Abstract
Background
Non-adherence is widespread problem. Adherence is a crucial point for the success and the safe use of therapies. The objective of this overview (review of reviews) was to identify factors that influence adherence in chronic physical conditions.
Methods
A systematic literature search was performed in Medline and Embase (1990 to July 2013). Publications were screened according to predefined inclusion criteria. The study quality was assessed using AMSTAR. Both process steps were carried out independently by two reviewers. Relevant data on study characteristics and results were extracted in piloted standardized tables by one reviewer and checked by a second. Data were synthesized using a standardized quantitative approach by two reviewers.
Results
Seven systematic reviews were included. Higher education and employment seem to have a positive effect on adherence. Ethnic minorities seem to be less adherent. Co-payments and higher medication cost seems to have negative effect on adherence. In contrast financial status/income and marital status seem to have no influence on adherence. The effect of therapy related factors was mostly unclear or had no effect. Only the number of different medications in heart failure patients showed the tendency of an effect. Indicators of regime complexity showed consistently a negative effect direction. Duration of disease seems to have no effect on adherence. There is the tendency that higher or middle age is associated with higher adherence. But in more than half of the reviews the effect was unclear. There is no clear effect of physical as well as mental comorbidity. Only one review showed the tendency of an effect for mental comorbidity. Also for gender the effect is not clear because the effect direction was heterogenic between and within the systematic reviews.
Conclusion
The presented overview shows factors than can potentially have influence on adherence. Only for a few factors the influence on adherence was consistent. Most factors showed heterogeneous results regarding statistical significance and/or effect direction. However, belonging to an ethnic minority, unemployment and cost for the patient for their medications showed consistently a negative effect on adherence which indicates that there is a social gradient.
Electronic supplementary material
The online version of this article (doi:10.1186/2049-3258-72-37) contains supplementary material, which is available to authorized users.
Keywords: Adherence, Compliance, Systematic review, Oral medications, Oral therapy
Background
Adherence, can be defined as “the extent to which a patient acts in accordance with the prescribed interval and dose of a dosing regimen” [1]. Non-adherence is widespread problem in chronic conditions [2, 3]. Adherence is not only a crucial point for the success, but also for the safe and effective use of many therapies [4–6]. Moreover non-adherence can cause substantial costs [3]. In chronic conditions adherence is especially important because medication has to be taken for a long time.
Adherence is a multifactorial problem that can be influenced by various factors. The factors can be roughly divided in the following five dimensions: Social and economic, health care system, health condition, therapy and patient [3]. Furthermore non-adherence can be intentional (e.g. decided not to take because of adverse events) and non-intentional (e.g. forgetfulness).
The objective was to identify factors that influence adherence to oral medications in patients suffering from physical chronic conditions. Because of the large amount of literature in this field it is difficult for researchers and clinicians to know all relevant studies for all physical chronic conditions. Thus, we decided to perform a review of reviews (overview) which is a new form of evidence synthesis to answer the research question. An overview can provide a comprehensive picture of the evidence while keeping the information flood manageable [7]. Furthermore we decided to focus on factors that are not associated with the indication or therapy to enhance universal applicability of the results.
Methods
Sources
A systematic literature search was performed in MEDLINE (via Pubmed) and Embase (via Embase). The search strategy combined various terms and medical subject headings related to adherence, and oral medical medication (the full search strategies for each database are available in Additional file 1). The search was limited to a publication date after 1990. The search was performed on July the 15th 2013.
Study selection
To be eligible for this review the studies had to meet the following inclusion criteria:
Patients: Adult patients with physical chronic condition (i.e. studies that analysed only children, patients with acute conditions or mental illness were excluded).
Medication: Oral intake.
Exposure: Potentially adherence influencing factor (exposure is not controlled by the investigator).
Outcome Adherence (right dose, right timing and/or right frequency of intake).
Study type: Systematic review (definition: systematic literature search in at least one electronic database and assessment of risk of bias of included studies) of quantitative studies.
Publication language: English or German.
It was decided to focus on factors unrelated to the health care system (e.g. type of insurances), condition (e.g. symptoms) and medication (e.g. side effects) i.e. factors that are strongly related to health care system, condition or medications were excluded. For example side effects or symptoms are strongly associated with the respective medication disease respectively and can consequently have different influence on adherence. The purpose of focusing on disease and medication unrelated factors was on the one hand to insure comparability, consistency and clarity of results and on the other hand to identify factors that are widely applicable in clinical practice. A preliminary unsystematic search was performed to identify articles on adherence influencing factors. Based on identified articles a list of potential adherence influencing factors was prepared and chosen which factors are unrelated to the disease and therefore should be included in the analysis. The decision to include a factor was made independently by two reviewers and discussed until consensus in case of discrepancies. The following factors were eligible: age, gender, ethnic status, education, employment, financial status/income, marital status/not living alone, social support, measure of intake complexity (e.g. number of tablets, number of medications, frequency of intake), duration of therapy, duration of disease, comorbidity, co-payments, medication costs, insurance status (insured/not ensured).
Furthermore it was decided to include systematic reviews only if the risk of bias of included primary studies was assessed, documented and relatable to the respective factor because an examination of the evidence for an effect of a potential adherence influencing factor is only possible considering the validity of evidence of the primary studies.
Two reviewers performed the study selection independently according to the inclusion criteria above. Titles and abstracts of all articles were screened. The full-texts of all potentially relevant articles were than obtained and screened in detail. Any differences between the reviewers were discussed until consensus. In addition to identify grey literature the reference lists of all full-texts were cross-checked and an additional search in Google scholar performed.
Methodological quality assessment
The methodological quality of included studies was assessed using the AMSTAR instrument that was found to be valid and reliable to assess SRs [8, 9]. Each assessment question was rated with “yes”, “no”, “unclear” or “not applicable”. The instrument was applied independently by two reviewers. Disagreements were resolved in a discussion. The question “was the scientific quality of the included studies assessed and documented” was skipped because we formulated quality assessment of included studies as inclusion criterion (see inclusion criterion 5).
Data extraction and synthesis
Data were extracted by one reviewer in standardized summary tables and were verified by a second reviewer. Any disagreements were discussed till consensus. For each systematic review, characteristics were extracted on the condition/medication (marked bold), inclusion and exclusion criteria for primary studies (only other than our applied inclusion criteria) and search period limits. Results were extracted according to the type of evidence synthesis. In the case of a narrative synthesis, results were abstracted by modified vote counting [10]. This contained data to the effect direction, all comparisons showing this effect direction, statistically significant comparisons showing this effect direction and total number of comparisons for this factor. This method has been suggested for presenting results of qualitative synthesis, overcoming problems arising when simple vote counting is used by relying either on the number of comparisons with a positive direction of effect or the number of comparisons reaching statistical significance. For all meta-analyses, pooled effect sizes and measures with confidence intervals and test and measure for statistical heterogeneity were extracted. All data in the tables were commutated so that the influence on adherence refers throughout to an increase of the respective factor independently whether the factor is positive (e.g. educational level) or negative (adverse events). A p-level of less than 0.05 was considered statistical significant. Overlaps (multiple included primary studies) were assessed in case of multiple systematic reviews on the same indication.
For all factors a summary evaluation of the influence was made. Two reviewers rated the evidence for an effect, considering the number of included systematic reviews for a comparison, the effect direction, the significance and consistency (within and between systematic reviews) of results, the methodological quality of systematic reviews and the methodological quality/risk of bias of included primary studies. If only one high quality study was included in a comparison that showed a statistical significant positive effect for a certain factor, this was rated as tendency for a positive effect. Discrepant judgments were discussed until consensus.
Results
Description of included systematic reviews
The electronic literature search resulted in 1604 hits after duplet removal (EndNote ×5). Of these 72 titles and abstracts seemed potential relevant and full-text versions of the publications were screened in detail. Seven studies met all inclusion criteria and were finally included in this overview [11–17]. The process of study selection is illustrated in the flowchart (see Figure 1). Most systematic reviews were excluded because a risk of bias assessment was not performed or the quality of evidence was not attributable to the influencing factors (inclusion criteria 5).
Figure 1.
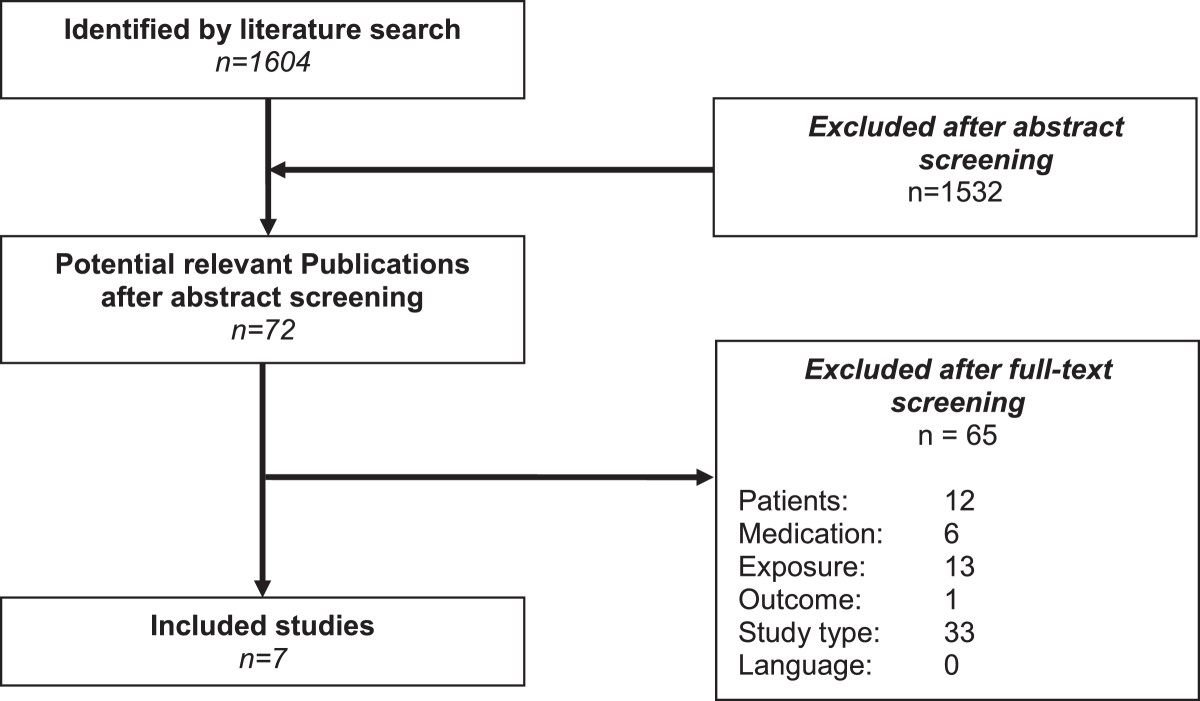
Flow- chart of study selection.
The included systematic reviews assessed adherence influencing factors in the following conditions/medications; chronic malignant pain, Parkinson disease, heart failure, inflammatory arthritis, prophylactic treatment of hemophilia A or B, intake of oral anti-cancer agents [11–15, 17]. Sinoott et. al did not restrict the condition or medication but included all studies that analysed public insured patients which were exposed to co-payments for medications [16]. Overlaps were not assessed, because all included systematic reviews were on different indications. The characteristics of the included systematic reviews are presented in Table 1.
Table 1.
Study characteristics
| Study | Search period | Inclusion criteria* |
|---|---|---|
| Broekmans [11] | Not limited – 12/2006 | Adult patients with chronic non -malignant pain |
| Adult patients with prescribed pain medication | ||
| Original research | ||
| Daley [12] | Not limited – 01/2012 | Patients with Parkinson |
| All ranges and duration of anti- parkinsonian treatments | ||
| All age ranges | ||
| Published in English | ||
| Presenting quantitative/qualitative data | ||
| Oosterom-Calo [13] | Not limited – 08/2010 | ≥50% heart failure patients |
| Quantitative results were reported | ||
| Studies of at least fair quality | ||
| Evaluations of interventions were not the main purpose | ||
| No descriptive study | ||
| No review paper | ||
| Published in English | ||
| Pasma [14] | Not limited – 02/2011 | Inflammatory arthritis patients |
| Used a reproducible definition or validated instrument to measure adherence | ||
| Provided a statistical measure to reflect the strength of the association between the determinant and adherence | ||
| No letters, editorials, reviews, RCTs, case reports, qualitative studies and opinion articles | ||
| Schrijvers [15] | Not reported – 15/2012 | Haemophilia A or B |
| Prophylactic treatment | ||
| All age groups | ||
| Sinnott [16] | 1946 – 09/2012 | Participants received healthcare from a public insurance scheme |
| Comparator group was the same population/similar population who either didn’t pay copayments or experienced no increase in copayment | ||
| The intervention was copayment; either an increase in an existing copayment or the introduction of a copayment (no other types of cost-sharing, for example co-insurance) | ||
| Studies included were randomised controlled trials, controlled before and after studies, interrupted time series designs, repeated measures designs, and cohort designs | ||
| Verbrugghe [17] | NR | Oral anti- cancer drugs |
| Age ≥ 18 | ||
| Strong or moderate methodological quality | ||
| Written in English, French, German or Dutch | ||
| Original research articles published between 1990 and April 2012 | ||
| Studies not conducted in developing countries | ||
| All study designs |
*Indication & medication marked bold.
Methodological quality of included systematic reviews
Overall the quality of included systematic reviews was moderate to high One systematic review satisfied all quality criteria [16] and two did not fulfil only one quality criterion [12, 17]. At most three items were not answered with “no” or “unclear” (three studies [11, 14, 15]). In three systematic reviews the study selection and/or data extraction was not performed in duplicate or the item was rated as unclear because of missing information [11, 14, 15]. The most frequent flaw was that a list of excluded studies was not provided. The assessment questions relating to combing findings and publication bias were rated as not applicable in 6 studies because a quantitative data-synthesis was not performed. The quality of each systematic review is presented in Table 2.
Table 2.
Results of the study quality assessment
| Assessment question | á priori design | Two reviewers | Literature search | Status of publication | List of studies | Study characteristics | Conclusions | Combining findings | Publication bias | Conflict of interest |
|---|---|---|---|---|---|---|---|---|---|---|
| Study | ||||||||||
| Broekmans [11] | + | ? | + | + | - | + | + | O | O | - |
| Daley [12] | + | + | + | + | - | + | + | O | O | + |
| Oosterom-Calo [13] | + | + | - | + | - | + | + | O | O | + |
| Pasma [14] | + | ? | + | + | - | + | + | O | O | - |
| Schrijvers [15] | + | - | + | ? | - | + | + | O | O | + |
| Sinnott [16] | + | + | + | + | + | + | + | + | + | + |
| Verbrugghe [17] | + | + | + | + | - | + | + | O | O | + |
+ = yes; − = no; O = not applicable; ? = unclear.
Effect of adherence influencing factors
The following factors were included in the analysis: age, comorbidity, co-payments, duration of disease, duration of therapy, education, ethnic status, gender, regime complexity (e.g. number of daily intake, number of tablets, frequency of intake, daytime of intake), marital status (including domestic partnerships), medication costs, different medications, social support, financial status/income, employment.
The results of individual systematic reviews are presented in Additional file 2 and the evidence synthesis of individual systematic reviews is presented in Table 3.
Table 3.
Evidence synthesis
| Factor | Relationship | ||
|---|---|---|---|
| Indication/therapy | Effect direction | Evidence for effect | |
| Social and economic | |||
| Education | Chronic pain | O | + |
| Parkinson | ↑ | O | |
| Heart failure* | O | O | |
| Oral cancer therapy | ↑ | O | |
| Employed | Inflammatory arthritis | O | + |
| Ethnic status | Parkinson | Ethnic minorities < others | + |
| Inflammatory arthritis | White > others | + | |
| Oral cancer therapy | Non-white > others | O | |
| Financial status/income | Parkinson | ↑ | - |
| Heart failure* | O | - - | |
| Oral cancer therapy | ↑ | O | |
| Married/not living alone | Parkinson | ↑ | - |
| Oral cancer therapy | ↕ | O | |
| Social support | Heart failure* | ↕ | O |
| Inflammatory arthritis | ↑ | O | |
| Therapy related | |||
| Duration of therapy | Oral cancer therapy | ↓ | O |
| Frequency of intake | Parkinson | ↓ | O |
| Heart failure* | O | - - | |
| Inflammatory arthritis | O | - - | |
| Number of pills taken per day | Heart failure* | O | O |
| Different medications | Chronic pain | ↕ | O |
| Parkinson | ↓ | O | |
| Inflammatory arthritis | ↓ | O | |
| Oral cancer therapy | ↓ | O | |
| Heart failure* | O | + | |
| Taking medication at meals | Oral cancer therapy | ↑ | O |
| Disease related | |||
| Duration of disease | Chronic pain | O | - - |
| Inflammatory arthritis | ↓ | - | |
| Oral cancer therapy | ↓ | O | |
| Patient related | |||
| Age | Chronic pain | ↑ | O |
| Parkinson | ↑ | + | |
| Heart failure* | ↑ | + | |
| 35-56 > others | + | ||
| Inflammatory arthritis | ↑ | O | |
| 55-64 > others | + | ||
| Hemophilia | ↑ | O | |
| Oral cancer therapy | ↕ | O | |
| Comorbidity (not specified) | Inflammatory arthritis | ↑ | O |
| Comorbidity (physical) | Heart failure* | ↕ | O |
| Comorbidity (mental) | Parkinson | ↓ | O |
| Heart failure* | ↓ | + | |
| Gender (female) | Chronic pain | ↑ | + |
| Heart failure* | ↕ | O | |
| Oral cancer therapy | ↕ | O | |
| Health care system factors | |||
| Co-payments | Inflammatory arthritis | ↓ | + |
| Not restricted | ↓ | ++ | |
| Oral cancer therapy | ↓ | O | |
| Medication costs | Inflammatory arthritis | ↓ | + |
| Oral cancer therapy | ↓ | O | |
Effect direction.
↑: positive (all studies showed a positive effect on adherence); ↓: negative (all studies showed a negative effect on adherence); ↕: heterogenic (i.e. at least one study showed a contrary effect direction); O: unclear reported/ not reported.
Evidence for effect.
++: clear effect.
+: tendency of effect.
--: no effect.
-: tendency of no effect.
O: unclear effect.
*Inclusion criteria for studies: ≥50% of population are heart failure patients.
Social and economic factors
The evidence for effect of education was mostly judged as unclear. Only in chronic malignant pain there is a tendency that education has effect on adherence [11]. However the effect direction was not reported in this systematic review. In the systematic reviews that reported effect direction, education showed a positive influence on adherence [13, 17].
Employment was only analysed in the systematic review on inflammatory arthritis [14]. There is a tendency of effect but the effect direction was not reported.
In Parkinson diseases there is the tendency that ethnic minorities are less adherent [12]. In inflammatory arthritis whites seem to be more adherent [14].
Financial status seems to have no effect on adherence. The three systematic reviews that analysed this outcome showed no effect, the tendency of no effect, or unclear effect respectively [12, 13, 17].
The effect of social support was unclear in both systematic reviews that analysed this exposure [13, 14].
Therapy related factors
Duration of therapy was only analysed in the systematic review on oral anticancer agents [17]. The effect direction was negative. But the evidence for effect was unclear.
The effect direction of frequency of intake was negative in one primary study on patients with Parkinson diseases [12]. However, the study was not statistical significant. There is no effect of frequency of intake on adherence in patients with heart failure or inflammatory arthritis patients [14].
The effect of tablets taken per day in heart failure patients is unclear, because one of the two studies that analysed this factor was statistical significant and one was not. Moreover effect direction was not reported [13].
Most of the systematic reviews that analysed intake of different medications showed a negative effect on adherence (Parkinson, oral anticancer therapy, inflammatory arthritis) [12, 14, 17]. In patients with chronic malignant pain the effect direction was heterogenic for this factor [11]. For taking different medications there was some evidence for effect in heart failure patients. But effect direction was unclear [13].
Taking medications at meals showed a positive effect direction on adherence in patients taking oral anticancer agents. The evidence for effect was unclear [17].
Duration of diseases seems to have no influence on adherence in patients with chronic malignant pain and tendentially not in patients with inflammatory arthritis [11, 14]. For patients taking oral anticancer agents the effect direction was negative, but evidence for effect was unclear [17].
Patient related factors
With one exception [17], all studies showed a positive effect of higher age or middle age on adherence. There was a tendency for effect in patients with Parkinson and heart failure [12, 13]. In patients with inflammatory arthritis the effect of higher age was unclear. Patients aged between 35–56 showed higher adherence than other age groups in one study [14]. For the other indications (chronic malignant pain, hemophilia, oral anticancer therapy) the evidence of effect was unclear [11, 15, 17].
The evidence for effect of comorbidity was mostly unclear [12–14]. Only in heart failure patients there was the tendency of negative effect of mental comorbidities [13].
The effect direction of gender was heterogeneous in patients with heart failure and patients on oral anticancer therapy [13, 17]. In patients with chronic malignant pain there was the tendency of higher adherence in women [11].
Health care system factors
Co-payments and medication costs showed a negative effect direction in all systematic reviews. For both factors there was a tendency of effect in inflammatory arthritis patients and unclear effect in patients taking oral anticancer agents [14, 17]. The meta-analysis on co-payments in public insurance systems showed a clear negative effect [16].
Discussion
Seven relevant systematic reviews could be identified that satisfied all inclusion criteria. The quality of the systematic reviews was moderate to high.
Of the social economic factors higher education and employment seem to have a positive effect on adherence. Moreover ethnic minorities seem to be less adherent. In contrast financial status/income and marital status seem to have no influence on adherence. The effect of therapy related factors was mostly unclear or had no effect. Only the number of different medications in heart failure patients showed the tendency of an effect. However indicators of regime complexity showed consistently a negative effect direction. Duration of disease seems to have no effect on adherence. There is the tendency that higher or middle age is associated with higher adherence. But in more than half of the reviews the effect was unclear. There is no clear effect of physical as well as mental comorbidity. Only one review showed the tendency of an effect for mental comorbidity. Also for gender the effect is not clear because the effect direction was heterogenic between and within the systematic reviews. Co-payments and higher medication cost seems to have negative effect on adherence.
The results of the excluded systematic reviews on other chronic conditions are broadly in accordance with the results of the presented analysis. However most of these did not assess the risk of bias of included primary studies and results could therefore not be assessed systematically [18–23].
Although the quality of systematic reviews was moderate to high, there is a loss of information in our overview because of poor reporting in the systematic reviews. Thus, we often had to rate the evidence for effect as unclear not because of a “real” lack of evidence. For example it was not possible to include the effect size and number of included patients in the evidence synthesis because with one exception [16] none of the studies provide information on these. It appears that some studies report only significant comparisons. These impression is also in accordance with prior research [24]. For such reviews that did not mention the total number of comparisons for a certain factor but only the statistical significant comparisons it is impossible to make a summary estimation of effect. Consequently the evidence for effect in the systematic review of Daley et al. and Verbrugghe et al. had to be judged as unclear, throughout [12, 17].
Another limitation is that there were no information on adjustments of analysis, although confounding is a major problem in observational study designs [25, 26]. So data had to be extracted irrespective of the adjustments with might cause some of the heterogeneity of results. Furthermore no information can be derived on the relation between the factors. Thus, assumptions like that the effect of co-payments and medication costs is stronger in low income groups cannot be proven.
In continuous variables source of heterogeneity are probably the different categorizations. For example studies that analysed age with different categorizations showed higher adherence in the middle aged. In contrast studies that analysed age as linear showed often no statistical significance results and effect directions were partly even conflicting. Also for the factors that describe the complexity of intake regime different definitions and categorizations are probably a source for heterogeneity of results (e.g. frequency of intake, number of different medications). For such factors a well-chosen categorization (e.g. by testing different categorizations), definition and clear reporting of analysis is warranted for a substantial evaluation.
In the overview symptomatic as well as asymptomatic conditions were considered. Research has shown, probably because of the lower perceived importance of taking medications correctly that adherence is lower in asymptomatic conditions than in symptomatic conditions [27, 28]. The differences in adherence might be another reason for between study heterogeneity that is not controlled for.
A further probable source for heterogeneity is that there is no differentiation between intentional and non-intentional adherence. Indeed prior research revealed that non-adherence is mostly non-intentional [29]. However, the influence of an factor can differ between intentional and non-intentional adherence [30].
The overview has some methodological limitations. Firstly there is a risk for publication bias because we did not search other languages than English and German. Secondly the overview focuses only on therapy implementation. I.e. initiation and discontinuation of therapy were not considered [31]. Thirdly we assessed the included systematic reviews of prognostic factors with AMSTAR, which was originally developed for the quality assessment of systematic reviews of health interventions and which was only validated in randomised controlled trials.
These review aimed to provide a comprehensive overview of factors that can have influence on adherence for a wide variety of indications. Surprisingly, despite the mass of literature on adherence we could identify only systematic reviews on seven different chronic physical conditions that satisfied all inclusion criteria. The results possibly can be transferred to other conditions because we considered only condition and therapy unrelated factors in physical chronic conditions. This suggestion is confirmed by the results of systematic reviews on other chronic conditions [18–23]. However, it should be considered in the interpretation of data that the generalizability is limited because of this fact.
Only condition and therapy unrelated factors were analysed in this overview. There are also factors that are related to the respective condition (e.g. concomitant medications, symptoms) or therapy (e.g. side effects. complex treatment regimens) [7, 27]. In some conditions such factors may even be the most important cause of non-adherence. Moreover we analysed only selected factors. There are other therapy and condition unrelated factors that might have also an effect on adherence. E.g. for the physician- patient relationship, health literacy and positive beliefs about medication a positive effect on adherence is described for some chronic conditions. Again for long distance from treatment setting, forgetfulness and clinical asymptomatic course/disease mostly a negative influence on adherence is reported [3].
Most determinates of social status showed an effect on adherence. Apparently lower social status has a negative effect on adherence. This knowledge can contribute to identify vulnerable groups for non-adherence that are potential candidates for programs to enhance adherence [32]. The knowledge of adherence influencing factors can furthermore support the development of interventions and public health programs that are tailored to specific patient needs and that attempt to enhance adherence by reducing adherence barriers (e.g. counselling interventions tailored to education status, reduction of co-payments) [33].
Moreover the knowledge on adherence influencing factors can contribute to the development of risk factor based screening tools. Similar instruments have been developed for some indications [34].
Conclusion
The presented overview indicates factors than can potentially have influence on adherence. Only for a few factors the influence on adherence was consistent. Belonging to an ethnic minority, unemployment and cost for the patient for their medications showed a negative effect on adherence which indicates that there is a social gradient. This indicates that future programs to enhance adherence should focus on social disadvantaged groups. For example a possibility could be to reduce co-payments for vulnerable groups.
Most of the other analysed factors showed heterogeneous results regarding statistical significance and/or effect direction. The results of this overview should therefore be considered as indication for factors that can have an influence on adherence in chronic physical conditions. To be of sufficient significance to make decisions in clinical practice, the factors have to be evaluated in detail for the specific context of the decision.
Further systematic reviews that assesses the risk of bias of included studies to allow an estimation of the validity of results and that are transparently reported are needed. Especially on the factors for that the evidence for effect were mostly rated unclear further research is needed.
Electronic supplementary material
Additional file 1: Search strategy.(DOCX 16 KB)
Additional file 2: Results of individual systematic reviews.(DOCX 15 KB)
Below are the links to the authors’ original submitted files for images.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
TM: development of study concept, literature search, selection of literature, analysis of literature, draft of manuscript, final approval of the version submitted. TJ: selection of literature, data extraction, quality assessment, review of manuscript, final approval of the version submitted. DP: selection of literature, analysis of literature, review of manuscript, final approval of the version submitted.
Contributor Information
Tim Mathes, Email: Tim.Mathes@uni-wh.de.
Thomas Jaschinski, Email: Thomas.Jaschinski@uni-wh.de.
Dawid Pieper, Email: Dawid.Pieper@uni-wh.de.
References
- 1.Cramer JA, Roy A, Burrell A, Fairchild CJ, Fuldeore MJ, Ollendorf DA, Wong PK. Medication compliance and persistence: terminology and definitions. Value Health: J Int Soc Pharmacoeconomics Outcomes Res. 2008;11(1):44–47. doi: 10.1111/j.1524-4733.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- 2.Osterberg L, Blaschke T. Adherence to Medication. N Engl J Med. 2005;353(5):487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization . Adherence to long-term therapies: evidence for action. Geneva, Switzerland: World health Organization; 2003. [Google Scholar]
- 4.The ABC Project team . Final report of the ABC project (Deliverable 71) 2012. Ascertaing barriers for compliance: policies for save, effective and cost-effective use medicines in Europe. [Google Scholar]
- 5.DiMatteo MR, Giordani PJ, Lepper HS, Croghan TW. Patient adherence and medical treatment outcomes: a meta-analysis. Med Care. 2002;40(9):794–811. doi: 10.1097/00005650-200209000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Simpson SH, Eurich DT, Majumdar SR, Padwal RS, Tsuyuki RT, Varney J, Johnson JA. A meta-analysis of the association between adherence to drug therapy and mortality. BMJ. 2006;333(7557):15. doi: 10.1136/bmj.38875.675486.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mathes T, Pieper D, Antoine SL, Eikermann M. Adherence influencing factors in patients taking oral anticancer agents: a systematic review. Cancer Epidemiol. 2014;38(3):214–226. doi: 10.1016/j.canep.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 8.Shea B, Grimshaw J, Wells G, Boers M, Andersson N, Hamel C, Porter A, Tugwell P, Moher D, Bouter L. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7(1):10. doi: 10.1186/1471-2288-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shea BJ, Hamel C, Wells GA, Bouter LM, Kristjansson E, Grimshaw J, Henry DA, Boers M. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J Clin Epidemiol. 2009;62(10):1013–1020. doi: 10.1016/j.jclinepi.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Grimshaw J, McAuley LM, Bero LA, Grilli R, Oxman AD, Ramsay C, Vale L, Zwarenstein M. Systematic reviews of the effectiveness of quality improvement strategies and programmes. Qual Saf Health Care. 2003;12(4):298–303. doi: 10.1136/qhc.12.4.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Broekmans S, Dobbels F, Milisen K, Morlion B, Vanderschueren S. Medication adherence in patients with chronic non-malignant pain: is there a problem? Eur J pain (Lond, Eng) 2009;13(2):115–123. doi: 10.1016/j.ejpain.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 12.Daley DJ, Myint PK, Gray RJ, Deane KH. Parkinsonism Relat Disord. 2012. Systematic review on factors associated with medication non-adherence in Parkinson’s disease. [DOI] [PubMed] [Google Scholar]
- 13.Oosterom-Calo R, van Ballegooijen AJ, Terwee CB, Te Velde SJ, Brouwer IA, Jaarsma T, Brug J. Determinants of adherence to heart failure medication: a systematic literature review. Heart Fail Rev. 2013;18(4):409–427. doi: 10.1007/s10741-012-9321-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pasma A, Van’t Spijker A, Hazes JM, Busschbach JJ, Luime JJ. Semin Arthritis Rheum. 2013. Factors associated with adherence to pharmaceutical treatment for rheumatoid arthritis patients: A systematic review. [DOI] [PubMed] [Google Scholar]
- 15.Schrijvers LH, Uitslager N, Schuurmans MJ, Fischer K. Barriers and motivators of adherence to prophylactic treatment in haemophilia: a systematic review. Haemophilia: J World Federation Hemophilia. 2013;19(3):355–361. doi: 10.1111/hae.12079. [DOI] [PubMed] [Google Scholar]
- 16.Sinnott SJ, Buckley C, O’Riordan D, Bradley C, Whelton H: The Effect of Copayments for Prescriptions on Adherence to Prescription Medicines in Publicly Insured Populations; A Systematic Review and Meta-Analysis. PLoS One. 2013, 8 (5): [DOI] [PMC free article] [PubMed]
- 17.Verbrugghe M, Verhaeghe S, Lauwaert K, Beeckman D, Van Hecke A. Determinants and associated factors influencing medication adherence and persistence to oral anticancer drugs: A systematic review. Cancer Treat Rev. 2013;39(6):610–621. doi: 10.1016/j.ctrv.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 18.Atkinson MJ, Petrozzino JJ. An evidence-based review of treatment-related determinants of patients’ nonadherence to HIV medications. AIDS Patient Care STDS. 2009;23(11):903–914. doi: 10.1089/apc.2009.0024. [DOI] [PubMed] [Google Scholar]
- 19.Davies MJ, Gagliardino JJ, Gray LJ, Khunti K, Mohan V, Hughes R. Real-world factors affecting adherence to insulin therapy in patients with Type 1 or Type 2 diabetes mellitus: a systematic review. Diabet Med. 2013;30(5):512–524. doi: 10.1111/dme.12128. [DOI] [PubMed] [Google Scholar]
- 20.Thorneloe RJ, Bundy C, Griffiths CE, Ashcroft DM, Cordingley L. Adherence to medication in patients with psoriasis: a systematic literature review. Br J Dermatol. 2013;168(1):20–31. doi: 10.1111/bjd.12039. [DOI] [PubMed] [Google Scholar]
- 21.Dew MA, DiMartini AF, De Vito DA, Myaskovsky L, Steel J, Unruh M, Switzer GE, Zomak R, Kormos RL, Greenhouse JB. Rates and risk factors for nonadherence to the medical regimen after adult solid organ transplantation. Transplantation. 2007;83(7):858–873. doi: 10.1097/01.tp.0000258599.65257.a6. [DOI] [PubMed] [Google Scholar]
- 22.Jackson CA, Clatworthy J, Robinson A, Horne R. Factors associated with non-adherence to oral medication for inflammatory bowel disease: a systematic review. Am J Gastroenterol. 2010;105(3):525–539. doi: 10.1038/ajg.2009.685. [DOI] [PubMed] [Google Scholar]
- 23.Gellad WF, Grenard J, McGlynn EA. A Review of Barriers to Medication Adherence: A Framework for Driving Policy Options. In: Corporation TR, editor. Technical report. Pittsburgh: RAND; 2009. [Google Scholar]
- 24.Hayden JA, Chou R, Hogg-Johnson S, Bombardier C. Systematic reviews of low back pain prognosis had variable methods and results: guidance for future prognosis reviews. J Clin Epidemiol. 2009;62(8):781–796. doi: 10.1016/j.jclinepi.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 25.Jepsen P, Johnsen SP, Gillman MW, Sorensen HT. Interpretation of observational studies. Heart. 2004;90(8):956–960. doi: 10.1136/hrt.2003.017269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rothman K, Greenland S, Lash T. Modern epidemiology. 3. Philadelphia: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 27.Mathes T, Antoine S-L, Pieper D. Factors influencing adherence in Hepatitis-C infected patients: a systematic review. BMC Infect Dis. 2014;14(1):203. doi: 10.1186/1471-2334-14-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vasamreddy CR, Dalal D, Dong JUN, Cheng A, Spragg D, Lamiy SZ, Meininger G, Henrikson CA, Marine JE, Berger R, Calkins H. Symptomatic and Asymptomatic Atrial Fibrillation in Patients Undergoing Radiofrequency Catheter Ablation. J Cardiovasc Electrophysiol. 2006;17(2):134–139. doi: 10.1111/j.1540-8167.2006.00359.x. [DOI] [PubMed] [Google Scholar]
- 29.Lehane E, McCarthy G. Intentional and unintentional medication non-adherence: A comprehensive framework for clinical research and practice? A discussion paper. Int J Nurs Stud. 2007;44(8):1468–1477. doi: 10.1016/j.ijnurstu.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 30.Gadkari A, McHorney C. Unintentional non-adherence to chronic prescription medications: How unintentional is it really? BMC Health Serv Res. 2012;12(1):98. doi: 10.1186/1472-6963-12-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vrijens B, De Geest S, Hughes DA, Przemyslaw K, Demonceau J, Ruppar T, Dobbels F, Fargher E, Morrison V, Lewek P, Matyjaszczyk M, Mshelia C, Clyne W, Aronson JK. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol. 2012;73(5):691–705. doi: 10.1111/j.1365-2125.2012.04167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akerblad AC, Bengtsson F, Holgersson M, von Knorring L, Ekselius L. Identification of primary care patients at risk of nonadherence to antidepressant treatment. Patient Prefer Adherence. 2008;2:379–386. doi: 10.2147/ppa.s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harmon G, Lefante J, Krousel-Wood M. Overcoming barriers: the role of providers in improving patient adherence to antihypertensive medications. Curr Opin Cardiol. 2006;21(4):310–315. doi: 10.1097/01.hco.0000231400.10104.e2. [DOI] [PubMed] [Google Scholar]
- 34.Balfour L, Tasca GA, Kowal J, Corace K, Cooper CL, Angel JB, Garber G, Macpherson PA, Cameron DW. Development and validation of the HIV Medication Readiness Scale. Assessment. 2007;14(4):408–416. doi: 10.1177/1073191107304295. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Search strategy.(DOCX 16 KB)
Additional file 2: Results of individual systematic reviews.(DOCX 15 KB)


