Abstract
Background
Normal hepatocytes exhibit low-affinity hexokinase (glucokinase [HKIV]), but during oncogenesis, there is a switch from HKIV to HKII expression. The aims of this study were to compare the immunoexpression of HKII in non-dysplastic cirrhosis (NDC), liver cell change/dysplasia in cirrhosis (LCD), HCC, and normal liver control tissues, and to correlate HKII expression with clinical and histopathological parameters.
Design
Immunohistochemistry was performed on a liver cancer progression tissue array consisting of specimens from explants with cirrhosis, including 45 tissue samples with HCC, 108 without HCC, 143 with LCD, and 8 normal liver control tissues. HKII expression was quantified as positive pixel counts/square millimeter (ppc/mm2) by image analysis.
Results
There was a stepwise increase in HKII level from normal liver tissue to NDC, to LCD, and to HCC (p = 0.001). HKII levels were significantly higher in areas of LCD versus NDC (p ≤ 0.001), and in LCD and HCC versus NDC (p = 0.007). HKII levels were similar in LCD and HCC (p = 0.124). HKII levels were higher in grade 2–4 versus grade 1 HCCs (p = 0.044), and in pleomorphic versus non-pleomorphic HCC variants (p = 0.041). Higher levels of HKII expression in LCD and HCC versus NDC and in higher tumor grade remained significant in multivariate analysis.
Conclusions
Higher levels of HKII immunoexpression in LDC and HCC compared with NDC suggest that upregulation of HKII occurs during the process of hepatocarcinogenesis in humans. In HCC, higher levels of HKII are associated with more aggressive histological features.
Keywords: Hepatocellular carcinoma, Liver cell change/dysplasia in cirrhosis, Non-dysplastic cirrhosis, Hexokinase II immunohistochemistry, Tissue microarray, Image analysis
Introduction
Data from the SEER program registries showed that the incidence of liver cancer increased between 1975 and 2005 at an annual percent change of 4.1 % in men and 3.8 % in women [1]. Hepatocarcinogenesis results from a series of pro-oncogenic molecular events and morphological alterations during the course of liver disease progression. In most cases, the sequence of hepatocarcinogenesis includes the development of cirrhosis, followed by dysplasia, and HCC [2]. An increase in hepatitis C virus (HCV) cirrhosis has played a major role in the rise in HCC and now accounts for 25–50 % of cases in the USA [3, 4]. There is growing evidence that insulin resistance and inflammatory factors implicated in the development of nonalcoholic fatty liver disease (NASH) can promote hepatocarcinogenesis [5–10]. Given the increasing incidence of HCC, it is critical to better understand molecular events associated with tumor development in order to identify effective biomarkers and therapeutic targets.
In the absence of liver disease, normal adult hepatocytes express only the low-affinity glucokinase (HKIV) for the regulation of glucose homeostasis in the feeding and fasting states [11]. A transformation from HKIV to high-affinity HKII expression allows for the high glycolytic rates that occur in anaerobic metabolism during tumorigenesis and tumor progression [12–14]. Levels of mitochondrial hexokinase can be enhanced up to 200-fold in metabolically active malignant cells in comparison with normal tissues [15].
HKII was characterized in benign versus malignant hepatocytes in rat immunohistochemistry [16] and DNA genomic studies [17]. HKII is not expressed in the majority of adult human normal tissues, but is upregulated by oncogenic signaling and insulin. HKII was found to be enhanced in a number of human cancers including HCC [18–22]. In HCC, there is an isoform change from the low-affinity glucokinase to the high-affinity HKII. The expression of HKII in HCC cells distinguishes them from normal hepatocytes. Recent observations in humans have shown an association between increased HKII immuno-expression and higher HCC tumor grade, stage [23, 24], and poorer patient survival [24, 25]. However, it is not known when HKII expression becomes up-regulated in the sequence of hepatocarcinogenesis. The goals of this study were to compare the immunoexpression of HKII in non-dysplastic cirrhosis (NDC), liver cell change/dysplasia in cirrhosis (LCD), HCC, and normal liver control tissues, and to correlate HKII level with clinical and histopathological parameters.
Materials and Methods
Subjects
Subjects consisted of 153 patients who underwent liver transplantation at the University of Illinois Hospital and Health Sciences System between 2004 and 2012 and 8 normal controls. All subjects had available clinical data and archived liver tissue for immunohistochemistry (IHC). Histological sections of cirrhotic liver explants with and without LCD and HCC were examined, and corresponding areas of the tissue blocks were sampled such that the total number of specimens exceeded the number of explants. Forty-five specimens had cirrhosis with HCC, 108 had non-dysplastic cirrhosis (NDC) without HCC, and 143 of the cases had areas of LCD including 98 cases without HCC and 45 with HCC. Eight normal liver control samples were obtained from uninvolved, non-diseased liver tissues of benign resection specimens for hemangioma and focal nodular hyperplasia. The demographic and clinical features of all 153 subjects in the study population were obtained from review of electronic medical records.
Gross and Microscopic Evaluation of Liver Tissues
Explants were grossly examined and sectioned at 0.5-cm intervals according to the segmental distribution of the liver. The size, location, and number of tumor(s) were recorded. Samples were obtained for microscopy according to published guidelines [26], and sections were histopathologically evaluated by a pathology team (RC, GG, RP). Dysplastic hepatocytes were defined by the presence of abnormal nuclear size, exhibiting small cell change or large cell change [27–30] bizarre hyperchromatic or occasionally multiple nuclei; distribution in clusters, groups, or occupying a whole nodule; and absent liver cell plate expansion [31, 32]. Malignant hepatocytes consistent with HCC were identified by the presence of thickened liver cell plates, increased nucleocytoplasmic (N/C) ratio, atypical vesicular nuclei, and when present, bile production [33]. The tumors were evaluated for tumor type, grade, and stage following the guidelines of the AJCC [34]. Tumor pattern was classified as pseudoglandular, trabecular, solid, or combined [35]. Tumor variants were designated as non-pleomorphic (usual variant), pleomorphic, and combined according to criteria outlined by Ferrell [36].
Tissue Microarrays (TMA)
The adequacy, quality, and structural integrity of the paraffin-embedded tissue blocks were assessed and verified prior to inclusion in the study. Explant liver samples from HCC and cirrhosis cases provided ample tissue for construction of arrays. The tissue microarrays were designed and constructed based on prescribed practices [37] using the Beecher manual tissue arrayer.
Hematoxylin and eosin-stained adjacent tissue sections were reviewed and used to identify areas of NDC and LCD in liver explants with and without hepatocellular carcinoma and HCC lesions in liver explants with HCC. Cores from archived normal liver specimens provided non-disease liver controls. Donor core tissues with 0.6 mm diameter were obtained from corresponding regions in the donor paraffin block and embedded in a recipient block. A single donor core tissue from each lesion consisting of NDC, LCD, and when present, HCC, was sampled across a patient’s liver explant. The donor cores from all subjects were embedded in a tissue microarray (TMA) recipient block. A total of two TMA recipient blocks were prepared, one consisting of liver tissue cores obtained from liver explants of subjects without HCC (2 donor cores were obtained per subject representing NDC and LCD), and another TMA contained liver tissue cores of patients with HCC (3 donor cores were taken from each subject, representing NDC, LCD, and HCC). Each of the tissue micro-array cores was histologically examined to document and record tissue-sampling adequacy and diagnoses. Four-micron sections from the TMAs were placed on positively charged glass slides for IHC.
Immunohistochemistry (IHC)
Immunohistochemical staining was performed using the avidin–biotin-peroxidase complex method [38]. Formalin-fixed, paraffin-embedded histological sections were de-waxed in xylene and then rehydrated by passage through graded alcohol. The tissue arrays were rinsed and then incubated with a solution of 0.3 % hydrogen peroxide, followed by incubation with 2 % BSA and then 10 μM sodium citrate buffer at pH 6.0. Anti-hexokinase II (C64G5) rabbit mAb (Cell Signaling®), 300 μL, was added to slides and maintained at 4 °C overnight in a humidified chamber. Sections were washed with PBS and then incubated with a 1: 300 μL dilution of biotinylated antibody to rabbit, followed by washing and incubation with a 1:1,000 dilution of avidin and biotin in PBS. Avidin–biotin complex was washed off three times in buffered solution for 5 min each. 100–400 μL DAB was added, and slides were immersed in dH20. Sections were dehydrated by incubation in 95 % ethanol twice for 10 s each. This was repeated in 100 % ethanol twice for 10 s each. Additional washes with xylene were performed twice for 10 s each time. Slides were counterstained with hematoxylin and washed with dH20 twice for 5 min, and coverslips were mounted. HK II signals were described as concentrating primarily within the external mitochondrial membrane, resulting in a granular pattern of cytoplasmic staining [39].
Digital Imaging and Image Analysis
The Aperio system (ScanScope CS) was used for digital imaging and image analysis of HKII immunostained slides. Image analysis was performed using ImageScope® software from Aperio. An inclusion pen for selecting image analysis outputs was used to outline pertinent tissue samples representing hepatocytes. Non-hepatocyte tissues were isolated with an exclusion device. Each of the cores was histologically examined for the verification of sample adequacy and diagnoses. An HKII immuno-level was measured from each core, representing NDC, LCD, and when present, HCC and recorded in an excel database file. Scoring of hexokinase II was confined to the cytoplasm consistent with its characteristic staining features as described in published immunohistochemical validation studies for HKII [39]. Image analysis outputs of HKII immuno-levels were expressed in ppc/mm2.
Analysis
Data analysis was performed using SPSS (SPSS, Inc., version 17.0, Chicago, IL) and SAS 9.2 (Cary, NC). Descriptive statistics were used to evaluate for normalcy of data and for erroneous entries. For independent observations, normally and non-normally distributed continuous variables were analyzed using the Student t test and the Mann–Whitney U test, respectively. Pearson chi-square testing was used to compare categorical variables. Overall nonparametric Friedman tests were used to compare the ranks [40] of HKII scores among correlated samples within the same patients to examine the progression of staining over disease stage. We also conducted paired statistical analysis using nonparametric Wilcoxon singed-rank tests to perform pairwise comparison. ANOVA was used to evaluate the mean differences in HKII scores among different groups defined by tumor grade, tumor variant, tumor stage, and African American versus non-African American ethnicities. Multiple linear regression models were used to adjust for important covariates and potential confounders.
Results
Demographic and Clinical Features of Subjects
Clinical data are summarized in Table 1, stratified by HCC status. Subjects with HCC were older than those without HCC (p = 0.001). There were no significant differences in gender, ethnicity, cause of liver disease, or body mass index (BMI), between patients with and without HCC.
Table 1.
Clinical features of liver transplant patients with and without hepatocellular carcinoma (HCC)
| Clinical features | Subjects without HCC (n = 108) | Subjects with HCC (n = 45) | p value |
|---|---|---|---|
| Mean age in years (SD) | 50.02 (±8.98) | 55.98 (±11.5) | 0.001 |
| Gender (%) | 0.420 | ||
| Female | 38.0 | 31.9 | |
| Male | 62.0 | 68.1 | |
| Ethnicity (%) | 0.604 | ||
| African American | 25.2 | 21.3 | |
| Non-Hispanic Caucasian | 37.4 | 46.8 | |
| Hispanic | 32.7 | 23.4 | |
| Other (%) | 4.7 | 8.5 | |
| Etiology (%) | 0.204 | ||
| Viral (HBV, HCV) | 53.7 | 57.8 | |
| EtOH | 23.1 | 17.8 | |
| NASH/cryptogenic | 11.1 | 6.7 | |
| Other | 12.0 | 17.0 | |
| Mean body mass index (BMI kg/m2)(SD) at transplant | 30.37 (±7.18) | 28.44 (±6.94) | 0.069 |
| Diabetes (DM) status (%) | 23.1 DM (?) | 22.2 DM (?) | 0.306 |
Pairwise variables for mean age in years, gender % (male, female), ethnicity % (African American, non-Hispanic Caucasian, Hispanic, and other race), etiology % (viral, ethanol, NASH/cryptogenic, other etiology), and diabetes status % (positive, negative). All pairwise variables p value were not significant, except for age, p value = 0.001
Bold value is statistically significant (p <0.05)
Physical Characteristics of Hepatocellular Carcinoma
The liver explants had a mean of 1.8 ± 1.2 tumor lesions with a mean size of 3.85 ± 2.88 cm. Morphological patterns were pseudoglandular (18.6 %), trabecular (46.5 %), solid (18.6 %), and combined (16.3 %). The distribution of tumor grades was grade 1 (32.6 %), grade 2 (37.2 %), and grades 3 and 4 (30.2 %). The tumor variants were divided as usual (39.5 %), clear cell (2.3 %), pleomorphic (11.6 %), combined pleomorphic (11.6 %), and combined non-pleomorphic (34.9 %) variants of HCC. The tumor stages were classified as stage I (50 %), stage II (38.9 %), stage III (8.3 %), and stage IV (2.8 %). The average tumor stage at the time of surgery was 1.64 ± 0.76. Lymphovascular invasion was identified in 9.6 % of the tumor cases.
Immunohistochemistry
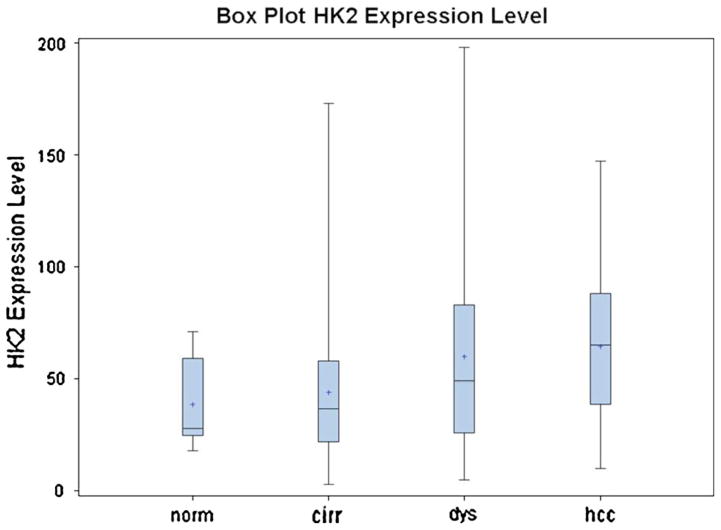
The staining characteristics of HKII are illustrated in Fig. 1. Staining for HKII was readily identified in all NDC, LCD, and HCC samples, was predominantly localized to the cytoplasm, and was characterized as finely granular. The mean level of HKII was 36.67 ± 15.20 (n = 8) in normal controls, 44.18 ± 32.71 (n = 108) in NDC, 59.94 ± 39.86 (n = 143) in LCD, and 64.42 ± 34.89 (n = 45) in HCC (p = 0.001). The box plot in Fig. 2 shows ranges and mean hexokinase II immuno-levels in normal liver control (n = 8) and non-dysplastic cells (n = 108), liver cell dysplasia (n = 143), and hepatocellular carcinoma (n = 45).
Fig. 1.
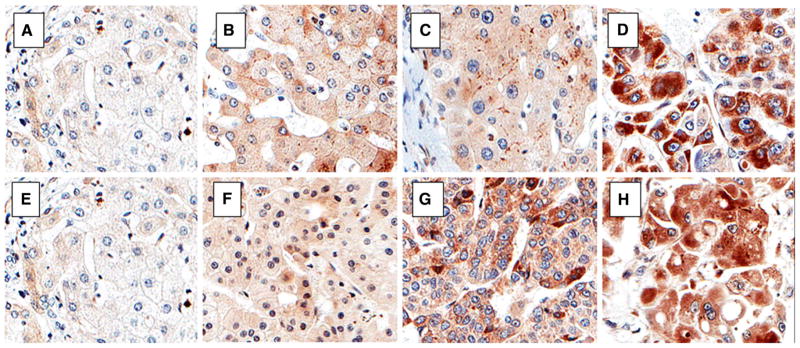
Hexokinase II immunoexpression in normal liver, cirrhosis, dysplasia, hepatocellular carcinoma, and advancing tumor grades. Hexokinase II immunoexpression is cytoplasmic. The upper panel shows images from a subject’s liver explant with a stepwise increase in HKII immunoexpression from non-dysplastic cirrhosis (b), to hepatocyte dysplasia (c), and hepatocellular carcinoma (HCC) (d) in comparison with a normal liver (a). The lower panel displays sections from various explants with increasing HKII immunoexpression from well (f), to moderately differentiated (g), to poorly differentiated HCC (h), in comparison with normal liver (e) (×20)
Fig. 2.
Bar and whiskers box plot illustrating pairwise raw data points of Hexokinase II Immunoexpression levels in positive pixel counts/mm2 in normal liver control (n = 8) and non-dysplastic cells (n = 108), liver cell dysplasia (n = 143), and hepatocellular carcinoma (n = 45) Obtained from subjects with fibrosis stage IV/0-IV liver explants (p = 0.001). Overall, there is a stepwise increase in mean HKII level (denoted as * in the plot) in positive pixel count ppc/mm2 in cirrhosis, to dysplasia, to HCC. Paired analysis by Wilcoxon signed-rank test for two samples within the same patient and nonparametric Friedman test for multiple samples within the same patient was used to examine the progression over disease stage
HKII levels were higher in areas of LCD versus NDC with a mean difference of 13.44 ± 23.30 (p ≤ 0.001) (n = 143) in all subjects with cirrhosis without and with HCC. Higher HKII expression was observed in areas of LCD versus NDC in patients with cirrhosis without HCC (p ≤ 0.001) (n = 98) and in cases with cirrhosis with HCC (p = 0.007) (n = 45). In patients with HCC, HKII levels were greater in areas of LCD and HCC than in NDC (mean difference 15 ± 23.93) (p = 0.007). There was no significant difference in HKII expression between areas of LCD and HCC (mean difference 7.60 ± 42.43) (p = 0.124).
HKII expression was higher in grade 2–4 HCC (71.40 ± 32.66) versus grade 1 HCC (48.96 ± 35.84) (p = 0.044), and in pleomorphic (85.22 ± 35.85) compared with non-pleomorphic HCC variants (57.03 ± 25.33) (p = 0.041). Stage II–IV HCC had higher HKII levels (71.95 ± 44.90) than stage I HCC (48.50 ± 33.07) (p = 0.047). In multivariate analysis adjusting for age, gender, ethnicity, cause of liver disease, BMI, and lymphovascular invasion, higher levels of HKII expression remained significantly associated with LCD and HCC versus NDC (p = 0.007) and higher (grades 2–4) versus lower tumor grade HCC (p = 0.044).
An exploratory analysis was performed to evaluate for associations between clinical factors and HKII expression. African Americans (AA) had higher levels of HKII expression compared with non-AA (NAA) in NDC (AA 62.10 ± 41.17 vs. NAA 36.45 ± 28.15, (p = 0.002) and in LCD (AA 72.97.10 ± 46.16 vs. NAA 54.80 ± 36.77, p = 0.020). HKII levels in HCC were similar between AA (75.63 ± 46.32) and NAA 59.64 ± 31.56) (p = 0.152). There is no significant relationship between race and tumor grade, tumor stage, or tumor variant. There was higher level of HKII in (DM+) in comparison with (DM−) in dysplastic lesions of the explanted livers of subjects without HCC (p = 0.018). There were no significant associations between DM and HKII levels in HCC lesions of subjects with HCC.
Discussion
The novel finding of this study was that HKII immunoexpression was similar in liver cell change/dysplasia and HCC and greater in liver cell dysplasia and HCC than in cirrhotic tissue without dysplasia. The results indicate that HKII level increases prior to the development of HCC and suggest that HKII expression should be evaluated as a potential biomarker for HCC risk in patients with cirrhosis. Hexokinase II has been identified in the glycolytic pathway that occurs in the hypoxic environment of rapidly growing tumors including HCC [13, 41]. Data from the current study raise the compelling question of whether HKII is involved in the transition from cirrhosis to dysplasia, perhaps by providing an energy source for proliferation of dysplastic hepatocytes while inhibiting apoptosis [42, 43] Mechanistic studies are needed to assess for a causal relation between HKII expression and progression to HCC.
The strengths of this study include well-characterized liver tissue samples from a robust cohort of patients at risk for hepatocellular carcinoma and the use of tissue arrays and image analysis. Application of tissue arrays in research has been reported to provide an efficient and cost-effective means to evaluate immunohistochemistry (IHC) in large numbers of cases [44–46]. The specimens were stained simultaneously under the same conditions, reducing the possibility that inter-batch differences in staining technique impacted the results. In contrast to previous works that reported HKII expression in only 45–56 % of samples [24, 25], the current study identified HKII expression in all 45 HCC specimens with an average value of 64.42 (±34.89) ppc/mm2. The differences between the results cannot be explained by variation in antibody or IHC methodology. Here, the use of image analysis provided an objective and sensitive method to quantify immunohistochemical staining that has not been used in previous studies of HKII.
The retrospective, cross-sectional design that limits the findings to associations is a weakness of the current study. Moreover, immunohistochemistry provides information regarding HKII expression, but molecular studies identifying mutational alterations of genes and transcription factors that control HKII over-expression are needed. Sampling adequacy is a frequently raised concern in tissue microarray studies. Protein expression attained from a single 0.6-mm tissue micro-array core has been shown to correlate with the results obtained from whole mount sections. In a study designed to validate the use of tissue microarray as a tool for molecular profiling of cancer biomarkers, up to 95 % concurrence of protein expression from 97 breast tumors was identified between single 0.6 mm cores and whole mount sections, yielding a strong statistical Kappa value of >0.90 [47].
Another item that merits discussion is the consequential upregulation of genes involved in glucose metabolism during adaptation to hypoxic conditions [48]. Molecular studies addressing the role of genes regulating glucose metabolism in tumorigenesis and tumor expansion are appealing as investigators have claimed that hypoxia fuels hepatocellular carcinoma expansion by way of HKII upregulation [48]. However, it is unlikely that HKII expression is induced only in response to hypoxia. Normal adult hepatocytes do not express HKII and only express glucokinase (HKIV) [49]. It is well established that in HCC, there is switch from glucokinase to HKII. HKII is a high-affinity hexokinase, which is required for the high metabolic demands of cancer cells [11–14]. In fact, all HCC cell lines do not express glucokinase and only show HKII in normoxic conditions [50].
While we found a significantly higher level of HKII in (DM+) in comparison with (DM−) in dysplastic lesions of the explanted livers of patients without HCC (p value = 0.018), there were no significant associations between DM and HKII levels in HCC lesions. HKII is known to be upregulated by insulin [51]. The low number of patients with (NAFLD)/cryptogenic cirrhosis in this series may contribute to one’s inability to detect differences in HKII levels between this group and other disease categories.
A number of studies have found that African Americans have an increased risk of HCC compared with others [52–54]. We therefore performed an exploratory analysis to compare HKII expression by race. Interestingly, African Americans had significantly higher levels of HKII in non-dysplastic and dysplastic hepatocytes in cirrhosis than other ethnic/racial groups. While we cannot exclude the possibility that these findings are due to confounding factors, the data provide a rationale for further investigations to assess for racial differences in HKII expression in the development of HCC.
In this study, higher levels of HKII were found to be associated with more aggressive histological features of HCC. Consistent with recent publications, we noted HKII immunoexpression was more pronounced in poorly differentiated, pleomorphic, and in higher stage HCC [23–25], thereby adding to the evidence that HKII is a likely marker for increased metabolic activity that occurs in HCC in humans. The potential use of HK II inhibitors for the treatment of advanced HCCs was described in a mouse tumor model [43], and the question remains whether HKII provides an attractive target for the development of new agents for the treatment of HCC in humans.
In conclusion, HKII expression was higher in HCC with more aggressive histological features and in more advanced-stage disease. Further, the level of HKII in liver cell dysplasia in cirrhosis was similar to that observed in HCC and greater than the degree of expression found in non-dysplastic cirrhosis. Our findings show that HKII levels rise earlier than the occurrence of hepatocellular carcinoma, providing basis for evaluating HKII as a potential biomarker for HCC risk in patients with cirrhosis.
Acknowledgments
This Project was supported by the University of Illinois at Chicago (UIC) Center for Clinical and Translational Sciences (CCTS) to G.G., N.H., and H.X. Award number ULRR029879 from the National Center for Research Resources, by Grants CA090764, AG025953 from the National Institutes of Health to N.H., and VA merit award BX000733 to N.H. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. The authors recognize the contribution of the Research Histology and Tissue Imaging Core at the University of Illinois at Chicago to the completion of this Project (GG/gbg08232014).
Footnotes
Conflict of interest None.
Contributor Information
Grace Guzman, Email: grace.guzman.md@gmail.com, Pathology, College of Medicine, Cancer Center, University of Illinois Hospital and Health Science System, 840 South Wood Street Room 130M/C 847, Chicago, IL 60612, USA.
Rohini Chennuri, Pathology, College of Medicine, Cancer Center, University of Illinois Hospital and Health Science System, 840 South Wood Street Room 130M/C 847, Chicago, IL 60612, USA.
Alexander Chan, Pathology, College of Medicine, Cancer Center, University of Illinois Hospital and Health Science System, 840 South Wood Street Room 130M/C 847, Chicago, IL 60612, USA.
Bryan Rea, Pathology, College of Medicine, Cancer Center, University of Illinois Hospital and Health Science System, 840 South Wood Street Room 130M/C 847, Chicago, IL 60612, USA.
Ada Quintana, Pathology, College of Medicine, Cancer Center, University of Illinois Hospital and Health Science System, 840 South Wood Street Room 130M/C 847, Chicago, IL 60612, USA.
Roshan Patel, Pathology, College of Medicine, Cancer Center, University of Illinois Hospital and Health Science System, 840 South Wood Street Room 130M/C 847, Chicago, IL 60612, USA.
Pei-Zhang Xu, Biochemistry and Molecular Genetics, College of Medicine, University of Illinois Hospital and Health Science System, Chicago, IL 60612, USA.
Hui Xie, Epidemiology and Biostatistics, School of Public Health, University of Illinois Hospital and Health Science System, Chicago, IL 60612, USA.
Nissim Hay, Biochemistry and Molecular Genetics, College of Medicine, University of Illinois Hospital and Health Science System, Chicago, IL 60612, USA.
References
- 1.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485–1491. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thorgeirsson SS, Grisham JW. Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet. 2002;31:339–346. doi: 10.1038/ng0802-339. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB. Hepatocellular carcinoma and hepatitis C in the United States. Hepatology. 2002;36:S74–S83. doi: 10.1053/jhep.2002.36807. [DOI] [PubMed] [Google Scholar]
- 4.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 5.Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2010;51:1820–1832. doi: 10.1002/hep.23594. [DOI] [PubMed] [Google Scholar]
- 6.Bugianesi E. Non-alcoholic steatohepatitis and cancer. Clin Liver Dis. 2007;11:191–207. x–xi. doi: 10.1016/j.cld.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Takamatsu S, Noguchi N, Kudoh A, et al. Influence of risk factors for metabolic syndrome and non-alcoholic fatty liver disease on the progression and prognosis of hepatocellular carcinoma. Hepatogastroenterology. 2008;55:609–614. [PubMed] [Google Scholar]
- 8.Guzman G, Brunt EM, Petrovic LM, Chejfec G, Layden TJ, Cotler SJ. Does nonalcoholic fatty liver disease predispose patients to hepatocellular carcinoma in the absence of cirrhosis? Arch Pathol Lab Med. 2008;132:1761–1766. doi: 10.5858/132.11.1761. [DOI] [PubMed] [Google Scholar]
- 9.Torres DM, Harrison SA. Nonalcoholic steatohepatitis and non-cirrhotic hepatocellular carcinoma: fertile soil. Semin Liver Dis. 2012;32:30–38. doi: 10.1055/s-0032-1306424. [DOI] [PubMed] [Google Scholar]
- 10.Rosmorduc O, Fartoux L. HCC and NASH: how strong is the clinical demonstration? Clin Res Hepatol Gastroenterol. 2012;36:202–208. doi: 10.1016/j.clinre.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 11.Chu CA, Fujimoto Y, Igawa K, et al. Rapid translocation of hepatic glucokinase in response to intraduodenal glucose infusion and changes in plasma glucose and insulin in conscious rats. Am J Physiol Gastrointest Liver Physiol. 2004;286:G627–G634. doi: 10.1152/ajpgi.00218.2003. [DOI] [PubMed] [Google Scholar]
- 12.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4:891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 13.Bustamante E, Pedersen PL. High aerobic glycolysis of rat hepatoma cells in culture: role of mitochondrial hexokinase. Proc Natl Acad Sci U S A. 1977;74:3735–3739. doi: 10.1073/pnas.74.9.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robey RB, Hay N. Mitochondrial hexokinases, novel mediators of the antiapoptotic effects of growth factors and Akt. Oncogene. 2006;25:4683–4696. doi: 10.1038/sj.onc.1209595. [DOI] [PubMed] [Google Scholar]
- 15.Robey RB, Hay N. Mitochondrial hexokinases: guardians of the mitochondria. Cell Cycle. 2005;4:654–658. doi: 10.4161/cc.4.5.1678. [DOI] [PubMed] [Google Scholar]
- 16.Dombrowski F, Filsinger E, Bannasch P, Pfeifer U. Altered liver acini induced in diabetic rats by portal vein islet isografts resemble preneoplastic hepatic foci in their enzymic pattern. Am J Pathol. 1996;148:1249–1256. [PMC free article] [PubMed] [Google Scholar]
- 17.Goel A, Mathupala SP, Pedersen PL. Glucose metabolism in cancer. Evidence that demethylation events play a role in activating type II hexokinase gene expression. J Biol Chem. 2003;278:15333–15340. doi: 10.1074/jbc.M300608200. [DOI] [PubMed] [Google Scholar]
- 18.Mathupala SP, Rempel A, Pedersen PL. Aberrant glycolytic metabolism of cancer cells: a remarkable coordination of genetic, transcriptional, post-translational, and mutational events that lead to a critical role for type II hexokinase. J Bioenerg Biomembr. 1997;29:339–343. doi: 10.1023/a:1022494613613. [DOI] [PubMed] [Google Scholar]
- 19.Brown RS, Goodman TM, Zasadny KR, Greenson JK, Wahl RL. Expression of hexokinase II and Glut-1 in untreated human breast cancer. Nucl Med Biol. 2002;29:443–453. doi: 10.1016/s0969-8051(02)00288-3. [DOI] [PubMed] [Google Scholar]
- 20.Engles JM, Quarless SA, Mambo E, Ishimori T, Cho SY, Wahl RL. Stunning and its effect on 3H-FDG uptake and key gene expression in breast cancer cells undergoing chemotherapy. J Nucl Med. 2006;47:603–608. [PubMed] [Google Scholar]
- 21.Kim JE, Ahn BC, Hwang MH, et al. Combined RNA interference of hexokinase II and (131)I-sodium iodide symporter gene therapy for anaplastic thyroid carcinoma. J Nucl Med. 2011;52:1756–1763. doi: 10.2967/jnumed.111.090266. [DOI] [PubMed] [Google Scholar]
- 22.Peng SY, Lai PL, Pan HW, Hsiao LP, Hsu HC. Aberrant expression of the glycolytic enzymes aldolase B and type II hexokinase in hepatocellular carcinoma are predictive markers for advanced stage, early recurrence and poor prognosis. Oncol Rep. 2008;19:1045–1053. [PubMed] [Google Scholar]
- 23.Guzman G, Chan A, Chennuri R, Patel R, Hay N, Cotler S. e-poster European Association for the Study of the Liver; Nice, France: Jun 28–29, 2011. High level of hexokinase 2 (HK2) is observed in diabetes (DM), biologically aggressive hepatocellular carcinomas (HCCs), and in the progression of HCCs. http://www.multiwebcast.com/easl/2011/nice/listing/by_poster#_t_189. [Google Scholar]
- 24.Kwee SA, Hernandez B, Chan O, Wong L. Choline kinase alpha and hexokinase-2 protein expression in hepatocellular carcinoma: association with survival. PLoS ONE. 2012;7:e46591. doi: 10.1371/journal.pone.0046591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gong L, Cui Z, Chen P, Han H, Peng J, Leng X. Reduced survival of patients with hepatocellular carcinoma expressing hexokinase II. Med Oncol. 2012;29:909–914. doi: 10.1007/s12032-011-9841-z. [DOI] [PubMed] [Google Scholar]
- 26.Ruby SG. Protocol for the examination of specimens from patients with hepatocellular carcinoma and cholangiocarcinoma, including intrahepatic bile ducts. Cancer Committee of the College of American Pathologists. Arch Pathol Lab Med. 2000;124:41–45. doi: 10.5858/2000-124-0041-PFTEOS. [DOI] [PubMed] [Google Scholar]
- 27.Park YN, Roncalli M. Large liver cell dysplasia: a controversial entity. J Hepatol. 2006;45:734–743. doi: 10.1016/j.jhep.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Roncalli M, Borzio M, Tombesi MV, Ferrari A, Servida E. A morphometric study of liver cell dysplasia. Hum Pathol. 1988;19:471–474. doi: 10.1016/s0046-8177(88)80499-4. [DOI] [PubMed] [Google Scholar]
- 29.Tiniakos DG, Brunt EM. Proliferating cell nuclear antigen and Ki-67 labeling in hepatocellular nodules: a comparative study. Liver. 1999;19:58–68. doi: 10.1111/j.1478-3231.1999.tb00011.x. [DOI] [PubMed] [Google Scholar]
- 30.Gong L, Li YH, Su Q, Chu X, Zhang W. Clonality of nodular lesions in liver cirrhosis and chromosomal abnormalities in monoclonal nodules of altered hepatocytes. Histopathology. 2010;56:589–599. doi: 10.1111/j.1365-2559.2010.03523.x. [DOI] [PubMed] [Google Scholar]
- 31.Anthony PP, Vogel CL, Barker LF. Liver cell dysplasia: a pre-malignant condition. J Clin Pathol. 1973;26:217–223. [PMC free article] [PubMed] [Google Scholar]
- 32.Anthony PP. Precursor lesions for liver cancer in humans. Cancer Res. 1976;36:2579–2583. [PubMed] [Google Scholar]
- 33.Rebello Pinto M, Koelma IA, Kumar D. Fine needle aspiration of focal liver lesions. Cytopathology. 1994;5:359–368. doi: 10.1111/j.1365-2303.1994.tb00441.x. [DOI] [PubMed] [Google Scholar]
- 34.Sobin LH, Compton CC. TNM seventh edition: what’s new, what’s changed: communication from the International Union Against Cancer and the American Joint Committee on Cancer. Cancer. 2010;116:5336–5339. doi: 10.1002/cncr.25537. [DOI] [PubMed] [Google Scholar]
- 35.Ferrell LD. Benign and Malignant Tumors of the Liver. In: Odze RD, Goldblum JR, Crawford R, editors. Surgical pathology of the GI tract, liver, biliary tract, and pancreas. Philadelphia: Saunders; 2004. pp. 999–1014. [Google Scholar]
- 36.Ferrell L. Malignant liver tumors that mimic benign lesions: analysis of five distinct lesions. Semin Diagn Pathol. 1995;12:64–76. [PubMed] [Google Scholar]
- 37.Vogel UF, Bueltmann BD. Simple, inexpensive, and precise paraffin tissue microarrays constructed with a conventional microcompound table and a drill grinder. Am J Clin Pathol. 2006;126:342–348. doi: 10.1309/F2Q38DXN1V1V4GQM. [DOI] [PubMed] [Google Scholar]
- 38.Hsu SM, Raine L, Fanger H. A comparative study of the peroxidase-antiperoxidase method and an avidin-biotin complex method for studying polypeptide hormones with radioimmunoassay antibodies. Am J Clin Pathol. 1981;75:734–738. doi: 10.1093/ajcp/75.5.734. [DOI] [PubMed] [Google Scholar]
- 39.Uhlen M, Oksvold P, Fagerberg L, et al. Towards a knowledge-based Human Protein Atlas. Nat Biotechnol. 2010;28:1248–1250. doi: 10.1038/nbt1210-1248. [DOI] [PubMed] [Google Scholar]
- 40.Theodorsson-Norheim E. Friedman and Quade tests: BASIC computer program to perform nonparametric two-way analysis of variance and multiple comparisons on ranks of several related samples. Comput Biol Med. 1987;17:85–99. doi: 10.1016/0010-4825(87)90003-5. [DOI] [PubMed] [Google Scholar]
- 41.Yasuda S, Arii S, Mori A, et al. Hexokinase II and VEGF expression in liver tumors: correlation with hypoxia-inducible factor 1 alpha and its significance. J Hepatol. 2004;40:117–123. doi: 10.1016/s0168-8278(03)00503-8. [DOI] [PubMed] [Google Scholar]
- 42.Majors BS, Betenbaugh MJ, Chiang GG. Links between metabolism and apoptosis in mammalian cells: applications for anti-apoptosis engineering. Metab Eng. 2007;9:317–326. doi: 10.1016/j.ymben.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 43.Kim W, Yoon JH, Jeong JM, et al. Apoptosis-inducing antitumor efficacy of hexokinase II inhibitor in hepatocellular carcinoma. Mol Cancer Ther. 2007;6:2554–2562. doi: 10.1158/1535-7163.MCT-07-0115. [DOI] [PubMed] [Google Scholar]
- 44.Shergill IS, Shergill NK, Arya M, Patel HR. Tissue microarrays: a current medical researchtool. CurrMed Res Opin. 2004;20:707–712. doi: 10.1185/030079904125003412. [DOI] [PubMed] [Google Scholar]
- 45.Jawhar NM. Tissue Microarray: a rapidly evolving diagnostic and research tool. Ann Saudi Med. 2009;29:123–127. doi: 10.4103/0256-4947.51806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hewitt SM. Tissue microarrays as a tool in the discovery and validation of predictive biomarkers. Methods Mol Biol. 2012;823:201–214. doi: 10.1007/978-1-60327-216-2_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang D, Salto-Tellez M, Putti TC, Do E, Koay ES. Reliability of tissue microarrays in detecting protein expression and gene amplification in breast cancer. Mod Pathol. 2003;16:79–84. doi: 10.1097/01.MP.0000047307.96344.93. [DOI] [PubMed] [Google Scholar]
- 48.Riddle SR, Ahmad A, Ahmad S, et al. Hypoxia induces hexokinase II gene expression in human lung cell line A549. Am J Physiol Lung Cell Mol Physiol. 2000;278:L407–L416. doi: 10.1152/ajplung.2000.278.2.L407. [DOI] [PubMed] [Google Scholar]
- 49.Patra KC, Wang Q, Bhaskar PT, et al. Hexokinase 2 is required for tumor initiation and maintenance and its systemic deletion is therapeutic in mouse models of cancer. Cancer Cell. 2013;24:213–228. doi: 10.1016/j.ccr.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gwak GY, Yoon JH, Kim KM, Lee HS, Chung JW, Gores GJ. Hypoxia stimulates proliferation of human hepatoma cells through the induction of hexokinase II expression. J Hepatol. 2005;42:358–364. doi: 10.1016/j.jhep.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 51.Gosmain Y, Lefai E, Ryser S, Roques M, Vidal H. Sterol regulatory element-binding protein-1 mediates the effect of insulin on hexokinase II gene expression in human muscle cells. Diabetes. 2004;53:321–329. doi: 10.2337/diabetes.53.2.321. [DOI] [PubMed] [Google Scholar]
- 52.Kemmer N, Neff G, Secic M, Zacharias V, Kaiser T, Buell J. Ethnic differences in hepatocellular carcinoma: implications for liver transplantation. Dig Dis Sci. 2008;53:551–555. doi: 10.1007/s10620-007-9872-7. [DOI] [PubMed] [Google Scholar]
- 53.Sloane D, Chen H, Howell C. Racial disparity in primary hepatocellular carcinoma: tumor stage at presentation, surgical treatment and survival. J Natl Med Assoc. 2006;98:1934–1939. [PMC free article] [PubMed] [Google Scholar]
- 54.Artinyan A, Mailey B, Sanchez-Luege N, et al. Race, ethnicity, and socioeconomic status influence the survival of patients with hepatocellular carcinoma in the United States. Cancer. 2010;116:1367–1377. doi: 10.1002/cncr.24817. [DOI] [PubMed] [Google Scholar]