Abstract
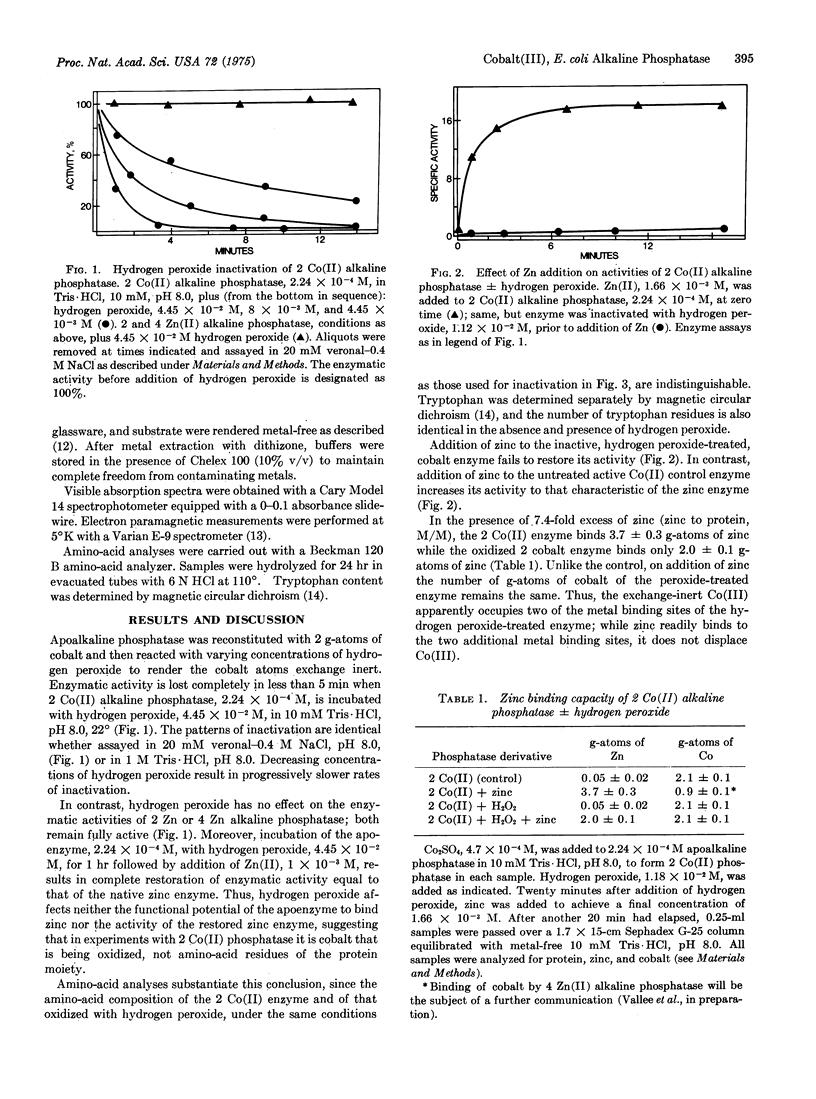
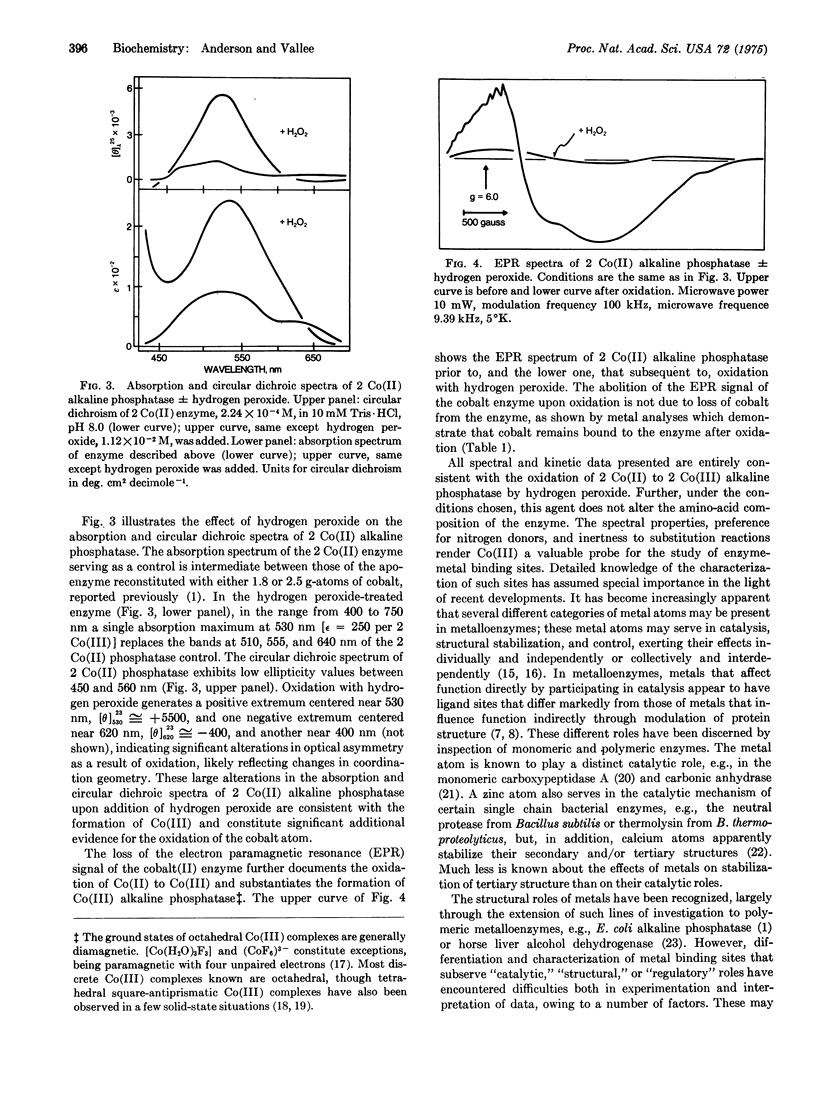
To facilitate the study of individual metal binding sites of polymeric metalloproteins, conversion of exchange-labile Co(II) in E. coli alkaline phosphatase (EC 3.1.3.1) to exchange-inert Co(III) was examined. Oxidation of Co(II) alkaline phosphatase with hydrogen peroxide results in a single absorption maximum at 530 nm and loss both of the characteristic electron paramagnetic signal and of enzymatic activity. Zinc neither reactivates this enzyme nor displaces the oxidized cobalt atoms. Metal and amino-acid analyses demonstrate that oxidation alters neither cobalt binding nor amino-acid composition of the enzyme. Al data are consistent with the conclusion that hydrogen peroxide oxidizes Co(II) in alkaline phosphatase to Co(III). Polymeric metalloenzymes can contain different categories of metal atoms serving in catalysis, structure stabilization, and/or control and exerting their effects independently or interdependently. The in situ conversion of exchange-labile Co(II) to exchange-stable (Co(III) offers a method to selectively and differentially "freeze" cobalt atoms at their respective binding sites. The accompanying spectral changes and concomitant retardation in ligand exchange reactions may be used to differentiate between specific metal binding sites that serve different roles in polymeric metalloenzymes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Drum D. E., Harrison J. H., 4th, Li T. K., Bethune J. L., Vallee B. L. Structural and functional zinc in horse liver alcohol dehydrogenase. Proc Natl Acad Sci U S A. 1967 May;57(5):1434–1440. doi: 10.1073/pnas.57.5.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmquist B., Vallee B. L. Tryptophan quantitation by magnetic circular dichroism in native and modified proteins. Biochemistry. 1973 Oct 23;12(22):4409–4417. doi: 10.1021/bi00746a018. [DOI] [PubMed] [Google Scholar]
- Kang E. P., Storm C. B., Carson F. W. Cobalt (3) carboxypeptidase A: preparation and esterase activity. Biochem Biophys Res Commun. 1972 Nov 1;49(3):621–625. doi: 10.1016/0006-291x(72)90456-1. [DOI] [PubMed] [Google Scholar]
- Keilin D., Mann T. Carbonic anhydrase. Purification and nature of the enzyme. Biochem J. 1940 Sep;34(8-9):1163–1176. doi: 10.1042/bj0341163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy F. C., Hill H. A., Kaden T. A., Vallee B. L. Electron paramagnetic resonance spectra of some active cobalt(II) substituted metalloenzymes and other cobalt(II) complexes. Biochem Biophys Res Commun. 1972 Sep 26;48(6):1533–1539. doi: 10.1016/0006-291x(72)90888-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Latt S. A., Holmquist B., Vallee B. L. Thermolysin: a zinc metalloenzyme. Biochem Biophys Res Commun. 1969 Oct 8;37(2):333–339. doi: 10.1016/0006-291x(69)90739-6. [DOI] [PubMed] [Google Scholar]
- PLOCKE D. J., VALLEE B. L. Interaction of alkaline phosphatase of E. coli with metal ions and chelating agents. Biochemistry. 1962 Nov;1:1039–1043. doi: 10.1021/bi00912a014. [DOI] [PubMed] [Google Scholar]
- Simpson R. T., Vallee B. L., Tait G. H. Alkaline phosphatase of Escherichia coli. Composition. Biochemistry. 1968 Dec;7(12):4336–4342. doi: 10.1021/bi00852a028. [DOI] [PubMed] [Google Scholar]
- Simpson R. T., Vallee B. L. Two differentiable classes of metal atoms in alkaline phosphatase of Escherichia coli. Biochemistry. 1968 Dec;7(12):4343–4350. doi: 10.1021/bi00852a029. [DOI] [PubMed] [Google Scholar]
- THIERS R. E. Contamination in trace element analysis and its control. Methods Biochem Anal. 1957;5:273–335. doi: 10.1002/9780470110218.ch6. [DOI] [PubMed] [Google Scholar]
- Tait G. H., Vallee B. L. Studies on the active center of alkaline phosphatase of E. coli. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1247–1251. doi: 10.1073/pnas.56.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VALLEE B. L., NEURATH H. Carboxypeptidase, a zinc metalloenzyme. J Biol Chem. 1955 Nov;217(1):253–261. [PubMed] [Google Scholar]
- Vallee B. L., Williams R. J. Enzyme action: views derived from metalloenzyme studies. Chem Br. 1968 Sep;4(9):397–402. [PubMed] [Google Scholar]
- Vallee B. L., Williams R. J. Metalloenzymes: the entatic nature of their active sites. Proc Natl Acad Sci U S A. 1968 Feb;59(2):498–505. doi: 10.1073/pnas.59.2.498. [DOI] [PMC free article] [PubMed] [Google Scholar]


