
| R_Hipp | 3485 | 3104 | 106.76 | 2888 | 3320 | 2314 | 3894 | |
| L_Amy | 1585 | 1920 | 117.24 | 1683 | 2157 | 1052 | 2787 | |
| R_Amy | 1507 | 2060 | 113.51 | 1830 | 2289 | 1220 | 2900 | |
| L_Thal | 5539 | 5879 | 252.94 | 5369 | 6389 | 3807 | 7951 | |
| R_Thal | 5927 | 6211 | 221.79 | 5764 | 6658 | 4394 | 8028 | |
| L_Cereb | 72849 | 66504 | 1191 | 64109 | 68898 | 56563 | 76444 | |
| R_Cereb | 65520 | 62772 | 1248 | 60263 | 65280 | 52360 | 73183 | |
| Brainstem | 24566 | 23568 | 661.58 | 22235 | 24901 | 18148 | 28988 | |
| WBV | 1285913 | 1378128 | 21001 | 1335925 | 1420330 | 1173045 | 1583210 | |
The Moderate Confidence Intervals (MCI) model predicts brain volumes for PI; the Conservative Confidence Intervals (CCI) model does so for a future observation (see Anatomical Image Processing section in the Methods). Both the Model’s Prediction and Standard Error columns are based on the MCI model. PI’s Raw Measurements, together with the confidence interval that they exceed, are highlighted in gray and black to indicate statistical significance for MCI and CCI, respectively. Both MCI and CCI determine increased volume only for the right subgenual region of the cingulate gyrus – an anatomical area involved in mentalizing, emotional processing, and autonomic arousal.
To compare PI’s anatomical data with that of our sample, we computed lower and upper 95% confidence limits using two methods. In the first, less-conservative method, we estimated variance using, var(V̑) = xT var(β̑)x, where xT = (1,PI’s age,PI’s sex,PI’s WBV). In the second, more conservative method, we estimated variance using var(V−V̑) = σ2 + xT var(β̑)x, where σ2 is the variance of measurement error in the linear regression. Whereas the Moderate Confidence Interval (MCI) method predicts brain volumes for PI, the Conservative Confidence Interval (CCI) method does so for a future observation (Kleinbaum, Kupper, Muller, & Nizam, 1998). In other words, MCI yields a confidence interval for the subpopulation, but CCI yields a prediction interval for a value to be drawn at random from the subpopulation. CCI limits are always wider than MCI’s, because MCI limits account only for variability in V̑, whereas CCI limits accommodate variability in both V̑ and in the future value of V. This is true even though V̑ is used as an estimate of the subpopulation mean as well as a predictor of the future value.
To establish statistical significance, the veridical values of PI’s regional volumes were compared with the confidence intervals drawn from the sample. We repeated the same analyses for WBV, with the exception that age and sex were entered as covariates, and WBV as a response. Our previous morphometric studies show that ethnic variation does not influence these anatomical measurements (Peterson et al., 2003).
Behavioral data
We used a number of standardized tests to characterize PI’s performance relative to comparable controls. The Wechsler Abbreviated Scale of Intelligence (WASI) and Wechsler Memory Scale –Revised (WMS-III) provided standard measures of PI’s general intelligence and declarative memory function, respectively. In addition, the Silverman and Eals Location Memory Task (Eals & Silverman, 1994; Silverman & Eals, 1992), Rotor Pursuit, and Mirror Tracing were administered to provide information about implicit and procedural learning and memory processes that may also characterize PI.
Implicit memory for objects and their locations was assessed using the Silverman and Eals Location Memory Task, which consists of one stimulus card and two response cards. The stimulus card depicts a spatial array of 27 common objects. One response card measures memory for object identities and depicts an array of the original 27 objects in their original locations and 20 added objects. The other response card measures memory for object location and depicts the original 27 objects with an exchange of position between 7 pairs of objects. The stimulus card is presented to the subject with no explicit instruction for the subject to note object locations. After 1 min, the first response card is presented and participants are told to mark any added objects (the object identity task). After completing that task, the second response card is presented and participants are told to mark any objects that have been moved to new locations or places on the card (the object location task). For each of the two tasks, errors include total omissions (false negatives) and total commissions (false positives). Accuracy (proportion correct) is calculated as 1−(omissions+commissions)/N.
Procedural learning processes were assessed with the Pursuit Rotor (Model 200010A, Lafayette Instruments) and Automatic Mirror Tracer (Model 58024A, LaFayette Instrument Company). The pursuit rotor task measures the ability to maintain contact between a stylus held in the preferred hand and a small target displayed on a rotating turntable. The task was divided into two trial blocks, each consisting of eight 20-s trials and a 20-s inter-trial interval. For each trial, scores could range from 0 to 2000 ms. Reminiscence, a measure of the consolidation of learning, was calculated as the difference between performance immediately before the rest (i.e., trial 8 of Block 1) and that after the rest (trial 1 of Block 2). The Mirror Tracer is an apparatus consisting of a mirror, a star pattern, and a metal screen. A stylus is used to trace the black star pattern that is hidden by the metal screen, using only the visual feedback that is provided by the mirrored image. The mirror-tracing task was divided into two trial blocks, each consisting of five trials. For each block of trials, the number of errors and the amount of time (ms) to complete the task was recorded.
RESULTS
Tables 1 and 2 show a comparison of PI’s behavioral data to that of a matched sample on a battery of standard tests examining implicit and procedural learning, and declarative memory, respectively.
TABLE 1.
Comparison of PI’s performance to matched controls on the Silverman, Rotor Pursuit, and Mirror Tracing tasks
| Task | PI | Group (18–25 years of age)
|
||
|---|---|---|---|---|
| Average | Range | No. of subjects | ||
| Silverman Task (proportion correct) | ||||
| Explicit/object task | .91 | .916 | .36–1.00 | 12 |
| Implicit/location task | .67 | .765 | .59–.93 | 12 |
| Rotor Pursuit | ||||
| Block2–Block1 Contact time (ms) | 2968 | 3785 | 1325–8718 | 6 |
| Mirror Tracing | ||||
| Block 1, errors | 4 | 32.3 | 1–69 | 6 |
| Block 1, ms | 267 | 239.3 | 89–357 | 6 |
| Bock 2, errors | 3 | 4.67 | 0–12 | 6 |
| Block 2, ms | 131 | 141.3 | 89–212 | 6 |
TABLE 2.
Comparison of PI’s performance to matched controls on the Wechsler Memory Scale - Revised (WMS-III)
| Immediate Tests
|
Delayed Memory Tests
|
General memory | Working memory | ||||
|---|---|---|---|---|---|---|---|
| Auditory | Visual | Memory | Auditory | Visual | Auditory recognition | ||
| 130 (121–134) | 71 (66–86) | 103 (95–111) | 117 (106–124) | 68 (63–83) | 120 (104–126) | 100 (92–108) | 155 (138–157) |
| 98 % | 03% | 58% | 87% | 02% | 91% | 50% | 99.9% |
Each data line shows PI’s index scores (which are measure equivalents of IQ scores), 95% confidence intervals, and percentiles, respectively.
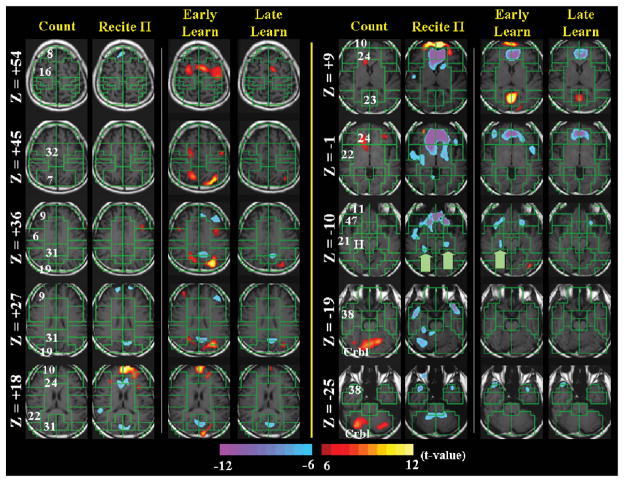
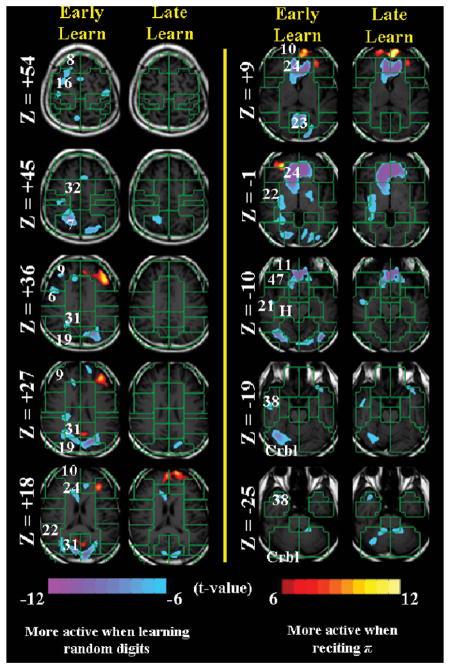
Figures 1–3 summarize the fMRI findings. Whereas Figure 1 shows the four experimental conditions, Figures 2 and 3 present direct statistical comparisons of early vs. late learning and of π recitation vs. both early and late recall of the 100 random digits, respectively.
Figure 1.
fMRI signal as a function of four experimental conditions at t>6. Brain slices are parallel to the anterior commissure–posterior commissure (AC-PC) line (Z = 0) and start at the top of the brain (top line left of the bold yellow line) in descending order (crossing over to the right hand side of the bold yellow line following Z = 18).
Figure 3.
A direct statistical comparison of π recitation (Experiment 1) vs. both early (first 4 runs of Experiment 2) and late (remaining 4 runs) recall of the 100 random digits at t > 6.
Figure 2.
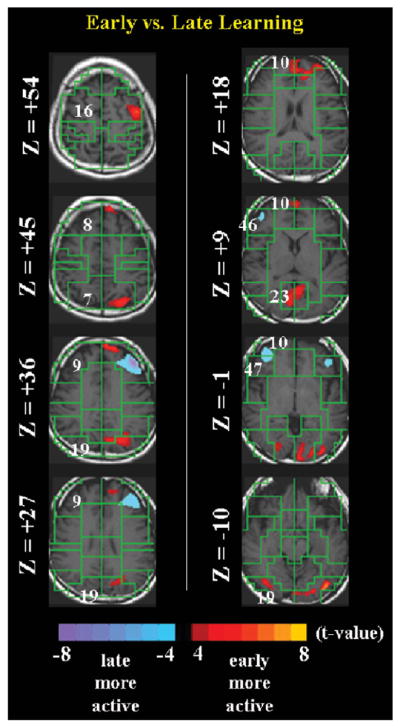
A direct statistical comparison of early (first 4 runs of Experiment 2) vs. late (remaining 4 runs of Experiment 2) learning at t>4.
Table 3 shows a comparison of PI’s neuroanatomical volumetric data to that of a comparable control group.
DISCUSSION
Behavioral data
The behavioral data show that PI’s most obvious strength was an exceptional working memory: score for working memory fell above the 99th percentile. PI’s general memory, in contrast, tested at the 50th percentile. (Table 2 shows PI’s Index scores, which are measure equivalents of IQ scores.) This result is perhaps not surprising because most tests of working memory use digits wherein PI may have applied a variation of the MOL. PI’s performance on the test of implicit memory (Silverman and Eals task) and procedural learning (mirror tracing and rotor pursuit tasks) was comparable to that of an age-matched group. This result is perhaps not unexpected because other mnemonists were not good at all types of memory, but PI’s superior performance on working memory subtests was consistent with previous reports of the working memory of superior memorists (Ericsson et al., 2004; Ishikawa, McGaugh, Sakata, & Nihon, 1996; Maguire et al., 2003; Presenti et al., 2001; Tanaka et al., 2002; Thompson et al., 1993).
On the other hand, PI’s scores for visual memory are most unusual. That a superior memorist would have such poor memory measures in any domain is extraordinary. Indeed, although interpreting these visual memory findings is difficult –after all, PI’s memory is highly eidetic, in line with MOL – related occurrences are not uncommon in the literature. For example, Alexander Luria spent 30 years studying Mr S, a synesthete who had a uniquely and astoundingly retentive memory, but who was virtually paralyzed when it came to understanding poetry, metaphorical thinking, or remembering words whose sound did not fit their meaning (Luria, 1968).
The MOL variant employed by PI involves the creation of images (including faces) and events that have highly emotional or affective content, followed by generation and rehearsal of these materials over several months prior to recall. Interestingly, findings from the Wechsler Memory Scale (WMS) demonstrated that PI had difficulty with neutral facial expressions and graphic depictions of common, everyday events. Thus, PI’s poor visual memory performance on these test items may relate to their relative lack of emotional valence. If so, these findings would suggest that PI’s extensive practice at processing extremely emotional material may have ultimately impaired encoding or recalling more neutral items and events.
fMRI data
Recall of the first 540 digits of π, compared to counting numbers in order, produced increased activity in the medial frontal gyrus (Broadman’s Area (BA) 10), as well as modest activation in the dorsolateral prefrontal cortex (BA 9) (Figure 1). In addition, decreased activity was seen in the ventral anterior cingulate cortex (BA 24), ventromedial prefrontal cortex (BA 11, 47), posterior cingulate (BA 31), and bilateral hippocampus (green arrows at Z = −10) during π recitation compared with counting.1 In accord with recent accounts, these relative ‘deactivations’ in the more engaging recall condition may reflect task-independent decreases in these regions during attention-demanding tasks (Greicius & Menon, 2004; Shulman et al., 1997). Consistent with their enhanced working memory when employing the intense associative processes of MOL, the superior memory capabilities of memorists such as PI may rely in particular on the recruitment of lateral frontal regions that are implicated in working memory and attention (Postle, Awh, Jonides, Smith, & D’Esposito, 2004). We detected only modest activation of lateral frontal cortex during the recall of π. This may indicate that memorists like PI use working memory processes (as indicated in the relative differences in activity in lateral frontal cortices) less for retrieval of well-learned sequences and more for encoding of novel digit strings. Alternatively, the differential activity in these regions during encoding and retrieval may reflect the greater novelty, effort, or arousal associated with generating the mnemonic associations used in MOL than in simply retrieving them after much intense rehearsal during memory consolidation.
To investigate directly the neural circuitry associated with encoding, we asked PI to memorize a series of 100 random digits using the MOL technique and compared brain activations during encoding of these 100 random digits to the activations during counting. Early in the process of encoding, we detected prominent activations in motor associative areas and midline frontal regions (BA 16), as well as in visual association areas around the precuneate/lingual/fusiform gyri (BA 31, 19, and 23). We also found medial frontal activation (BA 10) and deactivations, compared with counting, in the anterior- and posterior cingulate gyri (BA 24, 31) and in the hippocampus (green arrow at Z = −10). We had seen this latter pattern during recall of and similarly believe it to represent task-independent decreases during a relatively greater attention-demanding task. Compared with earlier encoding, during later encoding of the random 100-digit string, activation in the visual association cortex (BA 19, 23) decreased. Compared with early encoding, late encoding of the random number string was also associated with increased activity in a more anterolateral prefrontal region, as well as in the orbitofrontal cortex. We found what appear to be more brain areas activated during encoding of a novel digit string than during the recitation of π We suspect that these activations, which included frontoparietal regions, represent greater activity in and demands on working memory during encoding (particularly early encoding), compared with retrieval, in PI’s use of MOL (Walter et al., 2003).
These data are interesting in several respects. First, they are in line with previous findings of superior working memory, but not general memory, among superior memorists, particularly superior memorists using MOL (Ericsson, 1985; Ericsson 2003; Ericsson & Chase, 1982; Maguire et al., 2003). Second, consistent with a recent fMRI study of MOL in initially naïve subjects (Kondo et al., 2005), PI’s fMRI activations during memorization of a novel random number string suggest that the recruitment of working memory-related regions is considerably greater during encoding than during retrieval of remembered material. This interpretation of our data suggests at least two new directions for future investigations of individuals with superior memory. One direction is to assess whether the enhanced working memory of these individuals contributes to their superior memory by facilitating encoding or retrieval of novel material. The other, perhaps more probable, direction is to explore whether PI’s exceptional scores on working memory, as measured by the behavioral battery, is a consequence of MOL proficiency (i.e., working memory as an acquired skill rather than a naturally-superior ability).
If MOL seems to activate multimodal association areas, especially early in the process of encoding, perhaps less vigorous activity serves to maintain the already established associations. For example, a comparison of early with late learning (Figure 2) shows that association areas for visual processing and motor planning (e.g., BA 19, 16) were more active for early learning, and early activity in the mesial frontal cortex (BA 10) seems to migrate later to regions of the dorsolateral pre-frontal cortex (DLPFC) (BA 9, 46, and 47). This latter trend may imply that classical working memory regions (e.g., DLPFC) are more important in later encoding (or, alternatively, earlier in consolidation or retrieval) than in earlier encoding. Interpretation of encoding as deactivations of DLPFC regions is difficult, but it may be related to PI’s technique of invoking intense visual and emotional images upon generating the mnemonic associations during early encoding. As part of MOL, PI conjures up vivid and unusual images that likely engage multimodal association areas during early encoding.
These findings address the encoding of new information, but they do not account for PI’s extraordinary retrieval ability for the digits of π Interpretation of conspicuous fMRI signal changes that relate to the recitation of π but not to random digit encoding can be tenuous. It may be intuitively appealing that some activations (e.g., cuneus (BA 23) at Z = +9) are present during learning but not during π recall (i.e., recruiting visual imagery as part of the encoding but not the retrieval scheme). However, it is more difficult to explain that although the hippocampus deactivates bilaterally during the recitation of π (see Z = −10), this deactivation is missing from the left hippocampus and from both hippocampi during early and late learning, respectively. The absence of hippocampal activation is puzzling for new encoding but not for old retrieval. We can only speculate why the hippocampus appears to be more active during counting (baseline) than during retrieval, but perhaps the deactivation coupling of the (ventral) anterior- and posterior cingulate cortex relate to this concurrent hippocampal deactivation. In addition, our data may well differ from other accounts of superior memorists due the disparate experimental tests and baseline conditions across tasks (Kondo et al., 2005; Maguire et al., 2003).
Finally, comparing π recitation to random-digit learning, Figure 3 shows that whereas dorsolateral prefrontal cortex and medial frontal gyrus (BA 9 and 10, respectively) were more active for π recitation relative to early learning, only BA 10 was more active for π recitation compared to late learning. On the other hand, the ventral anterior cingulate cortex (BA 24) and visual areas (e.g., BA 19 and 31) were conspicuously more active for random digit learning, with some visual areas (e.g., BA 23) being more active in early than late learning. These data are consonant with PI’s encoding strategies.
Anatomical data
Comparing the volumetric measures of the neuroanatomical structures of PI with those of the sample (Table 3) shows that the only statistically significant (p<.05) difference, by both moderate and conservative estimates, occurs for the right subgenual region of the cingulate gyrus,2 an anatomical area located below the genu of the corpus callosum. Findings suggest that the anterior cingulate cortex and subgenual region plays a role in mentalizing (Frith & Frith, 2003), emotional processing (Bush, Luu, & Posner, 2000), and autonomic arousal (Critchley et al., 2003).
PI’s use of MOL relies greatly on creating images containing highly affective content. For example, as part of an interview, PI stated that the mnemonic technique uses emotion, color, synesthesia, sound, humor, vulgarity, destruction, sexuality, and exaggeration to make for more memorable images. In a more recent phone interview, PI said that ‘the more emotional and gruesome the scene, the easier the recall’. PI also stated that preparing and rehearsing for successful recall of tens of thousands digits of π involved several months’ preparation and was very ‘stressful’.
Volumetric differences in relevant brain structures have also been observed in other domains of mental processing ‘expertise’ (e.g., increased hippocampal volume may in part underlie the superior spatial memory abilities of London cab drivers (Maguire, Frackowiak, & Frith, 1997; Maguire et al., 2000)). We speculate that PI’s extensive self-generated processing of emotion-laden material may at least partially account for the observed volume increase in the right subgenual region.
Caveats and conclusion
The greatest contribution of this preliminary case report is its suggestions and directions for future research. We intentionally did not include in this study a control group of non-superior memorists: such non-experts would be MOL amateurs, and would undoubtedly use the method in a way different from PI. In addition, their MOL proficiency may rely on an alternative, less-established strategy that would likely not be comparable to PI’s, thus nullifying the comparison. Instead, we opted to elucidate the neural substrates of PI’s unusual mnemonic capability by using PI as PI’s own control. Although we did collect fMRI data on tests wherein both PI served as a self control and PI’s memory was not superior, those data were inadvertently lost through a computer malfunction.
While PI performed at 100% accuracy when tested outside of the magnet immediately after the scan, we can only assume that PI’s performance during the scan was comparably accurate. Nevertheless, that learning occurred during the scan is evident in PI’s accuracy immediately following the scan. Finally, we studied PI after having mastered MOL, so our findings cannot distinguish exceptional memory from the learning of effective memory strategies. Still, the bulk of the evidence suggests that an important component of a superior memory is the acquired skill in and diligent practice of mnemonic strategies (Ericsson 2003; Wilding & Valentine, 1997).
Given that cognitive testing of PI’s working memory performance (i.e., 99th percentile) is incongruent with PI’s general memory capabilities (i.e., 50th percentile), PI’s superior ability is likely a result of using memory strategies on these tests. Rather than being a general mnemonic device, our findings suggest that PI recruits MOL for the specific purpose of digit memorization. When asked why not memorize other information using the same stratagem, PI admitted to never trying to commit to memory anything other than the digits of π, which was a personal challenge prompted by a bet. To see whether PI can generalize PI-MOL, we plan to extend this initial report by future studies to test if PI can memorize other categories (e.g., words). Although it is our impression that PI’s unusually low visual memory scores are stable (i.e., PI was tested on multiple occasions), should our future investigations further affirm that inferior memory performance is a consistent characteristic of PI’s profile, these data would concur with previous results showing below par performance of other π-mnemonists on visual tasks (Biederman, Cooper, Fox, & Mahadevan, 1992). Furthermore, because our findings suggest a much stronger link with affect than classical MOL would suggest, we hope to further clarify the relationship between emotion and PI’s MOL-like system in upcoming experiments. Although we draw our current conclusions from an exceptional single case and do not claim population-based inferences, we intend this exploratory assay to encourage further investigation into superior memorists and the conception of working memory as a trainable, acquired skill, in both health and disease (Klingberg et al., 2005).
Supplementary Material
Acknowledgments
The authors thank Dr Jason Royal for editorial comments. This work was supported in part by NIMH grants MHK02-74677, MH59139, and MH068318, and funding from the Thomas D. Klingenstein & Nancy D. Perlman Family Fund and the Suzanne Crosby Murphy Endowment at Columbia University. In addition, Amir Raz thanks the Vancouver Coastal Health Research Institute for supporting his final efforts on this project.
Footnotes
The counting task, relative to subvocalizing the same number over and over again at the same rate, generated cerebellar activations (Z = −19 and −25): a result that may relate to a timing model that outlines the mechanisms of rhythm and temporal control (Ivry, Spencer, Zelaznik, & Diedrichsen, 2002).
Statistical significance holds when correcting for multiple comparisons using the moderate, but not conservative, confidence intervals (i.e., accounting for Type I errors).
References
- Azoulai S, Hubbard E, Ramachandran VS. Does synesthesia contribute to mathematical savant skills?. Paper presented at the Cognitive Neuroscience Society; New York. Apr 10, 2005. [Google Scholar]
- Bellezza FS. Mnemonic devices: Classification, characterization, and criteria. Review of Educational Research. 1981;51:247–275. [Google Scholar]
- Bellezza FS, Reddy BG. Mnemonic devices and natural memory. Bulletin of the Psychonomic Society. 1978;11:277–280. [Google Scholar]
- Biederman I, Cooper EE, Fox PW, Mahadevan RS. Unexceptional spatial memory in an exceptional memorist. Journal of Experimental Psychology: Learning, Memory and Cognition. 1992;18(3):654–657. doi: 10.1037//0278-7393.18.3.654. [DOI] [PubMed] [Google Scholar]
- Bor D, Duncan J, Wiseman RJ, Owen AM. Encoding strategies dissociate prefrontal activity from working memory demand. Neuron. 2003;37(2):361–367. doi: 10.1016/s0896-6273(02)01171-6. [DOI] [PubMed] [Google Scholar]
- Bower GH. Analysis of a mnemonic device. American Science. 1970;58:496–510. [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4(6):215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Chase WG, Ericsson KA. Skilled memory. In: Anderson JR, editor. Cognitive skills and their acquisition. Hillsdale, NJ: L. Erlbaum Associates; 1981. pp. 141–189. [Google Scholar]
- Critchley HD, Mathias CJ, Josephs O, O’Doherty J, Zanini S, Dewar BK, et al. Human cingulate cortex and autonomic control: Converging neuroimaging and clinical evidence. Brain. 2003;126(Pt 10):2139–2152. doi: 10.1093/brain/awg216. [DOI] [PubMed] [Google Scholar]
- de Zubicaray GI, Zelaya FO, Andrew C, Williams SC, Bullmore ET. Cerebral regions associated with verbal response initiation, suppression and strategy use. Neuropsychologia. 2000;38(9):1292–1304. doi: 10.1016/s0028-3932(00)00026-9. [DOI] [PubMed] [Google Scholar]
- Eals M, Silverman I. The hunter-gatherer theory of spatial sex differences: Proximate factors mediating the female advantage in location memory. Ethology and Sociobiology. 1994;15:95–105. [Google Scholar]
- Ericsson KA. Memory skill. Canadian Journal of Psychology[Special Issue: Skill] 1985;39(2):188. [Google Scholar]
- Ericsson KA. Exceptional memorizers: Made, not born. Trends in Cognitive Sciences. 2003;7(6):233. doi: 10.1016/s1364-6613(03)00103-7. [DOI] [PubMed] [Google Scholar]
- Ericsson KA, Chase WG. Exceptional memory. American Scientist. 1982;70(6):607. [PubMed] [Google Scholar]
- Ericsson KA, Delaney PF, Weaver G, Mahadevan R. Uncovering the structure of a memorist’s superior ‘basic’ memory capacity. Cognitive Psychology. 2004;49(3):191. doi: 10.1016/j.cogpsych.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R. Movement-related effects in fMRI time-series. Magnetic Resonance in Medicine. 1996;35(3):346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- Frith U, Frith CD. Development and neurophysiology of mentalizing. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 2003;358(1431):459–473. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Menon V. Default-mode activity during a passive sensory task: Uncoupled from deactivation but impacting activation. Journal of Cognitive Neuroscience. 2004;16(9):1484–1492. doi: 10.1162/0898929042568532. [DOI] [PubMed] [Google Scholar]
- Groninger LD. Mnemonic imagery and forgetting, Psychonomic Science. 1971;23:161–163. [Google Scholar]
- Ishikawa KO, McGaugh JL, Sakata H, Nihon D. Brain processes and memory. Proceedings of the 16th Nihon International Symposium on Brain Processes and Memory; Tokyo, Japan. 29 November–2 December 1995; Amsterdam/New York: Elsevier; 1996. [Google Scholar]
- Ivry RB, Spencer RM, Zelaznik HN, Diedrichsen J. The cerebellum and event timing. Annals of the New York Academy of Sciences. 2002;978:302–317. doi: 10.1111/j.1749-6632.2002.tb07576.x. [DOI] [PubMed] [Google Scholar]
- Kleinbaum DG, Kupper LL, Muller KE, Nizam A. Applied regression analysis and other multivariable methods. 3. Pacific Grove, CA: Duxbury Press; 1998. [Google Scholar]
- Klingberg T, Fernell E, Olesen PJ, Johnson M, Gustafsson P, Dahlstrom K, et al. Computerized training of working memory in children with ADHD: A randomized, controlled trial. Journal of the American Academy of Child & Adolescent Psychiatry. 2005;44(2):177–186. doi: 10.1097/00004583-200502000-00010. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Suzuki M, Mugikura S, Abe N, Takahashi S, Iijima T, et al. Changes in brain activation associated with use of a memory strategy: A functional MRI study. Neuroimage. 2005;24(4):1154–1163. doi: 10.1016/j.neuroimage.2004.10.033. [DOI] [PubMed] [Google Scholar]
- Luria AR. The mind of a mnemonist: A little book about a vast memory. Boston, MA: Harvard University Press; 1968. [Google Scholar]
- Maestu F, Simos PG, Campo P, Fernandez A, Amo C, Paul N, et al. Modulation of brain magnetic activity by different verbal learning strategies. Neuroimage. 2003;20(2):1110–1121. doi: 10.1016/S1053-8119(03)00309-4. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Frackowiak RS, Frith CD. Recalling routes around london: Activation of the right hippocampus in taxi drivers. Journal of Neuroscience. 1997;17(18):7103–7110. doi: 10.1523/JNEUROSCI.17-18-07103.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak RS, et al. Navigation-related structural change in the hippocampi of taxi drivers. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(8):4398–4403. doi: 10.1073/pnas.070039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Valentine ER, Wilding JM, Kapur N. Routes to remembering: The brains behind superior memory. Nature Neuroscience. 2003;6(1):90. doi: 10.1038/nn988. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Thomas P, Kane MJ, Scahill L, Zhang H, Bronen R, et al. Basal Ganglia volumes in patients with Gilles de la Tourette syndrome. Archives of General Psychiatry. 2003;60(4):415–424. doi: 10.1001/archpsyc.60.4.415. [DOI] [PubMed] [Google Scholar]
- Postle BR, Awh E, Jonides J, Smith EE, D’Esposito M. The where and how of attention-based rehearsal in spatial working memory. Research in Cognitive Brain Research. 2004;20(2):194–205. doi: 10.1016/j.cogbrainres.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Presenti M, Zago L, Crivello F, Mellet E, Samson D, Duroux B, et al. Mental calculation in a prodigy is sustained by right prefrontal and medial temporal areas. Nature Neuroscience. 2001;4(1):103. doi: 10.1038/82831. [DOI] [PubMed] [Google Scholar]
- Ross J, Lawrence KA. Some observations on a memory artifice. Psychonomic Science. 1968;13:107–108. [Google Scholar]
- Savage CR, Deckersbach T, Heckers S, Wagner AD, Schacter DL, Alpert NM, et al. Prefrontal regions supporting spontaneous and directed application of verbal learning strategies: Evidence from PET. Brain. 2001;124(Pt 1):219–231. doi: 10.1093/brain/124.1.219. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, et al. Common blood flow changes across visual tasks 2. Decreases in cerebral cortex. Journal of Cognitive Neuroscience. 1997;9(5):648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Silverman I, Eals M. Sex differences in spatial abilities: Evolutionary theory and data. In: Barkow JH, Cosmides L, Tooby J, editors. The adapted mind. New York: Oxford; 1992. pp. 533–549. [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: An approach to medical cerebral imaging. Stuttgart/New York: G. Thieme/Thieme Medical Publishers; 1988. [Google Scholar]
- Tanaka S, Michimata C, Kaminaga T, Honda M, Sadato N. Superior digit memory of abacus experts: An event-related functional MRI study. Neuroreport. 2002;13(17):2187–2191. doi: 10.1097/00001756-200212030-00005. [DOI] [PubMed] [Google Scholar]
- Thompson CP, Cowan TM, Frieman J. Memory search by a memorist. Hillsdale, NJ: L. Erlbaum Associates; 1993. [Google Scholar]
- Walter H, Bretschneider V, Gron G, Zurowski B, Wunderlich AP, Tomczak R, et al. Evidence for quantitative domain dominance for verbal and spatial working memory in frontal and parietal cortex. Cortex. 2003;39(4–5):897–911. doi: 10.1016/s0010-9452(08)70869-4. [DOI] [PubMed] [Google Scholar]
- Wilding JM, Valentine ER. Superior memory. Hove, UK: Psychology Press; 1997. [Google Scholar]
- Yates FA. The art of memory. Chicago, IL: University of Chicago Press; 1966. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.