Abstract
Habituation, or the relatively permanent waning of a response as a result of repeated stimulation, is a form of behavioural plasticity that allows animals to filter out irrelevant stimuli and to focus selectively on important stimuli. Individuals that fail to habituate might be at a disadvantage if they continue to respond to irrelevant stimuli; therefore, habituation can have adaptive significance. In this study we compared rates of behaviour over time toward three different ecologically-relevant stimuli (food, a male intruder and a gravid female) in threespine sticklebacks (Gasterosteus aculeatus). We detected evidence for habituation to the stimuli, and males in this study were especially aggressive toward both male and female conspecifics. Although there were some clear temporal patterns that could be detected by looking at average behaviour, not all individuals behaved in the same ‘average’ way. We detected substantial inter-individual variation in behaviour toward all three stimuli, inter-individual variation in rates of habituation to both male and female conspecifics, but no evidence for correlations between behaviours across stimuli (behavioural syndromes). These results suggest that individual animals vary in rates of habituation, and prompt hypotheses about the causes and consequences of variation in rates of habituation.
Keywords: personality, foraging, learning, coping styles, individual differences, temperament, aggression, courtship, linear mixed models
1. Introduction
Habituation is the relatively permanent waning of a response as a result of repeated stimulation (Thorpe, 1956). Habituation has adaptive value in situations where continued response to a constant stimulus would be energetically costly. For example, an animal should not continue to attack food if it is unobtainable (Peeke, 1983), a male should not keep attacking a neighbour if he can avoid the costs of fighting (‘dear enemy’: Brooks & Falls, 1975), and a male should not continue to court a female if she is not ready to mate (Hampton, 1984). By habituating, the animal can resume other important activities, and habituation allows animals to function in a dynamic environment. An animal that fails to habituate to a nonthreatening stimulus might maintain high levels of behaviour toward the stimulus, even when it might be adaptive to direct attention elsewhere.
Unlike acclimation, fatigue or sensory adaptation, habituation is an active learning process that helps animals focus on important information (Raderschall et al., 2011). Previous studies have suggested that variation in the rate of habituation is biologically meaningful and can be subject to natural selection (Hinde, 1970). Animals habituate faster to weaker stimuli (Rankin et al., 2009). For example, male sticklebacks slowly habituate to particularly attractive mates (Jenkins & Rowland, 2000; Rowland, 2000), and goslings slowly habituate to especially threatening predators (Canty & Gould, 1995). Intraspecific variation in rates of habituation is influenced by inherited genetic variation (Glowa & Hansen, 1994; Bolivar et al., 2000) and by the environment experienced during development: rats that were reared in more complex environments habituated faster to novelty compared to rats reared in less complex environments (Zimmermann et al., 2001). Moreover, individual differences in rates of habituation have been related to consistent individual differences in behaviour (‘personality’) in humans (O’Gorman, 1977; LaRowe et al., 2006; Anderson et al., 2011) and nonhuman animals. Calm penguins recover relatively quickly (Ellenberg et al., 2009), reactive great tits take longer to recover from a startle (Carere & van Oers, 2004) and exploratory wall lizards habituate faster to predators (Rodriguez-Prieto et al., 2011).
Threespine sticklebacks have been a favourite subject for studies of both habituation and individual differences in behaviour (Huntingford, 1976; Bell & Stamps, 2004; Bell, 2005, 2007; Dingemanse et al., 2007, 2009; Webster, 2007; Harcourt et al., 2009). Previous studies have shown that when presented with a rival male (Peeke, 1969; Peeke & Veno, 1973, 1976; Peeke et al., 1979; Peeke & Figler, 1997) or a potential mate (Peeke & Figler, 1997; Rowland, 2000), territorial male sticklebacks have an initially strong response that wanes over time, and that both males and females habituate to unobtainable food (Peeke, 1983, 1995). Other studies confirmed that habituation in sticklebacks to these stimuli is stimulus-specific and relatively permanent (Peeke & Veno, 1973; Rowland, 2000). Studies on sticklebacks (e.g., Peeke, 1982) have supported the dual process theory of habituation (Groves & Thompson, 1970) which posits that habituation involves two separate processes in the central nervous system that interact: a habituation process and a sensitization process. Stimuli elicit both processes and behavioural output reflects a summation of both processes. The habituation process is decremental and the sensitization process is initially incremental and then decremental.
In this study we measured the behaviour of individual adult sticklebacks toward different stimuli (food, a male intruder, a gravid female) over the course of minutes. Behavioural plasticity such as habituation over such short time periods is relevant for reproductive adult sticklebacks because sticklebacks on the breeding grounds are constantly engaged in a variety of activities, including foraging, mating, and for males, territorial defence and parental care. Activities on the breeding grounds are highly dynamic. Territorial males, for example, are routinely confronted by both rival intruders and potential mates while at the same time must forage to support the metabolic demands of territoriality and parental care (Huntingford et al., 2001). Therefore, individuals that quickly modulate behaviour to different stimuli over relatively short timescales such as minutes might be at an advantage. For example, habituation to a nonthreatening male might permit a territorial male to redirect his energies to other activities such as foraging or courtship. Continuous response to a nonthreatening conspecific is not only energetically wasteful, but might also decrease a male’s reproductive success if it prevents him from attacking more threatening intruders (Peeke & Figler, 1997). On the other hand, habituating too quickly to a male stimulus comes at the risk that the intruder might really be a threat to a male’s nest or to potential mates. If male–male competition for mates is high and if the energetic and predation costs of aggression are low, then it might benefit males to be very persistent in territorial aggression, i.e., to not habituate to territorial intruders (Jenkins & Rowland, 2000).
We measured individual sticklebacks’ responses to unobtainable food, a male intruder and a gravid female to address three specific aims. First, we characterized the overall shape of behaviour toward the three different stimuli over time, i.e., we determined whether average rates of behaviour toward each stimulus increased, decreased or did not change over time, and whether the shape of behaviour over time was nonlinear (Groves & Thompson, 1970; Rankin et al., 2009). Second, we asked whether there was consistent individual variation in behaviour and behavioural plasticity toward each stimulus over time using mixed models. Finally, we assessed whether individual differences in behaviour were correlated across stimuli (behavioural syndromes: Sih et al., 2004).
2. Material and methods
Marine threespine sticklebacks were collected from Bodega Harbor in Sonoma County, CA, USA in June 1999. Fish were transported to the laboratory and maintained on a natural photoperiod in saltwater holding tanks. They were fed frozen or live brine shrimp ad libitum daily. Male and female sticklebacks were moved from the holding tanks to individual saltwater aquaria (60 × 30 × 30 cm) that were fed by fresh saltwater. Water temperature was maintained at 18 ± 2°C and salinity at 32 ppt. Minor fluctuations in temperature might have contributed to individual differences in behaviour or behavioural plasticity (Biro et al., 2010), but we did not measure temperature during each behavioural observation; therefore, we cannot assess this possibility. Each aquarium had a substrate of fine gravel and sand. Males’ tanks included four stalks of eel grass forming a square in the centre of the tank and string algae from which the males built their nests. Saltwater was filtered and circulated by exterior air-driven filters. Behavioural observations of females started at least one week after they were transferred to the individual aquaria. Only males that had completed nests via ‘creeping through’, a behaviour that marks the onset of the courtship phase of reproduction (Wootton, 1984), were included in the study. By restricting the study to males with completed nests that did not contain eggs, all males were in the same stage of the reproductive cycle at the time of the behavioural observations. All of the males spawned after the experiment, indicating that they were sexually mature and receptive.
Nest-building and territoriality in this species are facilitated by visual interactions with neighbours (Peeke, 1982). Therefore, the fish were allowed visual access to fish in neighbouring tanks. Males had male neighbours and females had female neighbours in order to control for differences in behaviour caused by the sex of the neighbour (Peeke, 1983). By allowing sticklebacks visual access to their neighbours, this ensured that males were motivated to court females and defend their territory against intruders. Opaque dividers were inserted between adjacent aquaria one hour prior to each behavioural observation in order to prevent the behaviour of neighbours from influencing the behaviour of the focal fish during behavioural observations.
2.1. Experimental design
We observed the behaviour of individual male sticklebacks toward three different stimuli (food, a male intruder and a gravid female) presented sequentially in a fixed order with at least 24 h between observations. The response of a focal male to each stimulus was measured once. We recorded bites per minute toward all three stimuli. Because male sticklebacks both court females and can be aggressive toward them because females are often nest predators (Sevenster, 1961; van den Assem, 1967; Wilz, 1972), we recorded both rates of courtship (zig-zags) and aggression (bites) toward a gravid female stimulus. Focal females were only measured for their behaviour toward the food stimulus and were non-gravid.
To measure behaviour toward the food stimulus, 35 active, live Artemia were placed in a clear glass tube 56 mm in diameter containing saltwater. The Artemia actively swam throughout the glass tube. The tube was placed in the focal fish’s aquarium as close to the centre of the tank as possible. The number of times that the focal fish bit at the tube per minute was recorded for ten minutes after the first bite.
Males’ behavioural reactions to a male intruder was observed at least one day later (mean ± SE = 4.62 ± 0.533 days). A stimulus male in nuptial coloration was placed in a clear glass tube, 15 cm in diameter, containing saltwater. The tube was placed in the focal fish’s aquarium at least 15 cm from the nest. The number of bites per minute of the focal fish was recorded for 20 min after the first bite. Although some studies of sticklebacks have used dummies to measure aggression (Bakker, 1994), we elected to use live animals because preliminary observations indicated that live stimuli elicited stronger behavioural responses in the focal animals (see also Dzieweczynski & Forrette, 2011). In order to prevent repeated stress to the stimulus males, focal males were confronted by one of three randomly-selected stimulus males. Different stimulus males were used on each day of the experiment. On average, a stimulus male was used once per day. The maximum number of times a stimulus male was used on any given day was 3 times. The stimulus males were active throughout the behavioural observations, swimming up and down the glass tube.
Males’ behavioural reactions toward a gravid female was measured at least one day later (mean ± SE = 7.57 ± 1.241 days). A gravid female was placed in a clear glass tube, 15 cm in diameter, containing saltwater. As before, the tube was placed in the focal fish’s aquarium. Both the number of bites and the number of zig-zags per minute were recorded for twenty minutes after the first bite. During the behavioural observation males often crept through the nest and exhibited other nest-directed activities (not recorded). Each day, three different gravid stimulus females were used; stimulus females were replaced with new gravid females at the end of the day. On average, a stimulus female was used once per day. The maximum number of times a stimulus female was used on any given day was 3 times. The stimulus females maintained high rates of activity throughout the behavioural observation and often showed the ‘head up’ display, which indicates sexual receptivity (Rowland, 2000).
In total, we recorded the behaviour of 33 males and 35 females toward the food stimulus, 31 males toward the male intruder stimulus and 24 males toward the gravid female stimulus. 22 males were observed for their behavioural reactions to all three stimuli. The standard length of a subset of individuals was measured opportunistically (female standard length ± SE = 6.54 ± 0.08 cm, N = 18, male standard length ± SE = 6.34 ± 0.06 cm, N = 22). The procedures used in this study were approved by IACUC #8399 University of California, Davis, CA, USA.
2.2. Goals and data analysis
Our first goal was to characterize the overall shape of behaviour toward the three different stimuli over time. That is, we wished to determine whether average rates of behaviour toward each stimulus increased (sensitization), decreased (habituation) or did not change over time, and whether the shape of behaviour over time was nonlinear (Groves & Thompson, 1970; Rankin et al., 2009). To address this issue, we built three separate mixed models in SAS™ version 9.2 (SAS Institute, Cary, NC, USA). The first model considered bites toward the food as the dependent variable. Because we measured bites toward the food in both males and females, we included ‘sex’ as a fixed factor. The second model considered bites toward the male as the dependent variable. Because two behaviours were simultaneously recorded toward the female stimulus, we analysed them together in a third mixed multivariate model with two dependent variables (bites and zig-zags) (Snijders & Boster, 2012). The initial models included linear, squared and cubic fixed effect terms for ‘time’. By including polynomial terms for ‘time’, we could account for nonlinearity of behaviour over time. The behaviour data were +1 ln-transformed to meet model assumptions. We used an AR1 within-individual covariance structure because preliminary analyses showed that measurements of behaviour that were closer in time were more tightly correlated than measures further in time toward a stimulus and using AR1 type covariance structure consistently improved model fit according to likelihood ratio tests. Models were tested with type-1 sums of squares and time was centred around its mean in order to remove potential colinearity between the squared and linear term for time. Nonsignificant terms for time were sequentially removed, starting with higher-order terms.
The second goal was to determine whether there was variation among individuals in behaviour over time. To answer this question, we used mixed models with random slopes and intercepts (random regression) (Snijders & Boster, 2012) to quantify individual ‘behavioural reaction norms’ (Dingemanse et al., 2010) toward each stimulus using Proc Mixed in SAS. This approach is useful for characterizing how behaviour changes along a gradient, which is ‘time’ in this case (Dingemanse et al., 2010). This approach allowed us to determine for each stimulus whether individuals consistently differed in behaviour (intercepts), whether individuals differed in how behaviour changed over time (slopes, i.e., behavioural plasticity), and whether these two attributes might be related to one another (intercept–slope correlation). Because time was centred around its mean, individual variation in intercepts reflects individual variation in behaviour half-way through the behavioural observation.
For each stimulus, we tested for the significance of random effects by comparing models with the final fixed effect structure from Aim 1 in a hierarchical manner. Our general strategy was to first compare a model with the final fixed effect structure to a model with random intercepts. Then, we sequentially added random effects terms to the model as appropriate (see legends to Tables 4–6 for details). The strategy was slightly different for the model for response to a female stimulus because two behavioural variables were analyzed simultaneously (bites and zig-zags). For that stimulus, we built sequentially more complex models which either allowed random variances for the two behaviours to be the same, to vary or to covary (described further in the legend to Table 6). We used a log-likelihood ratio test to select the best model for each stimulus (Pinheiro & Bates, 2000). The covariance structure type was set as unstructured and the covariance matrix was allowed to vary, i.e., was not constrained to be positive definite (Martin et al., 2011). Covariances were converted to correlations to facilitate comparisons with other studies (Martin et al., 2011).
Table 4.
Test for significance of random effects of the models for bites at the food, N = 68 (number of fixed effects parameters = 5).
| AIC | Log(L) | Cov P | Test | Component tested | χ2,* | df | p | |
|---|---|---|---|---|---|---|---|---|
| 1 | 1540.38 | −768.19 | 2 | |||||
| 2 | 1495.14 | −744.57 | 3 | 1 vs. 2 | Intercept | 47.24 | 1 | <0.0001 |
| 3 | 1497.12 | −744.56 | 4 | 2 vs. 3 | Slope | 0.02 | 1 | 0.8929 |
AIC, Akaike Information Criterion (AIC); log(L), log likelihood; Cov P, the number of covariance parameters; df, degrees of freedom. The final model is indicated in bold.
Log-likelihood ratio tests.
There was not enough variation in the slopes for the squared and cubic term for time so that when these were added to the model as random effects, the random slopes were inestimable. Therefore, we only tested for variation among individuals in linear slopes.
Model 1 includes the final fixed effects structure with no random effects. Model 2 contains the same fixed effects as model 1 with random intercepts. Model 3 contains the same fixed and random effects as model 2 with random slopes.
Table 6.
Test for significance of random effects of the models for bites and zig-zags at the gravid female, N = 24 (number of fixed effects parameters = 4).
| AIC | Log(L) | Cov. P | Test | Component tested | χ2,* | df | p | |
|---|---|---|---|---|---|---|---|---|
| 1 | 2182.54 | −1087.27 | 4 | |||||
| 2 | 2172.70 | −1081.35 | 5 | 1 vs. 2 | Intercept (same variance) | 11.84 | 1 | <0.0001 |
| 3 | 2129.25 | −1058.63 | 6 | 2 vs. 3 | Slope (same variance) | 45.45 | 1 | <0.0001 |
| 4 | 2145.57 | −1066.78 | 6 | 2 vs. 4 | Intercept (different variance) | 29.13 | 1 | <0.0001 |
| 5 | 2133.20 | −1058.60 | 8 | 4 vs. 5 | Slope (different variance) | 16.37 | 2 | 0.0002 |
| 6 | 2108.31 | −1044.16 | 10 | 5 vs. 6 | COVI,S within behav | 28.89 | 2 | <0.0001 |
| 7 | 2088.89 | −1030.45 | 14 | 6 vs. 7 | COVI,S between behav | 27.42 | 4 | <0.0001 |
AIC, Akaike Information Criterion (AIC); log(L), log likelihood; Cov P, the number of covariance parameters; df, degrees of freedom. The final model is indicated in bold.
Log-likelihood ratio tests.
Model 1 includes the final fixed effects structure with no random effects. Model 2 contains the same fixed effects as model 1 with random intercepts for bites and zig-zags, assumed to have the same variance. Model 3 contains the same fixed and random effects as model 2 with random slopes for bites and zig-zags, assumed to have the same variance. Model 4 contains the same fixed and random effects as model 2 with random intercepts for bites and zig-zags, allowed to have different variances. Model 5 contains the same fixed and random effects as model 4 with random slopes for bites and zig-zags, which are allowed to have different variances. Model 6 contains the same fixed and random effects as model 5 but random effects within bites and zig-zags are allowed to have nonzero covariance. Model 7 contains the same fixed and random effects as model 6, but allows for all possible covariances between random effects between behaviours (i.e., COVI,I, COVS,S, COVI bites, S zz, COVI zz, S bites).
Recent studies have confirmed that random regression is a very data-hungry procedure most behavioural studies do not have sufficient statistical power to detect covariance between slopes and intercepts with great accuracy and precision (Martin et al., 2011; van de Pol, 2012), and the slope–intercept covariance tends to be overestimated when the sample size is small (van de Pol, 2012). Therefore, we are cautious in our interpretation of the covariance results.
Our third goal was to assess whether individual differences in behaviour were correlated across stimuli. For each stimulus, the number of behaviours (bites or zig-zags) over the course of the observation was summed. We tested whether total rates of behaviour were correlated across stimuli using Spearman rank correlations in SPSS version 19.
Another study showed that exposure to social stimuli can influence subsequent behaviour in sticklebacks: males that were presented with a male intruder for 5 min immediately increased rates of courtship after the male intruder was removed (Peeke & Figler, 1997). Therefore, it is possible that previous exposure to a stimulus might have influenced males’ subsequent behaviour to other stimuli in this study (i.e., a carryover effect: Diaz-Uriarte, 2002). If there was a carryover, we predicted that males that were recently presented with a stimulus would behave differently compared to males that had longer to recover between stimuli. Therefore, to test for carryover effects, we examined the relationship between the number of days that elapsed between exposure to one stimulus and behaviour (total number of bites or zig-zags) toward the subsequent stimulus using nonparametric Spearman rank correlations in SPSS version 19.
3. Results
3.1. Bites at the food stimulus
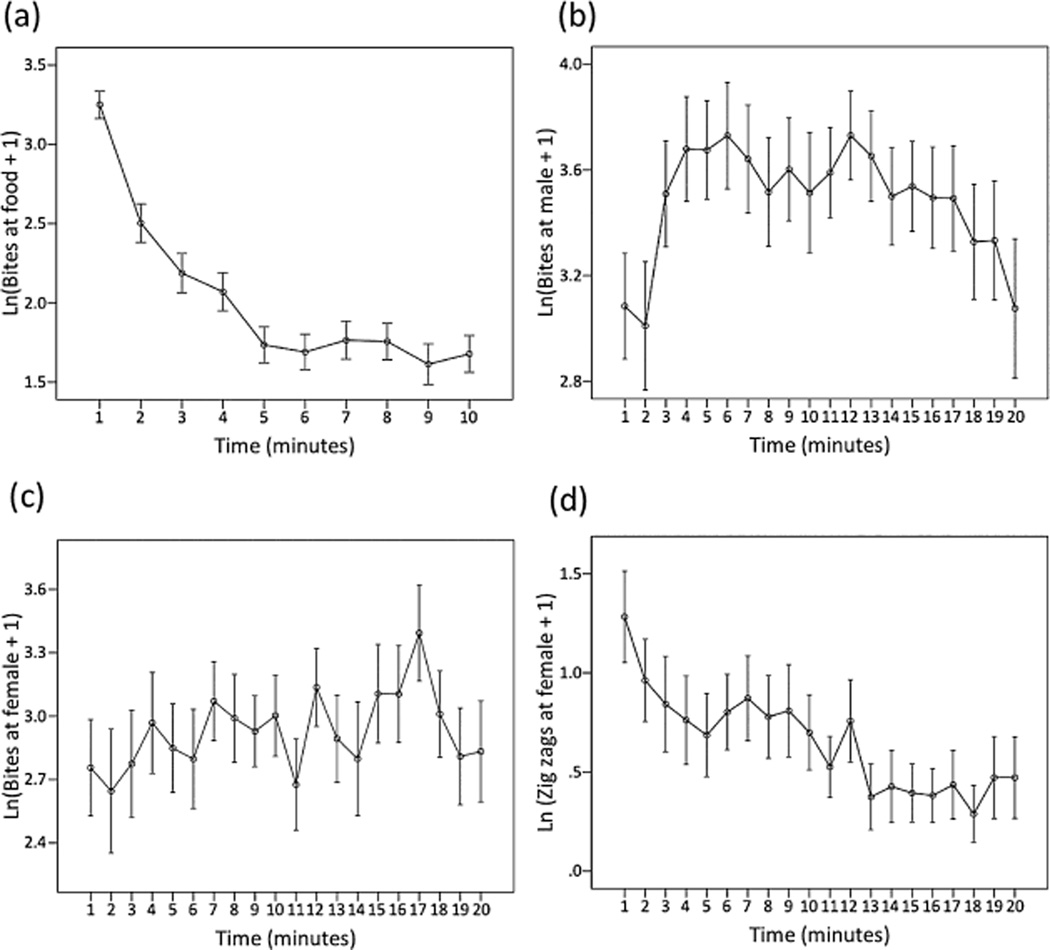
Initially, average rates of biting toward the food stimulus were high (average ± SE 30 ± 2.3 bites during the first minute), but quickly dropped and remained low after the fifth minute (Figure 1a), consistent with habituation and with previous studies of sticklebacks (Peeke, 1995).
Figure 1.
Average behaviour over time. The panels show means ± 1 standard error of the ln-transformed data. (a) Average number of bites at the food stimulus over 10 min; (b) average number of bites at a male intruder over 20 min; (c) average number of bites at a gravid female over 20 min; (d) average number of zig-zags at the gravid female over 20 min.
The rapid drop in bites at the food was nonlinear as indicated by the significant fixed effect of time3 (Tables 1–3). Males and females did not differ in overall rates of biting at the food (Tables 1–3), similar to (Peeke, 1995).
Table 1.
Results of the final model for bites at the food showing estimates of fixed effects and covariance parameter coefficients.
| Estimate ± SE | t1 | p | |
|---|---|---|---|
| Random effects | |||
| Intercept | 0.4334 ± 0.0921 | ||
| Residual | 0.4725 ± 0.0352 | ||
| Fixed effects | |||
| Intercept | 1.7858 ± 0.1339 | 13.34 | <0.0001 |
| Sex | −0.1423 ± 0.1751 | −0.81 | 0.4193 |
| Time | −0.0271 ± 0.0442 | −0.61 | 0.5409 |
| Time2 | −0.0249 ± 0.0167 | −1.48 | 0.1386 |
| Time3 | −0.0056 ± 0.0015 | −3.64 | 0.0003 |
Time refers to the coefficient for the linear term, Time2 refers to the coefficient for the squared term and Time3 refers to the coefficient for the cubed term. The parameter estimates show that there is significant variation among individuals intercepts (I), slopes (S, time) and the covariance between slopes and intercepts (COVI,S).
Table 3.
Results of the final model for bites and zig-zags at a gravid female showing estimates of fixed effects and covariance parameter coefficients.
| Estimate ± SE | Correlation | t1 | p | |
|---|---|---|---|---|
| Random effects | ||||
| Intercept – bites | 0.9239 ± 0.3299 | |||
| Slope – bites | 0.0032 ± 0.0013 | |||
| Intercept – zz | 1.0084 ± 0.3482 | |||
| Slope – zz | 0.0043 ± 0.0193 | |||
| COVI,S – bites | −0.0313 ± 0.0177 | −0.5686 | ||
| COVI,S – zz | −0.0560 ± 0.0193 | −0.8520 | ||
| COVI,I | −0.5973 ± 0.2679 | −0.6188 | ||
| COVS,S | −0.0041 ± 0.0193 | −1 | ||
| COVI bites, S zz | 0.0508 ± 0.0193 | 0.8067 | ||
| COVS bites, I zz | 0.0559 ± 0.0191 | 0.9731 | ||
| Residual (bites) | 0.5032 ± 0.0418 | |||
| Residual (zz) | 0.4430 ± 0.0338 | |||
| Fixed effects | ||||
| Intercept | 1.0556 ± 0.2214 | 4.77 | <0.0001 | |
| Time | −0.0376 ± 0.0150 | −2.49 | 0.0203 | |
| Behaviour | 1.7443 ± 0.3792 | 4.60 | 0.0001 | |
| Time × Behaviour | 0.0490 ± 0.0273 | 1.79 | 0.0863 |
The covariance between slopes and intercepts was converted to a correlation coefficient for ease of comparison with other studies. The fixed effect ‘Behaviour’ tests for differences in rates of zig-zags and bites; the Time × Behaviour term tests whether bites and zig-zags differed in how they changed over time. Shown are covariance parameter estimates for covariance between intercepts and slopes within each behaviour (e.g., CovI,S – bites) as well as between the two behaviours (e.g., CovI bites, S zz).
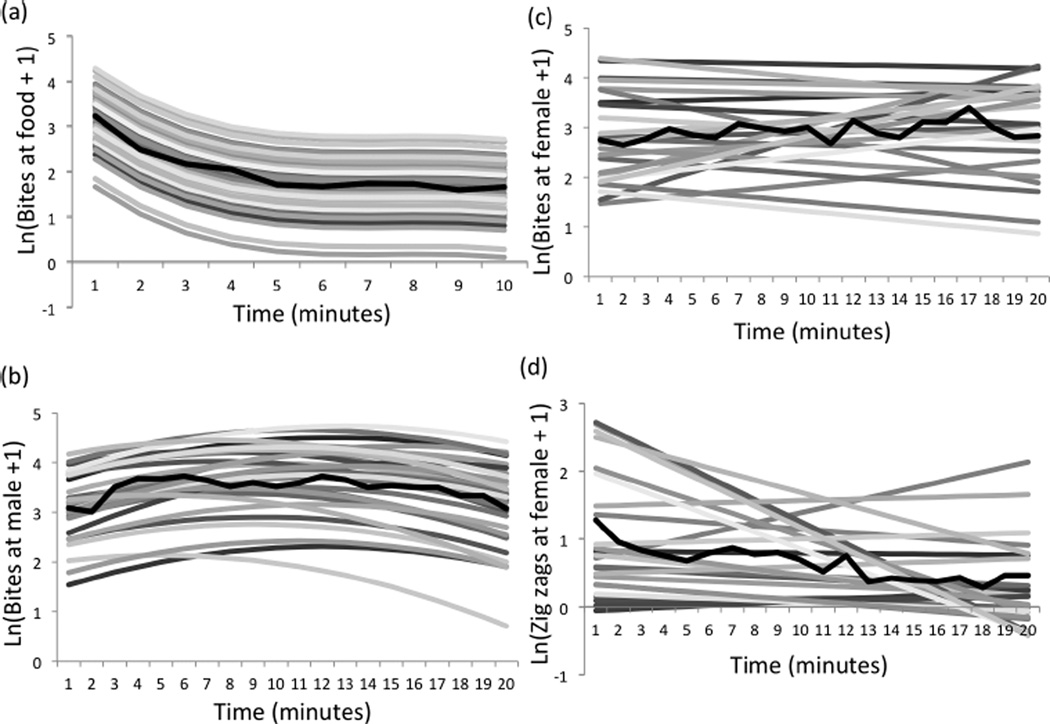
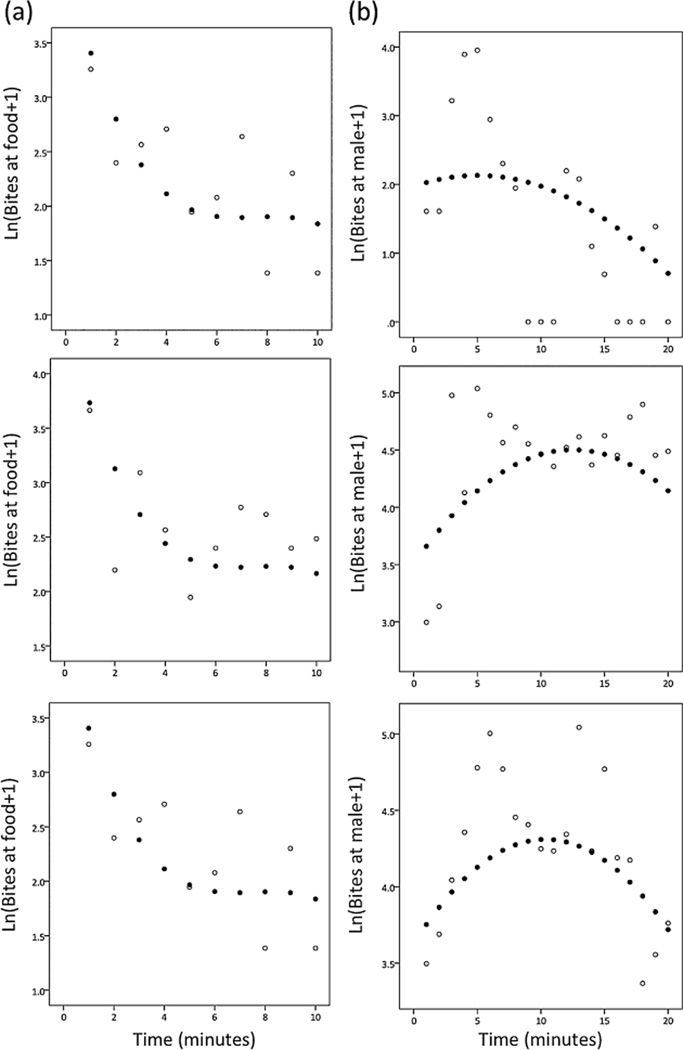
Although there were some clear temporal patterns that could be detected by looking at average behaviour toward the food stimulus, not all individuals behaved in the same ‘average’ way. Individuals consistently differed in behaviour toward the food stimulus, as evidenced by the final model that included random variation in intercepts (Table 4). We did not detect evidence for individual variation in behavioural plasticity (slopes) at the food stimulus (Table 4). The predicted behavioural reaction norms for each individual are in Figure 2, and examples showing the fit of the predicted reaction norms to the data are given in Figure A1.
Figure 2.
Individual differences in behaviour over time. Shown are the predicted values for each individual from the final model as well as the mean (observed) behaviour in bold. (a) Bites at the food stimulus; (b) bites at a male intruder; (c) bites at a gravid female; (d) zig-zags at the gravid female.
3.2. Bites at the male stimulus
Average bites at the male intruder were also nonlinear over time but showed evidence for sensitization followed by habituation (Figure 1b, Table 2), consistent with dual process theory and with other studies of the habituation of aggression toward a rival male in sticklebacks (Peeke, 1983). The average response to a male intruder was lowest in the first minute, peaked to 63 ± 9.04 bites per minute during the sixth minute and then gradually declined thereafter, with another minor peak at 12 min (Figure 1b).
Table 2.
Results of the final model for bites at a male intruder showing estimates of fixed effects and covariance parameter coefficients.
| Estimate ± SE | t1 | p | |
|---|---|---|---|
| Random effects | |||
| Intercept | 0.5469 ± 0.1701 | ||
| Slope of time | 0.0024 ± 0.0013 | ||
| Residual | 0.6514 ± 0.0755 | ||
| Fixed effects | |||
| Intercept | 3.6797 ± 0.1563 | 23.54 | <0.0001 |
| Time | 0.0172 ± 0.0132 | 1.30 | 0.2018 |
| Time2 | −0.0064 ± 0.0015 | −4.12 | <0.0001 |
Time refers to the coefficient for the linear term and Time2 refers to the coefficient for the squared term.
There was variation among males in aggression (intercepts) and in how aggression changed over time (slopes) according to the final model (Table 5, Figure 2b). Some of the individual variation in rates of aggression toward the male intruder could be explained by body size: larger males, on average, bit more at the male intruder (Table 7). We did not detect evidence for covariance between slope and intercepts for bites at the male intruder (Table 5).
Table 5.
Test for significance of random effects of the models for bites at a male intruder, N = 31 (number of fixed effects parameters = 3).
| AIC | Log(L) | Cov P | Test | Component tested | χ2,* | df | p | |
|---|---|---|---|---|---|---|---|---|
| 1 | 1386.99 | −691.49 | 2 | |||||
| 2 | 1370.22 | −682.11 | 3 | 1 vs. 2 | Intercept | 18.76 | 1 | <0.0001 |
| 3 | 1367.32 | −679.66 | 4 | 2 vs. 3 | Slope | 4.90 | 1 | 0.0268 |
| 4 | 1368.14 | −679.07 | 5 | 3 vs. 4 | COVI,S | 1.18 | 1 | 0.2778 |
AIC, Akaike Information Criterion (AIC); log(L), log likelihood; Cov P, the number of covariance parameters; df, degrees of freedom. The final model is indicated in bold.
Log-likelihood ratio tests.
Model 1 includes the final fixed effects structure with no random effects. Model 2 contains the same fixed effects as model 1 with random intercepts. Model 3 contains the same fixed and random effects as model 2 with random slopes. Model 4 contains the same fixed and random effects as model 3, allowing for nonzero covariance between intercepts (I) and slopes for time (S).
Table 7.
Spearman rank correlations between total number of behaviours over the 10- or 20-min observation period toward each stimulus and body size (standard length).
| Bites at the food |
Bites at the male |
Bites at the female |
Zig-zags at the female |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R | p | N | R | p | N | R | p | N | R | p | N | |
| Length | −0.197 | 0.229 | 39 | 0.449 | 0.036 | 22 | −0.103 | 0.714 | 15 | −0.166 | 0.553 | 15 |
| Bites at the food | 0.278 | 0.137 | 30 | 0.254 | 0.255 | 22 | 0.015 | 0.948 | 22 | |||
| Bites at the male | 0.154 | 0.483 | 23 | −0.172 | 0.433 | 23 | ||||||
| Bites at female | 0.270 | 0.202 | 24 | |||||||||
3.3. Behaviour toward the gravid female stimulus
3.3.1. Aggression (bites) toward the gravid female stimulus
Males maintained high rates of aggression (bites) toward the female stimulus throughout the 20-min observation (approximately 30 bites/min, Figure 1c). On average, males bit at the female less than they bit at the male, but rates of biting toward the female were as high as 42 bites/min (17th minute).
There was variation among males in overall rates of aggressive behaviour toward the female stimulus and in how individuals’ aggression changed over time (Figure 2c), as indicated by the final model which included variation in slopes and intercepts for bites (Table 6). Relative to the mean slope, a small, negative slope reflects faster exponential decline in aggression, which in turn reflects fast habituation. Therefore, the negative COVI,S – bites term in Table 3 suggests that males that were especially aggressive toward the female quickly decreased rates of biting over time.
3.3.2. Courtship (zig-zags) toward the female stimulus
Rates of courtship during the first minute were 5.4 ± 1.56 zig-zags per minute and then declined linearly with time (Figure 1d), consistent with habituation. Rates of courtship (zig-zags) were variable among individuals, as indicated by variation among individuals in intercepts (Table 3). There was also variation among males in rates of habituation (slopes, Table 3), indicating that some males decreased rates of courtship faster than others (Figure 2d).
The negative COVI,S – zz termin Table 3 suggests thatmales that courted the female more (higher intercepts) relatively quickly decreased rates of courtship over time (smaller, more negative slope) compared to the mean slope, i.e., habituated faster.
3.3.3. Courtship and aggression toward the gravid female
In general, males bit at the female stimulus more than they courted her (fixed effect of ‘behaviour’ in Table 3, compare Figure 1c and 1d). The marginally significant Behaviour × Time interaction suggests that the two behaviours differed in how they changed over time.
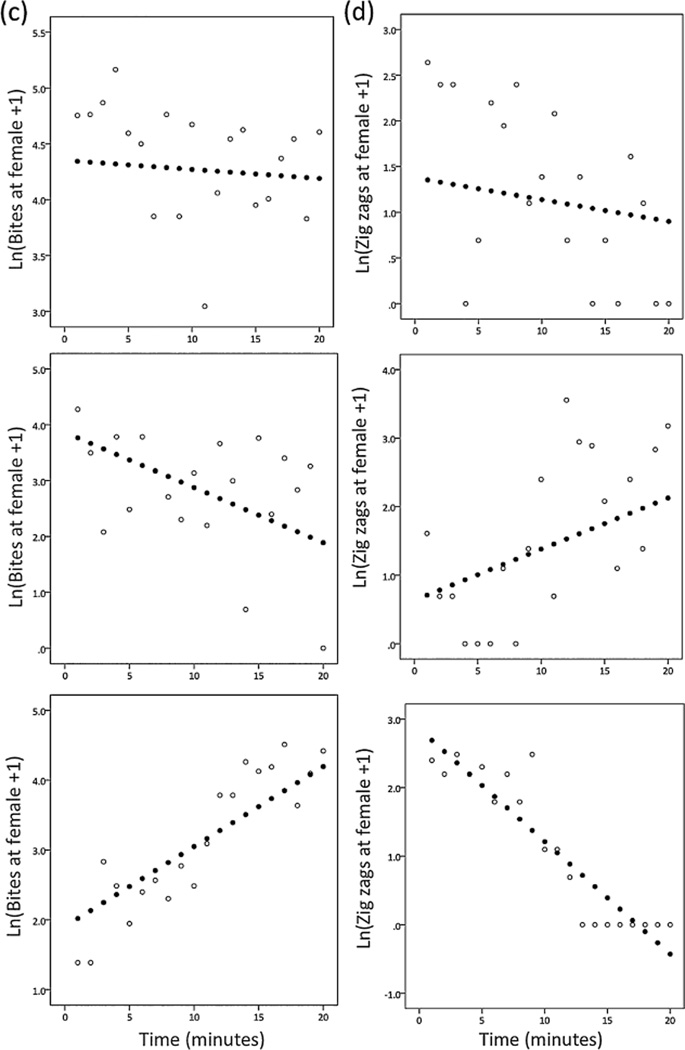
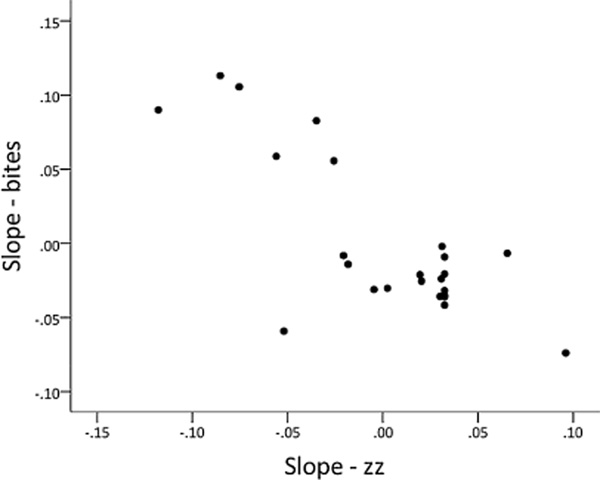
The random regression analysis suggested that within individual males, courtship and aggression toward the female stimulus were mutually inhibitory. Compared to the mean slope, males that rapidly decreased aggression toward the female quickly increased courtship behaviour over time, and vice versa, as suggested by the negative COVS,S term in Table 3. According to the final model, the two slopes were perfectly negatively correlated with each other, which might reflect an overparameterized model. We elected to keep the covariance terms between behaviours in the final model because they are of biological interest, and including covariances across the two behaviours improved model fit (Table 6). To evaluate this pattern further, we constructed separate univariate models for bites and zig-zags at the gravid female to evaluate their slopes independently of one another, and inspection of the estimated slopes for bites and zig-zags revealed that there were strongly negatively correlated (R =−0.779, N = 24, p <0.0001, Figure A2). Therefore, although the precise estimate of the COVS,S term should be treated with caution, we are more confident in the overall direction of the relationship (negative).
The results also suggest that some males might have been generally very active while interacting with a female. For example, males that were very aggressive toward the female maintained relatively high levels of courtship behaviour throughout the 20-min observation period (positive COVI bites, S zz term in Table 3).
3.4. Correlations across stimuli
When we summed rates of behaviour over time toward each stimulus, e.g., the total number of bites at the food, total number of bites at the male, etc., we did not detect a relationship between individual differences in behaviours across stimuli (Table 7). In addition, when comparing across individuals, there was no relationship between aggression (bites) and courtship (zig-zags) toward the gravid female (Table 7).
3.5. Carryover effects
We found no evidence for carryovers across stimuli. There was not a detectable relationship between the number of days that elapsed between the observation of behaviour toward the food stimulus and the observation of behaviour toward the male stimulus on levels of aggressive behaviour (bites) toward the male stimulus (r =−0.032, p = 0.872, N = 28). Similarly, there was no relationship between the number of days that elapsed between the observation of behaviour toward the male stimulus and the observation of behaviour toward the female stimulus on behaviour toward the female stimulus (bites: r = 0.027, p = 0.912, N = 19; zig-zags: r =−0.008, p = 0.973, N = 19).
4. Discussion
The first goal of this study was to characterize sticklebacks’ responses to different ecologically-relevant stimuli. We found that different stimuli evoked very different behavioural responses. In particular, sticklebacks from this population were very aggressive: rates of aggression to the male intruder were as high as 1 bite per second, which is higher than published estimates from other populations (Rowland, 1989; Peeke & Figler, 1997). Unlike other studies of habituation of territorial aggression in sticklebacks that have used sticklebacks from freshwater or brackish habitats (Rowland, 1989; Peeke & Figler, 1997), here, we studied sticklebacks from a marine habitat, where sheltered, vegetated areas that are suitable for nesting territories might be more limited. There might be greater male–male competition for territories in Bodega Harbor, which might explain the high levels of aggression observed in this study.
Another striking pattern was the relatively low level of courtship observed in this study compared to others (Peeke & Figler, 1997; Jenkins & Rowland, 2000), even though all males were in the ‘courtship phase’ of the breeding cycle (Wootton, 1984) and the females that were used as stimuli were gravid and appeared to be receptive. In some populations, male sticklebacks do not zig-zag at all, but instead lead the female directly to the nest (Foster, 1994). Therefore, it is possible that males from this population use other forms of courtship to attract females to their nest. It is also conceivable that the high level of territorial aggressiveness in males from this population inhibits their courtship behaviour.
The second goal of this study was to quantify individual variation in behaviour toward different stimuli. We detected substantial inter-individual variation in behaviour (variation in intercepts) toward all three stimuli, and found that some male sticklebacks habituated to male and female conspecifics faster than others (variation in slopes). This study adds to the growing body of literature showing that there is intraspecific variation in plasticity (Brommer et al., 2008; Martin & Reale, 2008; Dingemanse et al., 2009; Martin et al., 2011; Mathot et al., 2011; Westneat et al., 2011). A recurring theme of these studies is that when there is variation in plasticity (slopes), the extent of individual differences changes over time (Montiglio et al., 2010). However, most recent studies of individual variation in behavioural plasticity have measured behaviour over the course of days (Martin & Reale, 2008; Rodriguez-Prieto et al., 2010, 2011; Biro, 2012; Stamps et al., 2012) or months (Dingemanse et al., 2009, 2012b). In contrast, in this study, we measured behaviour over a relatively short period of time (min), similar to Montiglio et al. (2010) and Dingemanse et al. (2012a). Given the dynamic nature of activities on the breeding grounds, variation in behavioural plasticity over the course of relatively short time periods such as minutes is likely to be ecologically important for sticklebacks. Nesting males that fail to immediately habituate to irrelevant stimuli such as unobtainable food might miss out on courtship opportunities when a school of females suddenly appears. On the other hand, there might be costs of rapid habituation if it means that a male disregards a stimulus too quickly. For example, it might benefit males to be persistently aggressive and to maintain high levels of territorial defence in habitats where male–male competition is strong. Rates of habituation are probably shaped by natural selection and variation in rates of habituation among individuals within populations likely reflects a compromise between the costs and benefits of habituation.
Analysing behaviour over time offered a number of other insights that we would not have appreciated if we had simply looked at total rates of behaviour. For example, the total behavioural response to the food stimulus (total number of bites) was much greater than the courtship response to the gravid female. However, courtship behaviour took longer to habituate than foraging behaviour (Figure 1a): zig-zags declined gradually until the 14th minute, while bites at the food dropped rapidly such that by the 4th minute they were close to zero (Figure 1a versus 1d). If animals habituate rapidly to non-salient stimuli (Glowa & Hansen, 1994), this pattern suggests that the female stimulus was more salient to the males than the food was, even though the total response was much greater to the food.
Another intriguing pattern that was revealed by analysing behaviour over time was the relationship between courtship and aggression toward the gravid female. For male sticklebacks, females are both a threat and an opportunity because while females are potential mates, they also often cannibalize males’ nests (Wootton, 1984). When we looked at total behavioural responses, there was no relationship between aggression (bites) and courtship (zig-zags) toward the gravid female (Table 7, similar to Jenkins & Rowland (2000), but see Dzieweczynski et al. (2009)). But within individual males, courtship and aggression were negatively correlated over time. That is, we detected significant among-individual variation in the plasticity of both bites and zig-zags to the female, and negative covariance between the slopes (Tables 1–3). The analysis suggests that if a male increased courtship, he became less aggressive over time, and vice versa. In other words, some individuals increased rates of aggression over time (Figure A1c), and for those males, their courtship behaviour decreased over time. Other individuals decreased rates of aggression over time (Figure A1c), and those males simultaneously decreased rates of courtship (Figure 2c and 2d). One possible explanation for this finding is that the two behaviours are mutually exclusive, i.e., in order to increase rates of biting, a male had to decrease rates of zig-zagging. Another way to view the pattern is that perhaps males switched from courting the female to aggressively attempting to chase her out of the territory if they learned that the female was not receptive or was unobtainable. Another (complementary) explanation is that courtship and aggression were mutually inhibitory within individual males, consistent with classic ethological theory that there are multiple ‘motivations’ or ‘drives’ within an individual that can come into conflict with one another (Sevenster, 1961; van den Assem, 1967; Wilz, 1972). Indeed, studies on other organisms including sticklebacks have shown a trade-off between sex and aggression when males are presented with a male and female simultaneously: the presence of competitors causes males to decrease courtship (Kodric-Brown & Brown, 1984; Candolin, 1997; Santangelo et al., 2002; Dzieweczynski et al., 2009).
The third aim of this study was to determine if individual differences in behaviour were correlated across stimuli. We found no evidence for behavioural syndromes (Sih et al., 2004) when looking at total behavioural responses to the different stimuli. We did not detect any relationships between the total number of behaviours (bites or zig-zags) directed at food, the male intruder or the gravid female, suggesting that behaviour in these different contexts is independent. However, it would be worthwhile to apply a random regression approach to a larger sample of animals measured in all three contexts in order to determine whether there is a relationship between the shape of behaviour over time to different stimuli. In addition, we found no evidence for behavioural carryovers across contexts. Courtship behaviour was not influenced by the number of days since the focal male had been confronted by a male intruder, for example. An earlier study found that male sticklebacks exposed to a male intruder immediately increased rates of courtship (Peeke & Figler, 1997). Our results suggest that if there was a behavioural carryover across stimuli, it did not persist after 24 h.
In conclusion, we found very different average behavioural responses toward different stimuli — sticklebacks habituated to conspecifics, and they maintained very high levels of aggressiveness over time that might have spilled over to influence their courtship behaviour. However, we detected strong inter-individual variation in rates of habituation: some individual sticklebacks persistently attended to a stimulus, while other individuals quickly recovered. Given the adaptive significance of habituation — habituation allows animals to filter out irrelevant stimuli and to selectively focus on important stimuli — it is likely that individual variation found in this experiment is biologically meaningful, but future studies need to quantify its causes and consequences.
Acknowledgements
Thanks to Bodega Marine lab for financial support and Maria Muyot for help with statistics. Matt Grobis helped with the manuscript. Three referees offered helpful comments that improved the manuscript.
Appendix
Figure A1. Sample model fits. Shown are the final models fits (closed circles) to the data (open circles) for three randomly-selected individuals per behaviour. (A) Bites at the food stimulus; (B) bites at a male intruder; (C) bites at a gravid female; (D) zig-zags to the gravid female.
Figure A2. Scatterplot showing the estimated slopes for bites and zig-zags at the gravid female from the univariate models. Each data point represents a different individual.
References
- Anderson NE, Wan L, Young KA, Stanford MS. Psychopathic traits predict startle habituation but not modulation in an emotional faces task. Pers. Indiv. Diff. 2011;50:712–716. [Google Scholar]
- Bakker TCM. Evolution of aggressive behaviour in the threespine stickleback. In: Foster SA, Bell MA, editors. The evolutionary biology of the threespine stickleback. Oxford: Oxford University Press; 1994. pp. 345–380. [Google Scholar]
- Bell AM. Differences between individuals and populations of threespined stickleback. J. Evol. Biol. 2005;18:464–473. doi: 10.1111/j.1420-9101.2004.00817.x. [DOI] [PubMed] [Google Scholar]
- Bell AM. Future directions in behavioural syndromes research. Proc. Roy. Soc. Lond. B: Biol. 2007;274:755–761. doi: 10.1098/rspb.2006.0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell AM, Stamps JA. Development of behavioural differences between individuals and populations of sticklebacks, Gasterosteus aculeatus . Anim. Behav. 2004;68:1339–1348. [Google Scholar]
- Biro PA. Do rapid assays predict repeatability in labile (behavioural) traits? Anim. Behav. 2012;83:1295–1300. [Google Scholar]
- Biro PA, Beckmann C, Stamps JA. Small within-day increases in temperature affects boldness and alters personality in coral reef fish. Proc. Roy. Soc. Lond. B: Biol. 2010;277:71–77. doi: 10.1098/rspb.2009.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar VJ, Caldarone BJ, Reilly AA, Flaherty L. Habituation of activity in an open field: a survey of inbred strains and F-1 hybrids. Behav. Genet. 2000;30:285–293. doi: 10.1023/a:1026545316455. [DOI] [PubMed] [Google Scholar]
- Brommer JE, Rattiste K, Wilson AJ. Exploring plasticity in the wild: laying date-temperature reaction norms in the common gull. Proc. Roy. Soc. Lond. B: Biol. 2008;275:687–693. doi: 10.1098/rspb.2007.0951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks RJ, Falls JB. Individual recognition by song in white-throated sparrows. 1. Discrimination of songs of neighbors and strangers. Can. J. Zool. 1975;53:879–888. [Google Scholar]
- Candolin U. Predation risk affects courtship and attractiveness of competing threespine stickleback males. Behav. Ecol. Sociobiol. 1997;41:81–87. [Google Scholar]
- Canty N, Gould JL. The hawk-goose experiment — sources of variability. Anim. Behav. 1995;50:1091–1095. [Google Scholar]
- Carere C, van Oers K. Shy and bold great tits (Parus major): body temperature and breath rate in response to handling stress. Physiol. Behav. 2004;82:905–912. doi: 10.1016/j.physbeh.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Diaz-Uriarte R. Incorrect analysis of crossover trials in animal behaviour research. Anim. Behav. 2002;63:815–822. [Google Scholar]
- Dingemanse NJ, Barber I, Wright J, Brommer JE. Quantitative genetics of behavioural reaction norms: genetic correlations between personality and behavioural plasticity vary across stickleback populations. J. Evol. Biol. 2012a;25:485–496. doi: 10.1111/j.1420-9101.2011.02439.x. [DOI] [PubMed] [Google Scholar]
- Dingemanse NJ, Bouwman KM, van de Pol M, van Overveld T, Patrick SC, Matthysen E, Quinn JL. Variation in personality and behavioural plasticity across four populations of the great tit Parus major . J. Anim. Ecol. 2012b;81:116–126. doi: 10.1111/j.1365-2656.2011.01877.x. [DOI] [PubMed] [Google Scholar]
- Dingemanse NJ, Kazem AJN, Reale D, Wright J. Behavioural reaction norms: animal personality meets individual plasticity. Trends Ecol. Evol. 2010;25:81–89. doi: 10.1016/j.tree.2009.07.013. [DOI] [PubMed] [Google Scholar]
- Dingemanse NJ, van der Plas F, Wright J, Réale D, Schrama M, Roff DA, van der Zee E, Barber I. Individual experience and evolutionary history of predation affect expression of heritable variation in fish personality and morphology. Proc. Roy. Soc. Lond. B: Biol. 2009;276:1285–1293. doi: 10.1098/rspb.2008.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemanse NJ, Wright J, Kazem AJN, Thomas DK, Hickling R, Dawnay N. Behavioural syndromes differ predictably between 12 populations of three-spined stickleback. J. Anim. Ecol. 2007;76:1128–1138. doi: 10.1111/j.1365-2656.2007.01284.x. [DOI] [PubMed] [Google Scholar]
- Dzieweczynski TL, Forrette LM. Repeatability of decision-making behaviour in male threespine stickleback Gasterosteus aculeatus: effects of dummy vs. live stimuli. Curr. Zool. 2011;57:101–108. [Google Scholar]
- Dzieweczynski TL, Mack CL, Granquist RM. Lovers and fighters: male stickleback, Gasterosteus aculeatus differ in their responses to conflicting stimuli. Anim. Behav. 2009;78:399–406. [Google Scholar]
- Ellenberg U, Mattern T, Seddon PJ. Habituation potential of yellow-eyed penguins depends on sex, character and previous experience with humans. Anim. Behav. 2009;77:289–296. [Google Scholar]
- Foster SA. Evolution of reproductive behavior of theespine stickleback. In: Bell MA, Foster SA, editors. The evolutionary biology of the threespine stickleback. Oxford: Oxford University Press; 1994. pp. 381–395. [Google Scholar]
- Glowa JR, Hansen CT. Differences in response to an acoustic startle stimulus among 46 rat strains. Behav. Genet. 1994;24:79–84. doi: 10.1007/BF01067931. [DOI] [PubMed] [Google Scholar]
- Groves PM, Thompson RF. Habituation: a dual process theory. Psychol. Rev. 1970;77:419–450. doi: 10.1037/h0029810. [DOI] [PubMed] [Google Scholar]
- Hampton RE. A possible role of habituation in the sexual behavior of male mosquitofish. Anim. Behav. 1984;32:1262–1263. [Google Scholar]
- Harcourt JL, Ang TZ, Sweetman G, Johnstone RA, Manica A. Social feedback and the emergence of leaders and followers. Curr. Biol. 2009;19:248–252. doi: 10.1016/j.cub.2008.12.051. [DOI] [PubMed] [Google Scholar]
- Hinde RA. Animal behaviour: a synthesis of ethology and comparative psychology. 2nd edn. New York, NY: McGraw-Hill; 1970. [Google Scholar]
- Huntingford FA. The relationship between anti-predator behaviour and aggression among conspecifics in the three-spined stickleback. Anim. Behav. 1976;24:245–260. [Google Scholar]
- Huntingford FA, Chellappa S, Taylor AC, Strang RHC. Energy reserves and reproductive investment in male three-spined sticklebacks, Gasterosteus aculeatus . Ecol. Freshwater Fish. 2001;10:111–117. [Google Scholar]
- Jenkins JR, Rowland WJ. Stimulus-specific and response-specific habituation in courting stickleback: developmental and functional considerations. Behaviour. 2000;137:933–945. [Google Scholar]
- Kodric-Brown A, Brown JH. Truth in advertising — the kinds of traits favored by sexual selection. Am. Nat. 1984;124:309–323. [Google Scholar]
- LaRowe SD, Patrick CJ, Curtin JJ, Kline JP. Personality correlates of startle habituation. Biol. Psychol. 2006;72:257–264. doi: 10.1016/j.biopsycho.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Martin JGA, Reale D. Temperament, risk assessment and habituation to novelty in eastern chipmunks, Tamias striatus . Anim. Behav. 2008;75:309–318. [Google Scholar]
- Martin JGA, Nussey DH, Wilson AJ, Reale D. Measuring individual differences in reaction norms in field and experimental studies: a power analysis of random regression models. Methods Ecol. Evol. 2011;2:362–374. [Google Scholar]
- Mathot KJ, van den Hout PJ, Piersma T, Kempenaers B, Reale D, Dingemanse NJ. Disentangling the roles of frequency-vs. state-dependence in generating individual differences in behavioural plasticity. Ecol. Lett. 2011;14:1254–1262. doi: 10.1111/j.1461-0248.2011.01698.x. [DOI] [PubMed] [Google Scholar]
- Montiglio P-O, Garant D, Thomas D, Reale D. Individual variation in temporal activity patterns in open-field tests. Anim. Behav. 2010;80:905–912. [Google Scholar]
- O’Gorman JG. Individual differences in human physiological responses — review of theory, method and findings in a study of personality correlates in non-clinical populations. Biol. Psychol. 1977;5:257–318. doi: 10.1016/0301-0511(77)90017-5. [DOI] [PubMed] [Google Scholar]
- Peeke HVS. Habituation of conspecific aggression in 3-spined stickleback (Gasterosteus aculeatus) Behaviour. 1969;35:137–156. [Google Scholar]
- Peeke HVS. Stimulus- and motivation-specific sensitization and redirection of aggression in the three-spined stickleback (Gasterosteus aculeatus) J. Comp. Physiol. Psychol. 1982;96:816–822. [PubMed] [Google Scholar]
- Peeke HVS. Habituation, sensitization and redirection of aggression and feeding behavior in the 3-spined stickleback (Gasterosteus aculeatus) J. Comp. Psychol. 1983;97:43–51. [PubMed] [Google Scholar]
- Peeke HVS. Habituation of a predatory response in the stickleback (Gasterosteus aculeatus) Behaviour. 1995;132:1255–1266. [Google Scholar]
- Peeke HVS, Figler MH. Form and function of habituation and sensitization of male courtship in the three-spined stickleback (Gasterosteus aculeatus L.) Behaviour. 1997;134:1273–1287. [Google Scholar]
- Peeke HVS, Veno A. Stimulus specificity of habituated aggression in stickleback (Gasterosteus aculeatus) Behav. Biol. 1973;8:427–432. doi: 10.1016/s0091-6773(73)80083-5. [DOI] [PubMed] [Google Scholar]
- Peeke HVS, Veno A. Response independent habituation of territorial aggression in 3-spined stickleback (Gasterosteaus aculeatus) Z. Tierpsychol. 1976;40:53–58. doi: 10.1111/j.1439-0310.1976.tb00925.x. [DOI] [PubMed] [Google Scholar]
- Peeke HVS, Figler MH, Blankenship N. Retention and recovery of habituated territorial aggressive behavior in the 3-spined stickleback (Gasterosteus aculeatus) — roles of time and nest reconstruction. Behaviour. 1979;69:171–182. [Google Scholar]
- Pinheiro JC, Bates DM. Mixed-effects models in S and S-Plus. New York, NY: Springer- Verlag; 2000. [Google Scholar]
- Raderschall CA, Magrath RD, Hemmi JM. Habituation under natural conditions: model predators are distinguished by approach direction. J. Exp. Biol. 2011;214:4209–4216. doi: 10.1242/jeb.061614. [DOI] [PubMed] [Google Scholar]
- Rankin CH, Abrams T, Barry RJ, Bhatnagar S, Clayton DF, Colombo J, Coppola G, Geyer MA, Glanzman DL, Marsland S, McSweeney FK, Wilson DA, Wu C-F, Thompson RF. Habituation revisited: an updated and revised description of the behavioral characteristics of habituation. Neurobiol. Learn. Mem. 2009;92:135–138. doi: 10.1016/j.nlm.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Prieto I, Martin J, Fernandez-Juricic E. Habituation to low-risk predators improves body condition in lizards. Behav. Ecol. Sociobiol. 2010;64:1937–1945. [Google Scholar]
- Rodriguez-Prieto I, Martin J, Fernandez-Juricic E. Individual variation in behavioural plasticity: direct and indirect effects of boldness, exploration and sociability on habituation to predators in lizards. Proc. Roy. Soc. Lond. B: Biol. 2011;278:266–273. doi: 10.1098/rspb.2010.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland WJ. The effects of body size, aggression and nuptial coloration on competition for territories in male threespine sticklebacks. Anim. Behav. 1989;37:282–289. [Google Scholar]
- Rowland WJ. Habituation and development of response specificity to a sign stimulus: male preference for female courtship posture in stickleback. Anim. Behav. 2000;60:63–68. doi: 10.1006/anbe.2000.1462. [DOI] [PubMed] [Google Scholar]
- Santangelo N, Itzkowitz M, Richter M, Haley MP. Resource attractiveness of the male beaugregory damselfish and his decision to court or defend. Behav. Ecol. 2002;13:676–681. [Google Scholar]
- Sevenster P. A causal study of displacement activity (fanning in Gasterosteus aculeatus L.) Behaviour. 1961;S9:1–170. [Google Scholar]
- Sih A, Bell AM, Johnson JC. Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol. Evol. 2004;19:372–378. doi: 10.1016/j.tree.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Snijders TAB, Boster RJ. Multilevel analysis: an introduction to basic and advanced multilevel modeling. 2nd edn. London: Sage; 2012. [Google Scholar]
- Stamps JA, Briffa M, Biro PA. Unpredictable animals: individual differences in intraindividual variability (IIV) . Anim. Behav. 2012 [Google Scholar]
- Thorpe WH. Learning and instinct in animals. London: Methuen; 1956. [Google Scholar]
- van de Pol M. Quantifying individual variation in reaction norms: how study design affects the accuracy, precision and power of random regression models. Methods Ecol. Evol. 2012;3:268–280. [Google Scholar]
- van den Assem J. Territory in the three-spined stickleback. Behaviour. 1967;S16:1–164. [Google Scholar]
- Webster MM. Boldness is influenced by social context in threespine sticklebacks. Behaviour. 2007;144:351–371. [Google Scholar]
- Westneat DF, Hatch MI, Wetzel DP, Ensminger AL. Individual variation in parental care reaction norms: integration of personality and plasticity. Am. Nat. 2011;178:652–667. doi: 10.1086/662173. [DOI] [PubMed] [Google Scholar]
- Wilz KJ. Causal relationships between aggression and sexual and nest behaviors in three-spined stickleback. Anim. Behav. 1972;20:335–340. doi: 10.1016/s0003-3472(72)80055-1. [DOI] [PubMed] [Google Scholar]
- Wootton RJ. A functional biology of sticklebacks. Berkeley, CA: University of California Press; 1984. [Google Scholar]
- Zimmermann A, Stauffacher M, Langhans W, Wurbel H. Enrichmentdependent differences in novelty exploration in rats can be explained by habituation. Behav. Brain. Res. 2001;121:11–20. doi: 10.1016/s0166-4328(00)00377-6. [DOI] [PubMed] [Google Scholar]