Abstract
Despite the early promising results with the anti-angiogenic agent, bevacizumab, to prolong time to progression in patients with brain tumors, the optimal dose and drug combinations have not yet been defined. The purpose of this study was to characterize the bevacizumab dose–response relationship for brain tumors by measuring the contrast-agent enhanced tumor volumes and relative cerebral blood volume (rCBV) using dynamic susceptibility contrast (DSC) imaging. The studies, performed in the U87 brain tumor model using doses of bevacizumab ranging from 0 to 10 mg/kg, demonstrate that tumor growth and vascularity are inhibited at all doses used, compared to untreated controls. However, only the maximum dose showed a statistically significant difference in growth rate. Conversely tumor vascularity, as measured with rCBV, was inhibited equally well for all doses used with no clear indication that higher doses are more effective.
Keywords: Glioblastoma, Bevacizumab, MRI, rCBV, Dose–response
Introduction
Glioblastoma multiforme is one of the most aggressive cancers and is associated with high levels of angiogenesis. Despite the increase in therapies available, the median survival of patients with glioblastoma multiforme (GBM) remains less than 15 months [1]. Cancer therapies have been studied in several rodent models including the intracerebral human xenograft. Observations made in this model have led to clinical trials [2–4].
Promising results have been obtained with the anti-angiogenic agent, bevacizumab, for the treatment of brain tumor patients [5]. Recent successes of the combination of antiangiogenic therapies with radiation or chemotherapy have been shown in clinical and animal studies [5–8].
Despite these early promising results to prolong the time to progression, the optimal dose and drug combinations have not yet been defined [9]. Traditionally optimal dose has been based on maximal tumor-cell kill, which for chemotherapeutic agents, is set by using the maximum tolerated dose (MTD) [10]. However, the relationship between MTD and the optimal biologically active dose (OBD) for anti-angiogenic agents is unclear. Treatment efficacy varies with the tumor type studied and is not necessarily achieved at the maximum dose [10]. Consequently, the goal of this study was to use MRI to characterize the bevacizumab dose–response relationship for brain tumors by measuring the contrast-agent enhanced tumor volumes and relative cerebral blood volume (rCBV) using dynamic susceptibility contrast (DSC) imaging in a U87 glioblastoma tumor model, for a range of bevacizumab doses over an 8-day period.
Materials and methods
Cell culture
The U87MG (adult glioblastoma) cell line was purchased from American Type Culture Collection (Manassas, Virginia) and maintained in MEM with Earle’s salts, 10% fetal bovine serum (FBS), 0.1% penicillin/streptomycin, and 0.02% Fungizone. The cells were maintained in a humidified atmosphere containing 5% CO2 at 37°C.
Animals
Care of the animals before and during the experimental procedures was conducted in accordance with the policies of the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All protocols were approved by the Institutional Animal Care and Use Committee at the Medical College of Wisconsin and Zablocki Veterans Affairs Medical Center Veterinary Medical Unit.
Male RNU/RNUrats weighing approximately 250 g were obtained from Charles River Laboratories (Wilmington, Massachusetts) and housed in pairs in individually ventilated cages. Animals were fed autoclaved laboratory diet; food and RO hyperchlorinated water were available ad libitum.
Intracranial xenograft transplantation
Rats were anesthetized with ketamine (60 mg/kg), acepromazine (0.9 mg/kg) and xylazine (6 mg/kg) IP. Heads were immobilized and using an aseptic technique a 0.9 mm burr hole was drilled in the skull 1 mm anterior and 2 mm lateral to bregma on the right side. A 10 μl gas-tight syringe (Hamilton Company, Reno, Nevada) was used to inject 2 × 105 U87 cells into the right frontal lobe at a depth of 3 mm relative to the dural surface. The injection time was 5 min, after which the needle was in place for 5 min and retracted slowly for an additional 5 min. The skin was closed with cyanoacrylate.
Anti-angiogenic therapies
At 16 days post tumor cell inoculation the animals underwent baseline MRI scanning. Following baseline scanning, animals were treated with 2.5 (n = 7), 5 (n = 8), or 10 (n = 10) mg/kg of bevacizumab (Avastin, Genetech, South San Francisco, California) iv or saline vehicle (n = 9).
MRI studies
At 16, 18, 21, and 24 days after tumor cell inoculation, MRI studies were performed on a 9.4T Bruker AVANCE Scanner fitted with a linear transmit coil and a surface receive coil of 2 cm2 area. The rats were anesthetized with 1.5% isoflurane and immobilized with a fiberglass bite-bar. Temperature was monitored and maintained at 37 ± 1.5°C throughout the experiment. A RARE (rapid acquisition rapid echo) imaging sequence (TE/TR = 4 ms/8 ms; matrix = 256 × 256; FOV = 3.5 cm, slice = 17.5 mm) was used to acquire sagittal scout images. A T1-weighted spin-echo image was acquired (TE/TR = 11 ms/500 ms; matrix = 256 × 256; FOV = 3.5 cm; slice 2 mm). Five axial (rat coronal) imaging slices were chosen based on the RARE images and the tumor inoculation site. A loading dose of Gadodiamide (0.1 mmol/kg) was administered 10 min before the DSC (dynamic susceptibility contrast) scan, in order to diminish confound effects on the rCBV images due to contrast agent leakage [11–13]. A GRE-EPI (gradient-echo echo planar imaging) sequence (TE = 18.8 ms, TR = 500 ms, 5 NEX, α = 38.9°) was used to acquire the DSC data. Specifically, GRE-EPI images were collected continuously for a total of 2 min, for 1 min before, and then during and after a bolus injection of a 0.1 mmole/kg Gd contrast agent. Finally, a T1-weighted spin-echo image was acquired (TE/TR = 11 ms/500 ms; matrix = 256 × 256; FOV = 3.5 cm; slice 2 mm) to delineate enhancing tumor.
Leakage-corrected rCBV (relative cerebral blood volume) parameters were as previously described [14–16]. The tumor region of interest (ROI) was determined from the contrast enhancing region on the post-contrast T1 weighted image. Subsequently, the rCBV maps were standardized using a two-step piecewise linear transformation method as previously described [17]. Enhancing tumor volumes (reported in mm3) were determined from the post-contrast T1w images, in all slices showing enhancing tumor. At each imaging time point the mean percent change from baseline (day 16) are determined for tumor volume and rCBV for the standardized tumor ROI.
Statistical analysis
Data are presented as means ± SE. Generalized estimating equations (GEEs) were used to test the effects of dose, time and their interaction. GEEs are an alternative to repeated measures ANOVA when either the data is non-normal, some observations are missing, or the correlation structure over time needs to be accounted for (here the rats were observed on 4 days). The GEE analysis used the percent change from baseline as the outcome. A Spearman rank correlation was used to evaluate the data in Fig. 4. The 95% confidence interval was considered significant.
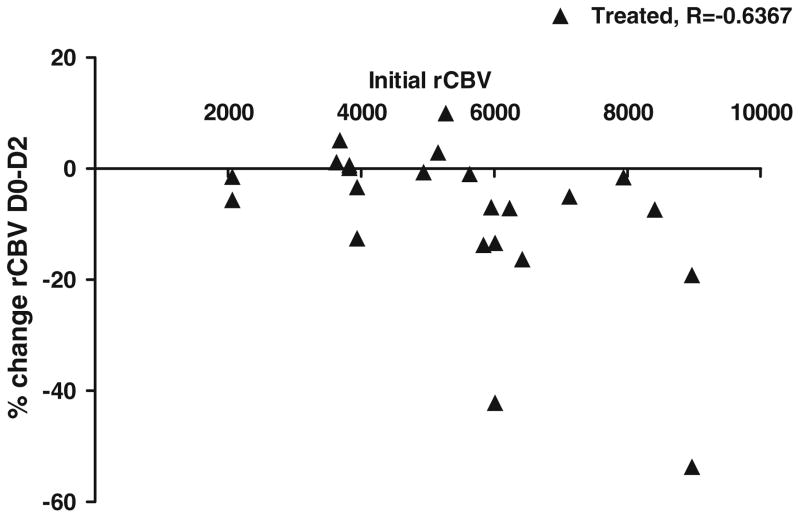
Fig. 4.
Initial decrease in relative cerebral blood volume following bevacizumab treatment dependent on initial blood volume. Data are presented median. P < 0.05 vehicle versus treated by Spearman rank correlation
Results
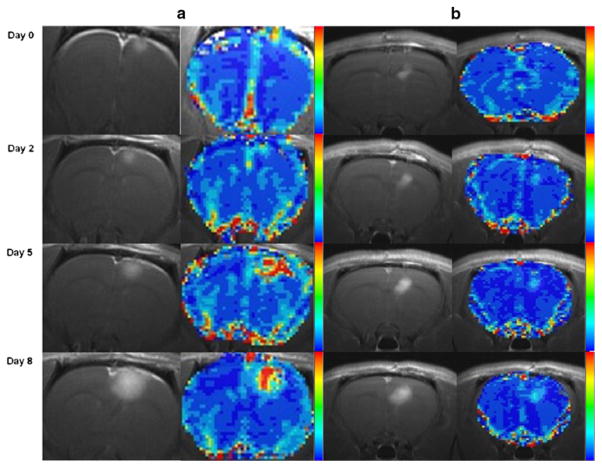
Figure 1 shows representative post-contrast T1 weighted images and the rCBV maps obtained in one rat treated with vehicle (a) and another treated with 5 mg/kg bevacizumab (b). In the post-contrast T1 weighted images, the tumor volumes increase in both treated and untreated animals, but to a greater extent in the untreated rat (Fig. 1a). The blood volume increases more rapidly in the untreated animal, as apparent on the rCBV maps.
Fig. 1.
a Representative images of post contrast T1 weighted image and rCBV maps from an untreated animal shown longitudinally (top to bottom). b Representative images of post contrast T1 weighted images and rCBV maps from an animal treated with 5 mg/kg bevacizumab shown at days 0, 2, 5, and 8 post-treatment
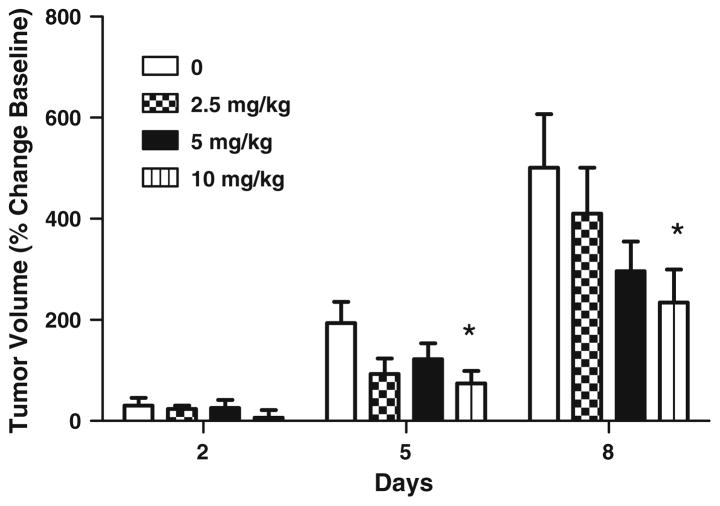
Figure 2 illustrates the percent change in tumor volumes as a function of bevacizumab dose for the three different post-treatment days. On day 2 post-treatment, enhancing tumor volume increased 30% in vehicle treated rats, which was not significantly different from the 6 to 24% changes in tumor volume for the treated groups. On day 5, on average the tumor growth for all treated groups was less than the untreated group; however, only the highest dose (10 mg/kg) significantly inhibited tumor volume. On post-treatment day 8, only the highest dose (10 mg/kg) demonstrated a significant inhibition of tumor volume compared to the untreated tumor volumes on that same day post-treatment. Overall the percent increases in tumor volumes were 500% for the untreated group, and 410, 296 and 234% for the 2.5, 5 and 10 mg/kg bevacizumab treatment groups. The GEE analysis showed a significant time effect (P < 0.01), a marginal average dose effect (P < 0.06) and a significant dose by time interaction (P < 0.042). This indicates that the dose effect increased significantly with time (days post-inoculation). Therefore, the tumor volumes did continue to increase over time even with treatment, but the growth rates were inhibited.
Fig. 2.
Bevacizumab inhibits the growth of the contrast-agent enhancing tumor volume. Animals were imaged at days 2, 5, and 8 post-treatment. Data are presented mean ± SE. * P < 0.05 vehicle versus treated
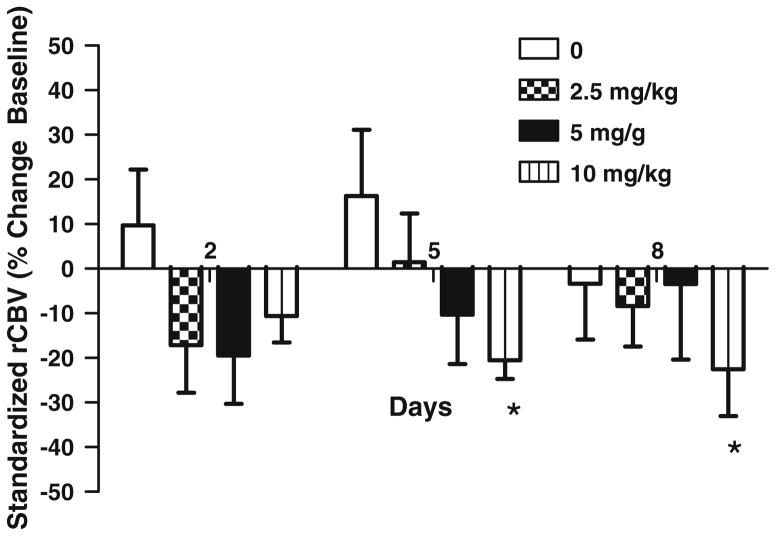
Figure 3 shows the percent change of the rCBV values for all rats as a function of bevacizumab dose for the three different treatment days. In untreated animals, rCBV is increased compared to baseline values at each time point, but not significantly. For rats treated with bevacizumab the rCBV decreased, relative to the baseline rCBV, on all days and doses. Similar to the increases in tumor volume over time (Fig. 2) the rCBV also increased over time but remained less than the untreated rCBV values. There was no significant terms in the overall GEE analysis: average time effect (P = 0.23), average dose effect (P = 0.19) and dose by time interaction (P = 0.24).
Fig. 3.
Effect of bevacizumab on relative cerebral blood volume. rCBV is calculated as the area under the ΔR2*(t) curves and then corrected for leakage. Animals were imaged at days 2, 5, and 8 post-treatment. Data are presented mean ± SE. * P < 0.05 vehicle versus treated
Although the mean rCBV values are consistently less for the treated rats compared to the untreated rats, only for the 10 mg/kg dose is the average rCBV significantly different (P < 0.03), than that for the untreated rats. This lack of statistically significant differences is likely due to the large variability in initial tumor sizes (1.2–93.7 mm3), despite well-controlled tumor cell inoculation procedures and use of animal models that are genetically equivalent. This may be explained in part by the result that treated animals with the largest decrease in median rCBV at day 2 also had the highest initial rCBV (Fig. 4). Therefore, the degree of rCBV changes will depend somewhat on the initial rCBV values. This relationship is significant for all doses combined (P = 0.001).
Discussion
The present studies characterize the bevacizumab dose–response relationship for the U87 brain tumor model by measuring the contrast-agent enhanced tumor volumes and relative cerebral blood volume (rCBV) using dynamic susceptibility contrast (DSC) imaging. The results demonstrate that: (1) Bevacizumab inhibits tumor growth and vascularity as indicated by both enhancing tumor volume and rCBV. (2) The inhibition of tumor volume with treatment is delayed in time relative to the inhibition in tumor rCBV, which occurred at the earliest measurement time point. (3) The optimal effective dose of anti-angiogenic therapy for malignant gliomas is not clearly indicated by either the tumor volume or rCBV measurements. While a bevacizumab dose of 10 mg/kg had the most inhibition of tumor volume at day 5 and 8, rCBV suggests that this dose was most effective in reducing rCBV at the day 5 and 8 post-treatment time point. Given these results, there is no clear indication that 10 mg/kg is the optimal dose. A lower dose, which is as equally effective as the higher dose, but will have fewer associated side-effects may be more optimal [18].
The lack of complete inhibition of tumor growth and vascularity with bevacizumab may be explained by the presence of host vascular endothelial growth factor (VEGF). Rodent cells recruited to the tumor can produce VEGF which is not bound by bevacizumab [19–23]. Liang and colleagues have created anti-VEGF antibodies that bind to murine and rat VEGF allowing a more complete inhibition of VEGF [19]. Though this may explain the lack of total inhibition in part, it does not fully explain the results. In fact these results are consistent with the clinical situation where bevacizumab has been shown to delay time to progression, but no improvement of overall survival in patients with glioblastoma [24–26]. The same mechanism of action explaining this clinical situation may also apply to these studies. Therefore, additional studies evaluating the time course of changes in the angiogenic and invasive phenotype of tumors are needed.
The fact that the inhibition in tumor volume is delayed relative to the inhibition of tumor rCBV is further evidence for the important role that rCBV measurements can play in the evaluation and optimization of treatment strategies. Specifically, while the standard criteria used to monitor therapy has been tumor size, with anti-angiogenic agents, this endpoint may be inappropriate since studies have shown an inhibition of angiogenesis that was detectable before measurable effects on tumor volume or in spite of increases in tumor volume [27, 28]. Therefore, an antiangiogenic therapy may not necessarily result in tumor shrinkage, but stabilize the tumor or return it to a dormant state. Measurements of tumor vascularity, as obtained with rCBV, can assist with the further elucidation of this issue as demonstrated here.
Furthermore, while in this study enhancing tumor volume proved to be a good (albeit delayed) indicator of treatment response, it is not consistently so in the clinical population [29, 30]. This discrepancy may be due to the effect of steroid administration which may decrease or even preclude contrast agent enhancement. In addition, anti-angiogenic therapies have direct effects on vascular permeability, inhibiting the efflux of contrast agent from the leaky tumor vasculature into the tumor tissue and eliminating contrast enhancement. Standard post contrast enhancing imaging would indicate a radiographic response; however, the response of the tumor is masked by the decrease in permeability. DSC-MRI is capable of tracking the tumor despite the decrease in contrast enhancement and provides valuable clinical information not obtainable with conventional gadolinium enhanced T1-weighted MRI [11, 31–33].
Additional studies are necessary to both fully evaluate and determine the optimal dose of bevacizumab, maximizing tumor effect while minimizing potential toxic side effects. This study represents a first step in that regard, using dosages that have been used and recommended in previous studies described in the literature [18, 34–37]. For example, Phase II clinical trials of several tumor types have used dosages ranging 3–20 mg/kg bevacizumab every 2 or 3 weeks. Many studies have shown lower doses to be more effective in treatment and reduced risk for renal adverse events. Clinical studies in colorectal cancer patients showed 5 mg/kg bevacizumab to decrease tumor perfusion, vascular volume, microvascular density, and interstitial fluid pressure [18]. Recent review of clinical studies has correlated an increased risk of proteinuria with 5 mg/kg bevacizumab in combination with chemotherapy compared to 2.5 mg/kg [38]. Successful combination studies of bevacizumab (10 mg/kg) and irinotecan showed increased progression free survival at 6 months and 63% radiographic response in recurrent glioblastoma patients. While in this study 10 mg/kg dose proved best in terms of maximal long-term tumor shrinkage and inhibiting vascularity at one time point, the inhibition of vascularity seemed to respond equally well at all doses at most post-treatment time points. Consequently, the higher dose used is not clearly superior to the lower doses used, and thus a dose of 5 mg/kg may prove optimal giving the best benefit to risk ratio. This topic requires further studies, which may include monitoring progression free survival (time from stopping treatment until tumor regrowth), overall survival, and the timing of therapy. In addition, tissue markers of response will likewise be measured in concert with imaging markers.
In summary, data from this study support the clinical research showing decreases in tumor volume and vascularity in a U87 glioblastoma model with bevacizumab therapy. The rCBV estimates of tumor perfusion provide useful information, different from that provided by tumor enhancement, which could aid in the full characterization and optimization of anti-angiogenic treatments. Furthermore, since most clinical studies incorporate bevacizumab in combination with chemotherapy, combination therapies will be studied in subsequent rCBV studies using our xenograft model.
Acknowledgments
The authors thank Genentech for the generous gift of Avastin and R. Harris and M. Runquist for technical assistance. This work was supported by National Institute of Health Grant CA-082500 to K.M. Schmainda and MCW Advancing Healthier Wisconsin/Translational Brain Tumor Research Program.
Contributor Information
Kimberly R. Pechman, Email: kpechman@mcw.edu, Translational Brain Tumor Program, Medical College of Wisconsin, Milwaukee, WI, USA. Department of Neurosurgery, Medical College of Wisconsin, 8701 Watertown Plank Road, Milwaukee, WI 53226, USA
Deborah L. Donohoe, Translational Brain Tumor Program, Medical College of Wisconsin, Milwaukee, WI, USA. Department of Radiology, Medical College of Wisconsin, Milwaukee, WI, USA
Devyani P. Bedekar, Translational Brain Tumor Program, Medical College of Wisconsin, Milwaukee, WI, USA. Department of Radiology, Medical College of Wisconsin, Milwaukee, WI, USA
Shekar N. Kurpad, Translational Brain Tumor Program, Medical College of Wisconsin, Milwaukee, WI, USA. Department of Neurosurgery, Medical College of Wisconsin, 8701 Watertown Plank Road, Milwaukee, WI 53226, USA. Clement J Zablocki VA Medical Center, Milwaukee, WI, USA
Raymond G. Hoffmann, Department of Pediatrics, Medical College of Wisconsin, Milwaukee, WI, USA
Kathleen M. Schmainda, Translational Brain Tumor Program, Medical College of Wisconsin, Milwaukee, WI, USA. Department of Radiology, Medical College of Wisconsin, Milwaukee, WI, USA. Department of Biophysics, Medical College of Wisconsin, Milwaukee, WI, USA
References
- 1.Stupp R, Mason WP, Van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Kurpad SN, Dolan ME, McLendon RE, Archer GE, Moschel RC, Pegg AE, Bigner DD, Friedman HS. Intraarterial O6-benzylguanine enables the specific therapy of nitrosourea-resistant intracranial human glioma xenografts in athymic rats with 1, 3-bis(2-chloroethyl)-1-nitrosourea. Cancer Chemother Pharmacol. 1997;39:307–316. doi: 10.1007/s002800050577. [DOI] [PubMed] [Google Scholar]
- 3.Rich JN, Sathornsumetee S, Keir ST, Kieran MW, Laforme A, Kaipainen A, McLendon RE, Graner MW, Rasheed BK, Wang L, Reardon DA, Ryan AJ, Wheeler C, Dimery I, Bigner DD, Friedman HS. ZD6474, a novel tyrosine kinase inhibitor of vascular endothelial growth factor receptor and epidermal growth factor receptor, inhibits tumor growth of multiple nervous system tumors. Clin Cancer Res. 2005;11:8145–8157. doi: 10.1158/1078-0432.CCR-05-0319. [DOI] [PubMed] [Google Scholar]
- 4.Heimberger AB, Archer GE, McLendon RE, Hulette C, Friedman AH, Friedman HS, Bigner DD, Sampson JH. Temozolomide delivered by intracerebral microinfusion is safe and efficacious against malignant gliomas in rats. Clin Cancer Res. 2000;6:4148–4153. [PubMed] [Google Scholar]
- 5.Vredenburgh JJ, Desjardins A, Herndon JE, II, Dowell JM, Reardon DA, Quinn JA, Rich JN, Sathornsumetee S, Gururangan S, Wagner M, Bigner DD, Friedman AH, Friedman HS. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13:1253–1259. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 6.Ansiaux R, Baudelet C, Jordan BF, Beghein N, Sonveaux P, De Wever J, Martinive P, Gregoire V, Feron O, Gallez B. Thalidomide radiosensitizs tumors through early changes in the tumor microenvironment. Cancer Res. 2005;11:743–750. [PubMed] [Google Scholar]
- 7.Huber PE, Bischof M, Jenne J, Heiland S, Peschke P, Saffich R, Grone HJ, Debus J, Lipson KE, Abdollahi A. Trimodal cancer treatment: beneficial effects of combined antiangiogenesis, radiation, and chemotherapy. Cancer Res. 2005;65:3643–3655. doi: 10.1158/0008-5472.CAN-04-1668. [DOI] [PubMed] [Google Scholar]
- 8.Winkler F, Kozin SV, Tong RT, Chae SS, Booth MF, Garkavtsev I, Xu L, Hicklin DJ, Fukumura D, di Tomaso E, Munn LL, Jain RK. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004;6:553–563. doi: 10.1016/j.ccr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 9.Tuma RS. Success of bevacizumab trials raises questions for future studies. J Natl Cancer Inst. 2005;97:950–951. doi: 10.1093/jnci/dji194. [DOI] [PubMed] [Google Scholar]
- 10.Bergsland E, Dickler MN. Maximizing the potential of bevacizumab in cancer treatment. Oncologist. 2004;9:36–42. doi: 10.1634/theoncologist.9-suppl_1-36. [DOI] [PubMed] [Google Scholar]
- 11.Donahue KM, Krouwer HG, Rand SD, Pathak AP, Marszalkowski CS, Censky SC, Prost RW. Utility of simultaneously acquired gradient-echo and spin-echo cerebral blood volume and morphology maps in brain tumor patients. Magn Reson Med. 2000;43:845–853. doi: 10.1002/1522-2594(200006)43:6<845::aid-mrm10>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 12.Schmainda KM, Rand SD, Joseph AM, Lund R, Ward BD, Pathak AP, Ulmer JL, Badruddoja MA, Krouwer HG. Characterization of a first-pass gradient-echo spin-echo method to predict brain tumor grade and angiogenesis. AJNR Am J Neuroradiol. 2004;25:1524–1532. [PMC free article] [PubMed] [Google Scholar]
- 13.Boxerman JL, Schmainda KM, Weisskoff RM. Relative cerebral blood volume maps corrected for contrast agent extravasation significantly correlate with glioma tumor grade, whereas uncorrected maps do not. AJNR Am J Neuroradiol. 2006;27:859–867. [PMC free article] [PubMed] [Google Scholar]
- 14.Paulson ES, Prah DP, Schmainda KM. Simultaneous measurement of DSC- and DCE-MRI parameters using dual-echo spiral with a standard dose of gadolinium. The International Society of Magnetic Resonance in Medicine annual meeting; Toronto, ON, Canada. 2008. [Google Scholar]
- 15.Paulson ES, Prah DP, Schmainda KM. Correction of confounding leakage and residual susceptibility effects in dynamic susceptibility contrast mr imaging using dual-echo SPIRAL. The American Society of Neuroradiology annual meeting; Chicago, IL. 2007. [Google Scholar]
- 16.Paulson ES, Prah DP, Schmainda KM. Compensation of confounding T1 and T2 dipolar and residual susceptibility effects in DSC-MRI using dual-echo SPIRAL. The International Society of Magnetic Resonance in Medicine annual meeting; Berlin, Germany. 2007. [Google Scholar]
- 17.Bedekar D, Jensen T, Schmainda KM. Standardization of relative cerebral blood volume (rCBV) image maps for ease of both inter- and intrapatient comparisons. Magn Reson Med. 2010;64:907–913. doi: 10.1002/mrm.22445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willett CG, Boucher Y, di Tomaso E, Munn LL, Tong RT, Chung DC, Sahani DV, Kalva SP, Kozin SV, Mino M, Cohen KS, Scadden DT, Hartford AC, Fischman AJ, Clark JW, Ryan DP, Zhu AX, Blaszkowsky LS, Chen HX, Shellito PC, Lauwers GY, Jain RK. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10:145–147. doi: 10.1038/nm988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang WC, Wu X, Peale FV, Lee CV, Meng YG, Gutierrez J, Fu L, Malik AK, Gerber HP, Ferrara N, Fuh G. Cross-species vascular endothelial growth factor (VEGF)-blocking antibodies completely inhibit the growth of human tumor xenografts and measure the contribution of stromal VEGF. J Biol Chem. 2006;281:951–961. doi: 10.1074/jbc.M508199200. [DOI] [PubMed] [Google Scholar]
- 20.Gerber HP, Kowalski J, Sherman D, Eberhard DA, Ferrara N. Complete inhibition of rhabdomyosarcoma xenograft growth and neovascularization requires blockade of both tumor and host vascular endothelial growth factor. Cancer Res. 2000;60:6253–6258. [PubMed] [Google Scholar]
- 21.Fukumura D, Xavier R, Sugiura T, Chen Y, Park EC, Lu N, Selig M, Neilsen G, Taksir T, Jain RK, Seed B. Tumor induction of VEGF promoter activity in stromal cells. Cell. 1998;94:715–725. doi: 10.1016/s0092-8674(00)81731-6. [DOI] [PubMed] [Google Scholar]
- 22.Kishimoto J, Ehama R, Ge Y, Kobayashi T, Nishiyama T, Detmar M, Burgeson RE. In vivo detection of human vascular endothelial growth factor promoter activity in transgenic mouse skin. Am J Pathol. 2000;157:103–110. doi: 10.1016/S0002-9440(10)64522-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barbera-Guillem E, Nyhus JK, Wolford CC, Friece CR, Sampsel JW. Vascular endothelial growth factor secretion by tumor-infiltrating macrophages essentially supports tumor angiogenesis, and IgG immune complexes potentiate the process. Cancer Res. 2002;62:7042–7049. [PubMed] [Google Scholar]
- 24.Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, Yung WK, Paleologos N, Nicholas MK, Jensen R, Vredenburgh J, Huang J, Zheng M, Cloughesy T. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 25.Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I, Garren N, Mackey M, Butman JA, Camphausen K, Park J, Albert PS, Fine HA. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Onco. 2009;27:740–745. doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ballman KV, Buckner JC, Brown PD, Giannini C, Flynn PJ, LaPland BR, Jaeckle KA. The relationship between six-month progression-free survival and 12-month overall survival end points for phase II trials in patients with glioblastoma multiforme. Neuro-oncol. 2007;9:29–38. doi: 10.1215/15228517-2006-025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindner DJ, Borden EC. Effects of tamoxifen and interferon- beta or the combination on tumor-induced angiogenesis. Int J Cancer. 1997;71:456–461. doi: 10.1002/(sici)1097-0215(19970502)71:3<456::aid-ijc25>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 28.Poptani H, Puumalainen AM, Gröhn OH, Loimas S, Kainulainen R, Ylä-Herttuala S, Kauppinen RA. Monitoring thymidine kinase and ganciclovir-induced changes in rat malignant glioma in vivo by nuclear magnetic resonance imaging. Cancer Gene Ther. 1998;5:101–109. [PubMed] [Google Scholar]
- 29.Norden AD, Young GS, Setayesh K, Muzikansky A, Klufas R, Ross GL, Ciampa AS, Ebbeling LG, Levy B, Drappatz J, Kesari S, Wen PY. Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology. 2008;70:779–787. doi: 10.1212/01.wnl.0000304121.57857.38. [DOI] [PubMed] [Google Scholar]
- 30.Iwamoto FM, Abrey LE, Beal K, Gutin PH, Resenblum MK, Reuter VE, DeAngelis LM, Lassman AB. Patterns of relapse and prognosis after bevacizumab failure in recurrent glioblastoma. Neurology. 2009;73:1200–1206. doi: 10.1212/WNL.0b013e3181bc0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weber MA, Zoubaa S, Schlieter M, Jüttler E, Huttner HB, Geletneky K, Ittrich C, Lichy MP, Kroll A, Debus J, Giesel FL, Hartmann M, Essig M. Diagnostic performance of spectroscopic and perfusion MRI for distinction of brain tumors. Neurology. 2006;66:1899–1906. doi: 10.1212/01.wnl.0000219767.49705.9c. [DOI] [PubMed] [Google Scholar]
- 32.Wagner Schuman ML, Bedekar D, Kvasnica K, Fishman M, Paulson ES, Rand SD, Krouwer HG, Schmainda KM. A multiparameter DSC study demonstrates the best predictor of brain tumor grade. The International Society of Magnetic Resonance in Medicine annual meeting; Toronto, ON, Canada. 2008. [Google Scholar]
- 33.Hu LS, Baxter LC, Smith KA, Feuerstein BG, Karis JP, Eschbacher JM, Coons SW, Makaji P, Yeh RF, Debbins J, Heiserman JE. Relative cerebral blood volume values to differentiate high-grade glioma recurrence from posttreatment radiation effect: direct correlation between image-guided tissue histopathology and localized dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging measurements. AJNR Am J Neuroradiol. 2009;30:552–558. doi: 10.3174/ajnr.A1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vredenburgh JJ, Desjardins A, Herndon JE, II, Marcello J, Reardon DA, Quinn JA, Rich JN, Sathornsumetee S, Gururangan S, Sampson J, Wagner M, Bailey L, Bigner DD, Friedman AH, Friedman HS. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25:4722–4729. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 35.Willett CG, Boucher Y, Duda DG, di Tomaso E, Munn LL, Tong RT, Kozin SV, Petit L, Jain RK, Chung DC, Sahani DV, Kalva SP, Cohen KS, Scadden DT, Fischman AJ, Clark JW, Ryan DP, Zhu AX, Blaszkowsky LS, Shellito PC, Mino-Kenudson M, Lauwers GY. Surrogate markers for antiangiogenic therapy and dose-limiting toxicities for bevacizumab with radiation and chemotherapy: continued experience of a phase I trial in rectal cancer patients. J Clin Oncol. 2005;23:8136–8139. doi: 10.1200/JCO.2005.02.5635. [DOI] [PubMed] [Google Scholar]
- 36.Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, Steinberg SM, Chen HX, Rosenberg SA. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herbst RS, O’Neill VJ, Fehrenbacher L, Belani CP, Bonomi PD, Hart L, Melnyk O, Ramies D, Lin M, Sandler A. Phase II study of efficacy and safety of bevacizumab in combination with chemotherapy or erlotinib compared with chemotherapy alone for treatment of recurrent or refractory non small-cell lung cancer. J Clin Oncol. 2007;25:4743–4750. doi: 10.1200/JCO.2007.12.3026. [DOI] [PubMed] [Google Scholar]
- 38.Wu S, Kim C, Baer L, Zhu X. Bevacizumab increases risk for severe proteinuria in cancer patients. J Am Soc Nephrol. 2010;21:1381–1389. doi: 10.1681/ASN.2010020167. [DOI] [PMC free article] [PubMed] [Google Scholar]