Abstract
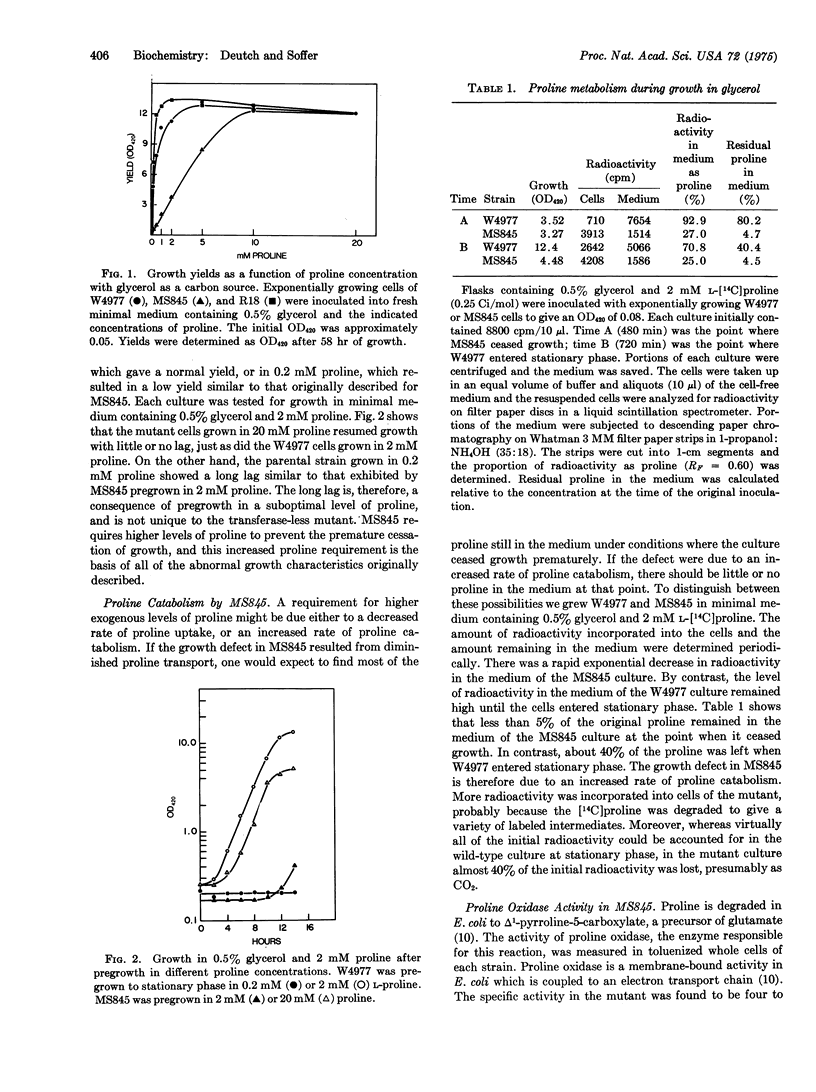
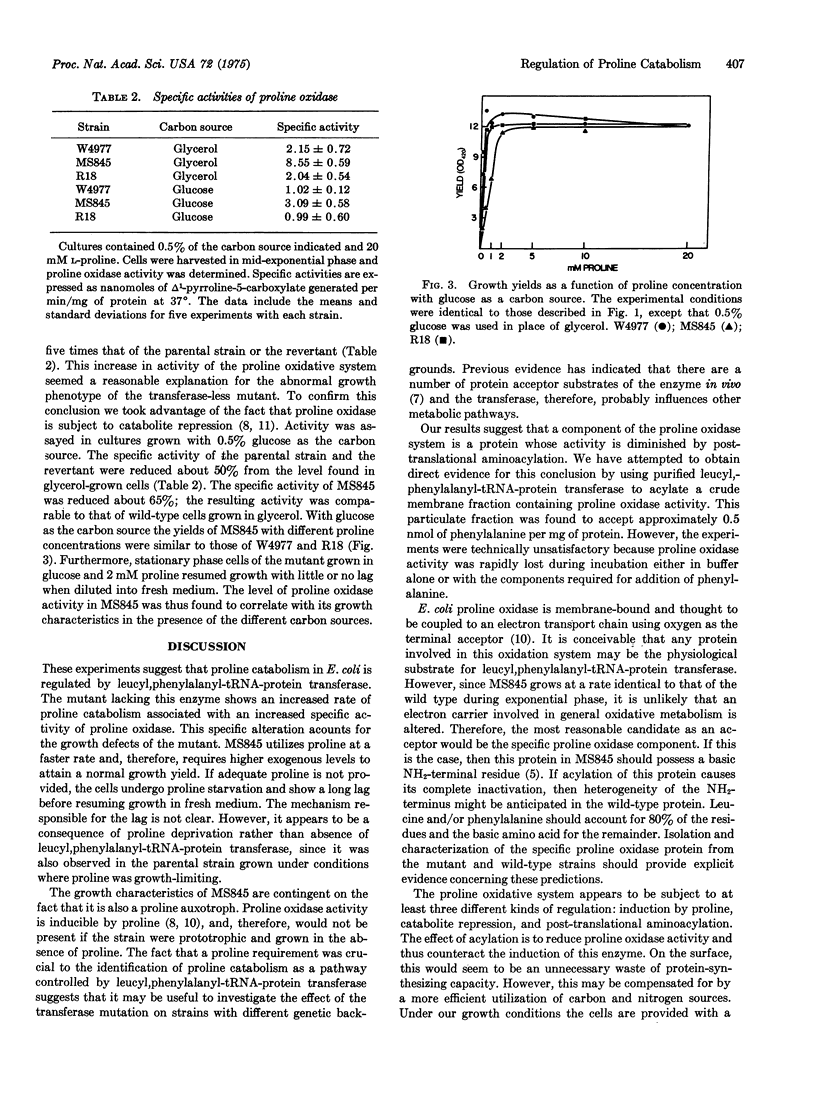
A mutant of Escherichia coli lacking leucyl,phenylalanyl-tRNA:protein leucyltransferase, EC 2.3.2.6) exhibited several abnormal growth characteristics relative to the wild type or a revertant when grown with glycerol as a carbon source. All three strains were auxotrophic for proline. The mutant required higher levels of this amino acid than did the other strains to attain a normal growth yield and metabolized exogenous [14C]proline more rapidly. The greater rate of proline utilization was associated with a 4-fold increase in specific activity of proline oxidase. When glucose rather than glycerol was employed as a carbon source, proline oxidase activity was reduced by catabolite repression and the growth ccharacteristics of the mutant were similar to those of the parental and revertant strains. These results suggest that the mutant growth phenotype is due to an altered rate of proline catabolism and constitue evidence for regulation of a specific metabolic pathway by leucyl,phenylalanyl-tRNA-protein transferase.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dendinger S., Brill W. J. Regulation of proline degradation in Salmonella typhimurium. J Bacteriol. 1970 Jul;103(1):144–152. doi: 10.1128/jb.103.1.144-152.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANK L., RANHAND B. PROLINE METABOLISM IN ESCHERICHIA COLI. 3. THE PROLINE CATABOLIC PATHWAY. Arch Biochem Biophys. 1964 Aug;107:325–331. doi: 10.1016/0003-9861(64)90338-8. [DOI] [PubMed] [Google Scholar]
- KAJI A., KAJI H., NOVELLI G. D. SOLUBLE AMINO ACID-INCORPORATING SYSTEM. I. PREPARATION OF THE SYSTEM AND NATURE OF THE REACTION. J Biol Chem. 1965 Mar;240:1185–1191. [PubMed] [Google Scholar]
- KREBS E. G., KENT A. B., FISCHER E. H. The muscle phosphorylase b kinase reaction. J Biol Chem. 1958 Mar;231(1):73–83. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leibowitz M. J., Soffer R. L. Enzymatic modification of proteins. VII. Substrate specificity of leucyl,phenylalanyl-transfer ribonucleic acid-protein transferase. J Biol Chem. 1971 Sep 10;246(17):5207–5212. [PubMed] [Google Scholar]
- Prival M. J., Magasanik B. Resistance to catabolite repression of histidase and proline oxidase during nitrogen-limited growth of Klebsiella aerogenes. J Biol Chem. 1971 Oct 25;246(20):6288–6296. [PubMed] [Google Scholar]
- Shapiro B. M., Stadtman E. R. The regulation of glutamine synthesis in microorganisms. Annu Rev Microbiol. 1970;24:501–524. doi: 10.1146/annurev.mi.24.100170.002441. [DOI] [PubMed] [Google Scholar]
- Soffer R. L. Aminoacyl-tRNA transferases. Adv Enzymol Relat Areas Mol Biol. 1974;40(0):91–139. doi: 10.1002/9780470122853.ch4. [DOI] [PubMed] [Google Scholar]
- Soffer R. L. Peptide acceptors in the leucine, phenylalanine transfer reaction. J Biol Chem. 1973 Dec 25;248(24):8424–8428. [PubMed] [Google Scholar]
- Soffer R. L., Savage M. A mutant of Escherichia coli defective in leucyl, phenylalanyl-tRNA-protein transferase. Proc Natl Acad Sci U S A. 1974 Mar;71(3):1004–1007. doi: 10.1073/pnas.71.3.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]


