Abstract
Transgenic mice with knock-in (KI) of a tryptophan hydroxylase 2 (Tph2) R439H mutation, analogous to the Tph2 R441H single-nucleotide polymorphism originally identified in a late life depression cohort, have markedly reduced levels of 5-hydroxytryptamine (5-HT). These Tph2KI mice are therefore interesting as a putative translational model of low endogenous 5-HT function that allows for assessment of adaptive changes in different anatomical regions. Here, we determined 5-HT2A receptor binding in several brain regions using in vitro receptor autoradiography and two different radioligands. When using the 5-HT2A receptor selective antagonist radioligand 3H-MDL100907, we found higher binding in the prefrontal cortex (10%, P=0.009), the striatum (26%, P=0.005), and the substantia nigra (21%, P=0.027). The increase was confirmed in the same regions with the 5-HT2A/C receptor agonist, 3H-CIMBI-36 (2-(4-Bromo-2,5-dimethoxyphenyl)-N-(2-methoxybenzyl)ethanamine). 5-HT2A receptors establish heteromeric functional receptor complexes with metabotropic glutamate 2 receptors (mGluR2), but binding levels of the mGluR2/3 ligand 3H-LY341495 were unaltered in brain areas with increased 5-HT2A receptor levels. These data show that in distinct anatomical regions, 5-HT2A receptor binding sites are up-regulated in 5-HT deficient mice, and this increase is not associated with changes in mGluR2 binding.
Introduction
Increased 5-hydroxytryptamine 2A (5-HT2A) receptor levels in depression patients and in suicide victims have been reported postmortem [2, 25, 32] and vulnerability factors of depression are positively correlated with frontolimbic 5-HT2A receptor binding potential in vivo [12]. Conversely, atypical antidepressants such as nefazodone and mianserin are potent 5-HT2A receptor antagonists [6], although such compounds also target other receptors and transporters.
One mechanism through which cerebral 5-HT2A receptors levels increase in depression could be an autoregulatory response to sustained low 5-HT levels. A moderate 5-HT depletion results in an increase and 5-HT2A receptor levels [5, 16] and chronic treatment with selective 5-HT reuptake inhibitors leads to a reduction in 5-HT2A receptor binding [15, 22, 23]. This has also been seen in humans as chronic but not acute treatment with 5-HT uptake inhibitors decreases cortical 5-HT2A receptor binding [24, 29]. It is still unclear if lower 5-HT is the only mechanism leading to an autoregulatory increase in 5-HT2A receptor level or other factors play a role.
A rare decrease-of-function mutation in the coding region of the gene for tryptophan hydroxylase 2 (Tph2) leading to an Arg441His substitution was recently discovered in depressed individuals [34]. Subsequently, a mouse model carrying the identical Arg439His substitution was generated by homologous combination [1]. These Tph2 knock-in (Tph2KI) mice have reduced synthesis-, tissue- and extracellular levels of 5-HT and exhibit increased depression- anxiety- and aggression-like behaviours [1, 18]. The Tph2KI mouse represents an excellent naturalistic model for the study of the long-term consequences of reduced 5-HT-neurotransmission, such as regulation of 5-HT2A receptors by ambient 5-HT levels.
More specific aims of these studies were to correlate any consequence of low 5-HT neurotransmission on 5-HT2A receptor binding to specific anatomical locations using two radioligands with different pharmacological activity on the receptor. Semi-quantitative autoradiography using 3H-MDL100907 was used to measured changes in several cortical and subcortical structures. To examine if changes were taking place in high-affinity 5-HT2A receptor binding sites, we also employed the recently discovered 5-HT2A receptor agonist radiotracer Cimbi-36 [9].
Several lines of evidence suggest that the metabotropic glutamate receptor 2 (mGluR2) establish dimers with 5-HT2A receptors [13, 27]. Activation of 5-HT2A receptor-associated mGluR2 modulates 5-HT2A receptor-mediated cell signalling in a fashion lessening the propensity for hallucinogenic effects [13, 26, 27]. The mGluR2 is therefore considered an important potential target for anti-psychotic treatment [10, 11, 14]. The stoichiometry between the 5-HT2A receptor and mGluR2 may therefore be functionally important [13, 27]. The two receptors exist as either homomeric or heteromeric receptors and activation of homomeric rather than heteromeric receptors would supposedly have different functional responses. In that perspective, we also investigated possible changes in mGluR2 binding using the mGluR2/3 ligand 3H-LY341495 [19] to see if increase in 5-HT2A receptor binding is accompanied by increase in mGluR2 binding.
Materials and Methods
Animals and tissue preparation
The generation of the Tph2KI mice has been described previously [1]. Littermate mice were housed 3–5 per cage with food and water available ad libitum on a 12-h light-dark cycle at an ambient temperature of 21+/− 2°C. The mice were quickly euthanized by cervical dislocation and the brains rapidly frozen on dry ice. The brains were sectioned on a cryostat in 12 µm coronal sections, thaw-mounted on superfrost plus glass slides (Thermo Scientific, Braunschweig, Germany), dried, and stored at −80°C until use. Glass slides with 4–5 brain sections were collected at seven rostro-caudal levels of the mouse brain containing the following seven anatomical regions: prefrontal cortex, striatum, hippocampus, hypothalamus, substantia nigra, brainstem, and cerebellum.
5-HT2A receptor autoradiography
3H-MDL100907 (77 Ci/mmol, kindly donated by Dr. Christer Halldin from Karolinska Institute, Stockholm, Sweden) was used for 5-HT2A receptor autoradiography [20]. 3H-CIMBI-36 (2-(4-Bromo-2,5-dimethoxyphenyl)-N-(2-methoxybenzyl)ethanamine) is a 5-HT2A receptor agonist [9], and the labelled compound (53 Ci/mmol) was also kindly provided by Dr. Christer Halldin from Karolinska Institute, Stockholm, Sweden.
The tissue sections were thawed for one hour and pre-incubated at room temperature in 50 mM Tris-HCl, pH 7.4 for 15 min. Incubation was performed in the same buffer containing 1.25 nM 3H-MDL100907 for 60 min, and non-specific binding (NSB) was assessed in adjacent sections in the presence of 10 µM ketanserin tartrate (Tocris Biosciences, Bristol, UK). Sections were washed 2 × 5 min in ice-cold 50 mM Tris-HCl followed by a 20 sec dip in ice-cold dH2O, and dried on a heating plate (60°C) for 10 min. Finally, sections were fixed in paraformaldehyde vapour overnight at 4°C, and dried for 3 hours in a desiccator at room temperature. The processed slides were together with 3H-microscales exposed to a tritium-sensitive BAS TR2040 phosphor imaging plate (Science Imaging Scandinavia AB, Nacka, Sweden) for 7 days at 4°C. The imaging plate was scanned on a BAS-2500 bioimaging analyser (Fujifilm Europe GmbH, Düsseldorf, Germany).
The buffer used for pre-incubation and incubation with 2 nM 3H-CIMBI-36 was 50 mM Tris-HCl containing 1% BSA and 4 mM CaCl2, pH 7.4. The incubation time used in this experiment was 120 min and the sections washed for 2 × 10 min in the incubation buffer and processed as above.
mGluR2/3 autoradiography
3H-LY341495 (40 Ci/mmol, American Radiolabeled Chemicals, Saint Louis, MO) was used for mGluR2/3 autoradiography [19, 21]. The general procedure was as described for 5-HT2A receptor autoradiography [20] with the following changes: Pre-incubation for 30 min was performed in 50 mM Tris-HCl, pH 7.4. Then the sections were incubated in 2 nM 3H-LY341495 in the same buffer as used for pre-incubation for 90 min. Non-specific binding was determined in the presence of 10 µM glutamate (Sigma-Aldrich, Denmark). Sections were washed for 2 × 30 sec in ice-cold 50 mM Tris-HCl, followed by a30 sec dip in ice-cold dH2O.
Image analysis of autoradiograms and statistical analysis
Autoradiograms were analysed with Quantity One (version 4.6.8; BioRad, Hercules, CA). Image optical density was determined in the areas of interest in at least three neighbour sections from each animal and background subtracted from each of these measures. The densities were converted to activity density in nCi/mg tissue equivalent using the linear range of 3H-microscales and then converted to radioligand binding in fmol/mg tissue equivalent using the specific activity of the ligands. The specific receptor binding was determined by subtracting non-specific binding from the total radioactivity in each animal, and the brain regional binding were analysed with Student’s t-test.
Results
5-HT2A receptors are up-regulated in PFC, striatum and substantia nigra
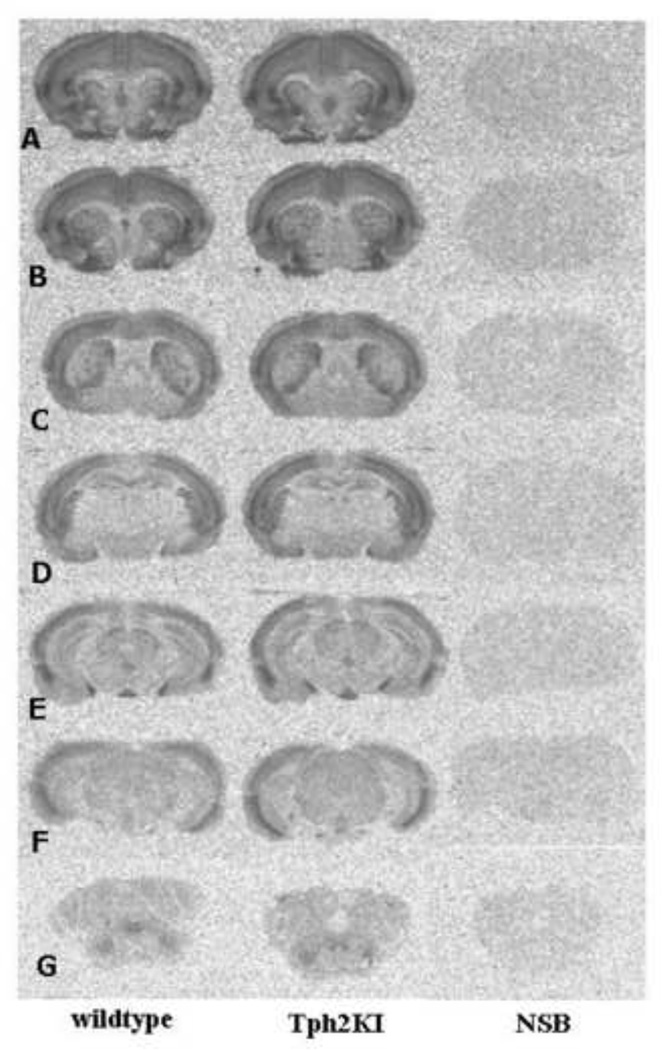
Receptor autoradiography using the ligand 3H-MDL100907 revealed the characteristic distribution of 5-HT2A receptor binding sites throughout the mouse brain. Binding was completely blocked by co-incubation with ketanserin (Fig. 1). High densities were observed presumably in layer III–IV throughout the neocortex, in the striatum, and in the interpeduncular nucleus. In the striatum, the labeling was heterogeneously distributed and appeared to be most dense in rostral and medial portions (Fig. 1C). Moderate 5-HT2A receptor levels were observed in the prefrontal cortex, frontal cortex, hippocampus, hypothalamus, parietal cortex. Low binding was observed in the substantia nigra, the brainstem, and the cerebellum (Table 1). Overall, higher levels of binding were detected in most of the regions in the Tph2KI mice though the magnitude was different between the brain areas (Table 1). A 10% increase in receptor binding was found in prefrontal cortex (P < 0.01) while the binding in the striatum (P < 0.005) and substantia nigra (P < 0.05) was increased with more than 20% compared to wildtype. Moderate increase was seen in all other regions, though one important exception was the hippocampus where a slight non-significant reduction in binding levels was observed.
Figure 1.
Binding of 3H-MDL100907 in wild type and Tph2KI mice. Representative autoradiograms throughout the mouse brain of 5-HT2A receptor binding. Specific 3H-MDL100907 binding (1.25 nM) and non-specific binding in the presence of 10 µM ketanserin. n=6–10.
Table 1.
5-HT2A receptor binding in wildtype and tph2KI mice. Densiometric measurements were performed on autoradiograms from sections incubated with 3H-MDL100907 in different brain regions. The data are expressed as binding in areas of interest and the relative change and statistical difference as revealed by Student t-tests are indicated. Significant changes are present in the prefrontal cortex, the striatum, and the substantia nigra.
| Wildtype (fmol/mg) |
n | Tph2KI (fmol/mg) |
n | % change | P-value | |
|---|---|---|---|---|---|---|
| Prefrontal cortex | 48.40 ± 1.16 | 7 | 53.24 ± 1.07 | 8 | 10.00 | 0.0088 ** |
| Frontal cortex | 39.54 ± 1.34 | 10 | 42.38 ± 1.33 | 8 | 7.18 | 0.1271 |
| Striatum | 20.54 ± 1.09 | 10 | 25.86 ± 1.21 | 8 | 25.90 | 0.0048 ** |
| Parietal cortex | 30.67 ± 1.38 | 6 | 33.45 ± 1.38 | 8 | 9.06 | 0.1898 |
| Hippocampus | 15.09 ± 0.75 | 7 | 14.25 ± 0.69 | 7 | −5.57 | 0.4202 |
| Hypothalamus | 12.55 ± 0.49 | 6 | 15.06 ± 1.10 | 7 | 20.00 | 0.0734 |
| Substantia nigra | 6.24 ± 0.34 | 8 | 7.52 ± 0.39 | 8 | 20.51 | 0.0271* |
| Brainstem | 7.48 ± 0.34 | 9 | 8.30 ± 0.40 | 8 | 10.96 | 0.1323 |
| Cerebellum | 2.66 ± 0.32 | 9 | 2.88 ± 0.30 | 7 | 8.27 | 0.6296 |
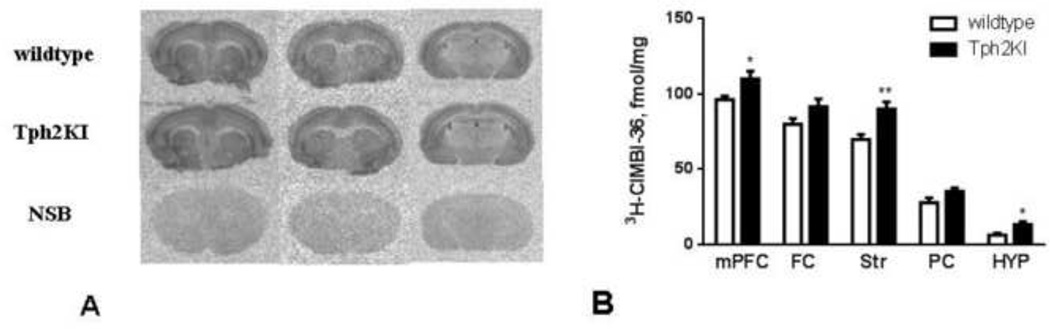
The 5-HT2A receptor agonist and radioligand 3H-CIMBI-36 was used to assess if the observed changes in 5-HT2A receptor binding involved high-affinity binding sites. Analysis of the receptor autoradiograms revealed overall the same distribution of binding as described for 3H-MDL100907 (Fig. 2A). However, due to 5-HT2C receptor binding [4], labeling of the choroid plexus was present using 3H-CIMBI-36 (Fig. 2A). Binding of 3H-CIMBI-36 was also significantly higher in the prefrontal cortex, striatum and hypothalamus in the Tph2KI mice (Fig. 2B). The remaining regions also contained a higher level of binding for 3H-CIMBI-36 in the Tph2KI mice, although not statistically significant (Fig. 2B).
Figure 2.
Receptor binding in wild-type and Tph2KI mice with the radioligand 3H-CIMBI-36 (2 nM). Examples of receptor autoradiograms using 3H-CIMBI-36 demonstrate binding in the cortex and the striatum. Non-specific binding was assessed in the presence of 10 µM ketanserin (A). The quantitative data are presented as means ± S.E.M (B). Student’s unpaired t-test. n=7–10.
mGluR2/3 binding is unaltered
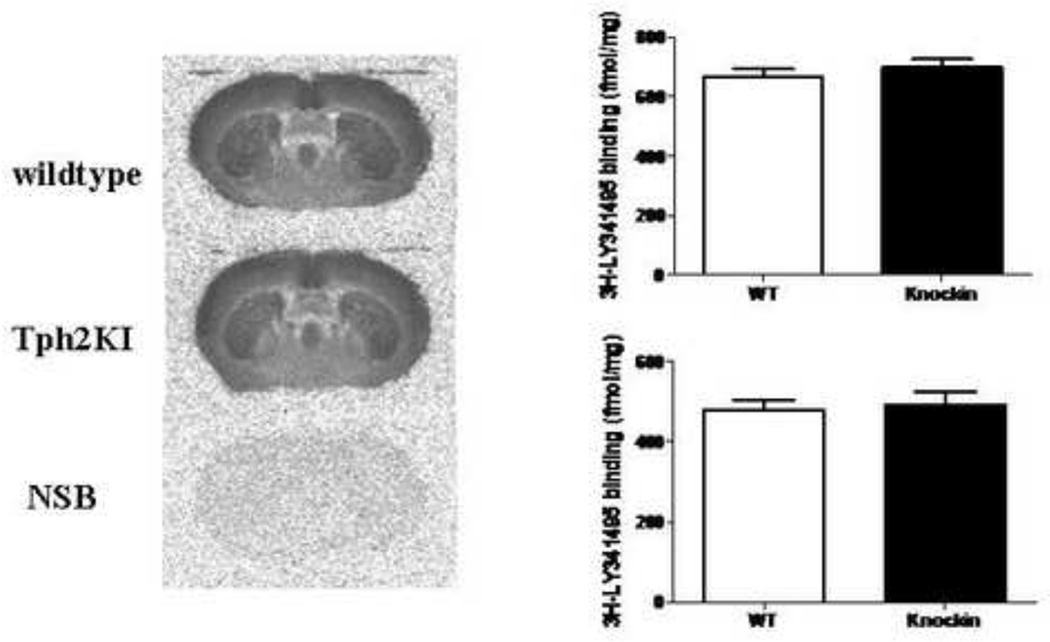
The ratio between 5-HT2A receptors and mGluR2 seems to be important for signalling mediated through the 5-HT2A receptor. In the same animals as used for 5-HT2A receptor binding, receptor autoradiography using 3H-LY341495 was conducted. Quantification of labelling was performed in the two areas where the most significant 5-HT2A receptor binding changes were observed, the prefrontal cortex and the striatum. However, mGluR2/3 binding as determined by 3H-LY341495 autoradiography was not different between wild type and Tph2KI mice (Fig. 3B).
Figure 3.
Receptor binding using 3H-LY341495 binding (2 nM) in the striatum. The quantitative data are presented as means ± S.E.M. Student’s unpaired t-test. n=8–10.
Discussion
We here report that 5-HT deficiency due to impaired endogenous 5-HT synthesis in the Tph2KI transgene mice results in significantly increased 5-HT2A receptor binding levels in the prefrontal cortex, striatum, and the substantia nigra. The increase in binding was detected using both 3H-MDL100907 and 3H-CIMBI-36; an antagonist and agonist radioligand with different efficacies on the 5-HT2A receptor, respecitively [4, 9, 20].
Similar effects on 5-HT2A receptors have been reported in other models where 5-HT levels are reduced by mechanical or neurotoxic lesions of the dorsal raphe, or by depletion of the 5-HT precursor tryptophan [8, 16, 31]. Furthermore, increase in 5-HT2A receptor binding has been reported but only after chronic and not acute tryptophan depletion [5]. Chronic tryptophan depletion increases 3H-ketanserin binding in the cortex, but not hippocampus [5]. Binding studies and detection of 5-HT2A receptor mRNA levels revealed increases in Bmax and mRNA suggesting that the number of 5-HT2A receptors is increased rather than binding properties [31]. The increase in binding also reflects a higher sensitivity to the 5-HT2A/C receptor agonist DOI in the Tph2KI mice as the number of head twitches in response to DOI is increased in the Tph2KI mice compared to wildtype [18].
Assuming that all 5-HT neurons produce less 5-HT and the reduction in neurotransmission may occur in all target areas, it is somewhat surprising that the 5-HT2A receptor is only changed in some brain areas and not in other. The region-specific regulations of 5-HT2A receptors could be explained by the synaptic localization of the 5-HT2A receptor. A common characteristic of the brain areas with significant alterations in 5-HT2A receptor binding is they receive a high density of 5-HT nerve fibres [17]. In the cortex, the receptor is primary located on glutamatergic pyramidal neurons, while in the hippocampus the majority of receptors are located on GABAergic interneurons [33]. Decreased 5-HT2A receptor binding has been found in the hippocampus of depressed individuals, while binding is increased in other regions [7, 30]. This is interesting, because the same pattern of receptor changes seen in the Tph2KI mice.
Another possibility is the synaptic distribution of 5-HT2A receptors change in response to 5-HT levels. In the prefrontal cortex, 5-HT2A receptors modulate the release of dopamine and glutamate [3, 28] and it is likely that 5-HT2A receptor antagonistic antidepressants may partly exert their therapeutic action by reducing the activity of pyramidal neurons in mPFC [6].
The differences in 5-HT2AR binding between transgenic and wildtype mice were seen both with the agonist and the antagonist, suggesting that the regulatory effect of the lower 5-HT levels also affected high-affinity binding sites.
In contrast to 5-HT2A receptor binding, the mGluR2/3 binding was not changed in the Tph2KI mice. The two receptors form heteromeric complexes [13] and activation of mGluR2 inhibits 5-HT2A receptor signalling [26]. This effect is considered to be an important mechanism through which mGluR2 agonist exert anxiolytic and anti-psychotic effects [10]. This suggests that it is only as monomers that 5-HT2A receptors upregulate in response to lower 5-HT levels.
Acknowledgements
This work was supported in part by grants from the Rigshospitalets Forskningsfond, the Danish Strategic Research Council COGNITO, The Lundbeck Foundation, and the National Institutes of Health MH79201.
References
- 1.Beaulieu JM, Zhang X, Rodriguiz RM, Sotnikova TD, Cools MJ, Wetsel WC, Gainetdinov RR, Caron MG. Role of GSK3 beta in behavioral abnormalities induced by serotonin deficiency. Proc Natl Acad Sci U S A. 2008;105:1333–1338. doi: 10.1073/pnas.0711496105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhagwagar Z, Hinz R, Taylor M, Fancy S, Cowen P, Grasby P. Increased 5-HT(2A) receptor binding in euthymic, medication-free patients recovered from depression: a positron emission study with [(11)C]MDL 100,907. Am J Psychiatry. 2006;163:1580–1587. doi: 10.1176/ajp.2006.163.9.1580. [DOI] [PubMed] [Google Scholar]
- 3.Bortolozzi A, Diaz-Mataix L, Scorza MC, Celada P, Artigas F. The activation of 5-HT receptors in prefrontal cortex enhances dopaminergic activity. J Neurochem. 2005;95:1597–1607. doi: 10.1111/j.1471-4159.2005.03485.x. [DOI] [PubMed] [Google Scholar]
- 4.Braden MR, Parrish JC, Naylor JC, Nichols DE. Molecular interaction of serotonin 5-HT2A receptor residues Phe339(6.51) and Phe340(6.52) with superpotent N-benzyl phenethylamine agonists. Mol Pharm. 2006;70:1956–1964. doi: 10.1124/mol.106.028720. [DOI] [PubMed] [Google Scholar]
- 5.Cahir M, Ardis T, Reynolds GP, Cooper SJ. Acute and chronic tryptophan depletion differentially regulate central 5-HT1A and 5-HT 2A receptor binding in the rat. Psychopharmacology. 2007;190:497–506. doi: 10.1007/s00213-006-0635-5. [DOI] [PubMed] [Google Scholar]
- 6.Celada P, Puig M, Amargos-Bosch M, Adell A, Artigas F. The therapeutic role of 5-HT1A and 5-HT2A receptors in depression. J Psychiatry Neurosci. 2004;29:252–265. [PMC free article] [PubMed] [Google Scholar]
- 7.Cheetham SC, Crompton MR, Katona CL, Horton RW. Brain 5-HT2 receptor binding sites in depressed suicide victims. Brain Res. 1988;443:272–280. doi: 10.1016/0006-8993(88)91621-6. [DOI] [PubMed] [Google Scholar]
- 8.Compan V, Segu L, Buhot MC, Daszuta A. Differential effects of serotonin (5-HT) lesions and synthesis blockade on neuropeptide-Y immunoreactivity and 5-HT1A, 5-HT1B/1D and 5-HT2A/2C receptor binding sites in the rat cerebral cortex. Brain Res. 1998;795:264–276. doi: 10.1016/s0006-8993(98)00316-3. [DOI] [PubMed] [Google Scholar]
- 9.Ettrup A, Hansen M, Santini MA, Paine J, Gillings N, Palner M, Lehel S, Herth MM, Madsen J, Kristensen J, Begtrup M, Knudsen GM. Radiosynthesis and in vivo evaluation of a series of substituted 11C-phenethylamines as 5-HT (2A) agonist PET tracers. Eur J Nucl Med Mol Imaging. 2011;38:681–693. doi: 10.1007/s00259-010-1686-8. [DOI] [PubMed] [Google Scholar]
- 10.Fell MJ, McKinzie DL, Monn JA, Svensson KA. Group II metabotropic glutamate receptor agonists and positive allosteric modulators as novel treatments for schizophrenia. Neuropharmacology. 2012;62:1473–1483. doi: 10.1016/j.neuropharm.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 11.Fell MJ, Svensson KA, Johnson BG, Schoepp DD. Evidence for the role of metabotropic glutamate (mGlu)2 not mGlu3 receptors in the preclinical antipsychotic pharmacology of the mGlu2/3 receptor agonist (−)-(1R,4S,5S,6S)-4-amino-2-sulfonylbicyclo[3.1.0]hexane-4,6-dicarboxylic acid (LY404039) J Pharmacol Exp Ther. 2008;326:209–217. doi: 10.1124/jpet.108.136861. [DOI] [PubMed] [Google Scholar]
- 12.Frokjaer VG, Mortensen EL, Nielsen FA, Haugbol S, Pinborg LH, Adams KH, Svarer C, Hasselbalch SG, Holm S, Paulson OB, Knudsen GM. Frontolimbic serotonin 2A receptor binding in healthy subjects is associated with personality risk factors for affective disorder. Biol Psychiatry. 2008;63:569–576. doi: 10.1016/j.biopsych.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez-Maeso J, Ang RL, Yuen T, Chan P, Weisstaub NV, Lopez-Gimenez JF, Zhou M, Okawa Y, Callado LF, Milligan G, Gingrich JA, Filizola M, Meana JJ, Sealfon SC. Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature. 2008;452:93–97. doi: 10.1038/nature06612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grillon C, Cordova J, Levine LR, Morgan CA., 3rd Anxiolytic effects of a novel group II metabotropic glutamate receptor agonist (LY354740) in the fear-potentiated startle paradigm in humans. Psychopharmacology. 2003;168:446–454. doi: 10.1007/s00213-003-1444-8. [DOI] [PubMed] [Google Scholar]
- 15.Gunther L, Liebscher S, Jahkel M, Oehler J. Effects of chronic citalopram treatment on 5-HT1A and 5-HT2A receptors in group- and isolation-housed mice. Eur J Pharmacol. 2008;593:49–61. doi: 10.1016/j.ejphar.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 16.Heal DJ, Philpot J, Molyneux SG, Metz A. Intracerebroventricular administration of 5,7-dihydroxytryptamine to mice increases both head-twitch response and the number of cortical 5-HT2 receptors. Neuropharmacology. 1985;24:1201–1205. doi: 10.1016/0028-3908(85)90155-8. [DOI] [PubMed] [Google Scholar]
- 17.Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- 18.Jacobsen JP, Siesser WB, Sachs BD, Peterson S, Cools MJ, Setola V, Folgering JH, Flik G, Caron MG. Deficient serotonin neurotransmission and depression-like serotonin biomarker alterations in tryptophan hydroxylase 2 (Tph2) loss-of-function mice. Mol. Psych. 2012;17:694–704. doi: 10.1038/mp.2011.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson BG, Wright RA, Arnold MB, Wheeler WJ, Ornstein PL, Schoepp DD. [3H]-LY341495 as a novel antagonist radioligand for group II metabotropic glutamate (mGlu) receptors: characterization of binding to membranes of mGlu receptor subtype expressing cells. Neuropharmacology. 1999;38:1519–1529. doi: 10.1016/s0028-3908(99)00053-2. [DOI] [PubMed] [Google Scholar]
- 20.Johnson MP, Siegel BW, Carr AA. [3H]MDL 100,907: a novel selective 5-HT2A receptor ligand. Naunyn Schmiedebergs Arch Pharmacol. 1996;354:205–209. doi: 10.1007/BF00178722. [DOI] [PubMed] [Google Scholar]
- 21.Kingston AE, Ornstein PL, Wright RA, Johnson BG, Mayne NG, Burnett JP, Belagaje R, Wu S, Schoepp DD. LY341495 is a nanomolar potent and selective antagonist of group II metabotropic glutamate receptors. Neuropharmacology. 1998;37:1–12. doi: 10.1016/s0028-3908(97)00191-3. [DOI] [PubMed] [Google Scholar]
- 22.Licht CL, Marcussen AB, Wegener G, Overstreet DH, Aznar S, Knudsen GM. The brain 5-HT4 receptor binding is down-regulated in the Flinders Sensitive Line depression model and in response to paroxetine administration. J Neurochem. 2009;109:1363–1374. doi: 10.1111/j.1471-4159.2009.06050.x. [DOI] [PubMed] [Google Scholar]
- 23.Maj J, Bijak M, Dziedzicka-Wasylewska M, Rogoz R, Rogoz Z, Skuza G, Tokarski T. The effects of paroxetine given repeatedly on the 5-HT receptor subpopulations in the rat brain. Psychopharmacology. 1996;127:73–82. doi: 10.1007/BF02805977. [DOI] [PubMed] [Google Scholar]
- 24.Meyer JH, Kapur S, Eisfeld B, Brown GM, Houle S, DaSilva J, Wilson AA, Rafi-Tari S, Mayberg HS, Kennedy SH. The effect of paroxetine on 5-HT(2A) receptors in depression: an [(18)F]setoperone PET imaging study. Am J Psychiat. 2001;158:78–85. doi: 10.1176/appi.ajp.158.1.78. [DOI] [PubMed] [Google Scholar]
- 25.Meyer JH, McMain S, Kennedy SH, Korman L, Brown GM, DaSilva JN, Wilson AA, Blak T, Eynan-Harvey R, Goulding VS, Houle S, Links P. Dysfunctional attitudes and 5-HT2 receptors during depression and self-harm. Am J Psychiat. 2003;160:90–99. doi: 10.1176/appi.ajp.160.1.90. [DOI] [PubMed] [Google Scholar]
- 26.Molinaro G, Traficante A, Riozzi B, Di Menna L, Curto M, Pallottino S, Nicoletti F, Bruno V, Battaglia G. Activation of mGlu2/3 metabotropic glutamate receptors negatively regulates the stimulation of inositol phospholipid hydrolysis mediated by 5-hydroxytryptamine2A serotonin receptors in the frontal cortex of living mice. Mol Pharm. 2009;76:379–387. doi: 10.1124/mol.109.056580. [DOI] [PubMed] [Google Scholar]
- 27.Moreno JL, Holloway T, Albizu L, Sealfon SC, Gonzalez-Maeso J. Metabotropic glutamate mGlu2 receptor is necessary for the pharmacological and behavioral effects induced by hallucinogenic 5-HT2A receptor agonists. Neurosci Lett. 2011;493:76–79. doi: 10.1016/j.neulet.2011.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pehek EA, Nocjar C, Roth BL, Byrd TA, Mabrouk OS. Evidence for the preferential involvement of 5-HT2A serotonin receptors in stress- and drug-induced dopamine release in the rat medial prefrontal cortex. Neuropsychopharmacology. 2006;31:265–277. doi: 10.1038/sj.npp.1300819. [DOI] [PubMed] [Google Scholar]
- 29.Pinborg LH, Adams KH, Yndgaard S, Hasselbalch SG, Holm S, Kristiansen H, Paulson OB, Knudsen GM. [18F]altanserin binding to human 5HT2A receptors is unaltered after citalopram and pindolol challenge. J Cerebr Blood Flow Metabol. 2004;24:1037–1045. doi: 10.1097/01.WCB.0000126233.08565.E7. [DOI] [PubMed] [Google Scholar]
- 30.Rosel P, Arranz B, Urretavizcaya M, Oros M, San L, Navarro MA. Altered 5-HT2A and 5-HT4 postsynaptic receptors and their intracellular signalling systems IP3 and cAMP in brains from depressed violent suicide victims. Neuropsychobiology. 2004;49:189–195. doi: 10.1159/000077365. [DOI] [PubMed] [Google Scholar]
- 31.Soria-Fregozo C, Perez-Vega MI, Gonzalez-Burgos I, Feria-Velasco A, Beas-Zarate C. Prefrontal serotonergic denervation induces increase in the density of 5-HT2A receptors in adult rat prefrontal cortex. Neurochem Res. 2008;33:2350–2357. doi: 10.1007/s11064-008-9740-7. [DOI] [PubMed] [Google Scholar]
- 32.Stanley M, Mann JJ. Increased serotonin-2 binding sites in frontal cortex of suicide victims. Lancet. 1983;1:214–216. doi: 10.1016/s0140-6736(83)92590-4. [DOI] [PubMed] [Google Scholar]
- 33.Vaidya VA, Marek GJ, Aghajanian GK, Duman RS. 5-HT2A receptor-mediated regulation of brain-derived neurotrophic factor mRNA in the hippocampus and the neocortex. J Neurosci. 1997;17:2785–2795. doi: 10.1523/JNEUROSCI.17-08-02785.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang X, Gainetdinov RR, Beaulieu JM, Sotnikova TD, Burch LH, Williams RB, Schwartz DA, Krishnan KR, Caron MG. Loss-of-function mutation in tryptophan hydroxylase-2 identified in unipolar major depression. Neuron. 2005;45:11–16. doi: 10.1016/j.neuron.2004.12.014. [DOI] [PubMed] [Google Scholar]