Abstract
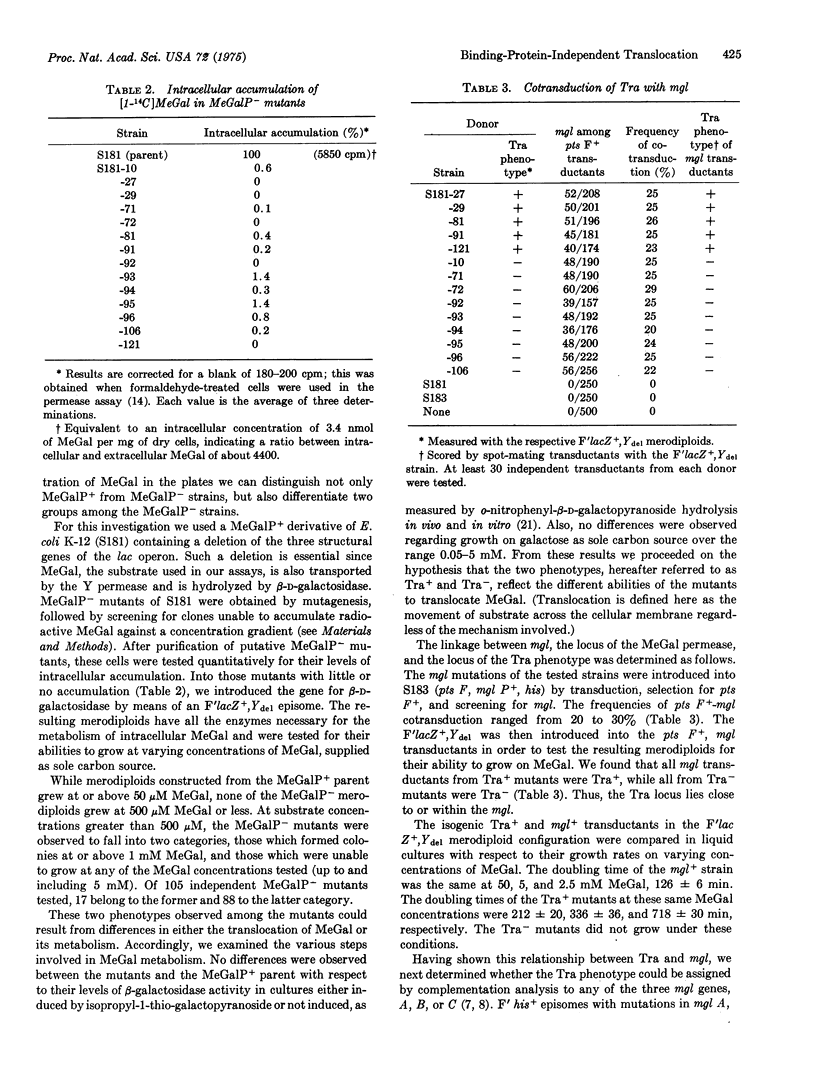
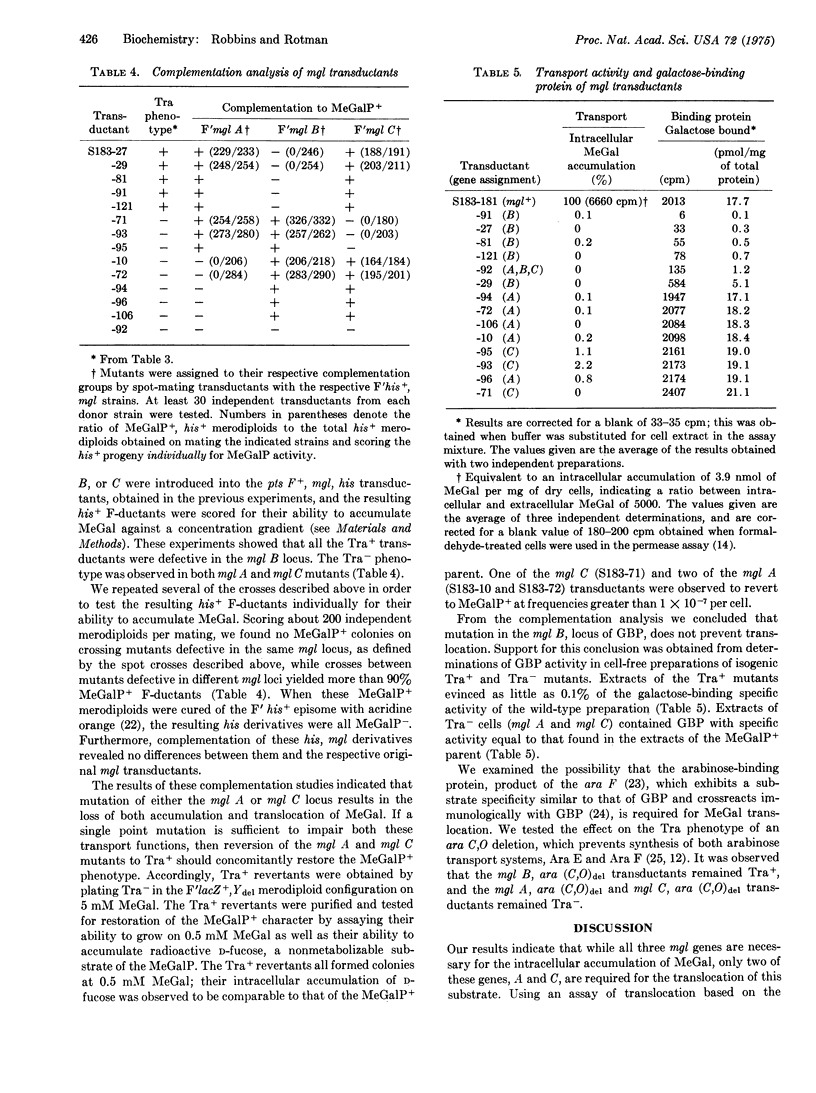
Three genes, mgl A, B, and C, are required for active transport of substrate by the methylgalactose permease of E. coli K12. We report here that only two of these genes are required for substrate translocation, as seen by the ability or inability of isogenic mgl mutants (referred to as Tra+ and Tra minus, respectively) to grow on methyl-beta-D-galactopyranoside, supplied as sole carbon source. Individual mutants of both the Tra+ and Tra minus classes exhibited no detectable intracellular accumulation of methyl-beta-D-galactopyranoside; thus, the Tra+ phenotype cannot be explained by the mutants' levels of residual active transport. The phosphotransferase (Pts), the beta-galactoside (LacY), and the arabinose (Ara E and Ara F) transport systems are not required for substrate translocation by Tra+ cells. The Tra+ phenotype was identified with mutants defective in the mgl B, locus of the galactose-binding protein, by genetic complementation; the Tra minus phenotype was observed with both mgl A and mgl C mutants. The conclusion that the galactose-binding protein is not required for substrate translocation was supported by direct assays of the mgl mutants' binding protein activity. Mutants capable of translocation all showed reduced galactose-binding protein activity; mutants incapable of translocation exhibited binding protein activity equal to that of the mgl+ parent.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anraku Y. Transport of sugars and amino acids in bacteria. I. Purification and specificity of the galactose- and leucine-binding proteins. J Biol Chem. 1968 Jun 10;243(11):3116–3122. [PubMed] [Google Scholar]
- Boos W. Bacterial transport. Annu Rev Biochem. 1974;43(0):123–146. doi: 10.1146/annurev.bi.43.070174.001011. [DOI] [PubMed] [Google Scholar]
- Boos W., Lengeler J., Hermann K. O., Unsöld H. J. The regulation of the beta-methylgalactoside transport system and of the galactose binding protein of Escherichia coli K12. Eur J Biochem. 1971 Apr 30;19(4):457–470. doi: 10.1111/j.1432-1033.1971.tb01336.x. [DOI] [PubMed] [Google Scholar]
- Boos W. Structurally defective galactose-binding protein isolated from a mutant negative in the -methylgalactoside transport system of Escherichia coli. J Biol Chem. 1972 Sep 10;247(17):5414–5424. [PubMed] [Google Scholar]
- Boos W. The galactose binding protein and its relationship to the beta-methylgalactoside permease from Escherichia coli. Eur J Biochem. 1969 Aug;10(1):66–73. doi: 10.1111/j.1432-1033.1969.tb00656.x. [DOI] [PubMed] [Google Scholar]
- Brown C. E., Hogg R. W. A second transport system for L-arabinose in Escherichia coli B-r controlled by the araC gene. J Bacteriol. 1972 Aug;111(2):606–613. doi: 10.1128/jb.111.2.606-613.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englesberg E., Irr J., Power J., Lee N. Positive control of enzyme synthesis by gene C in the L-arabinose system. J Bacteriol. 1965 Oct;90(4):946–957. doi: 10.1128/jb.90.4.946-957.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein W., Jewett S., Fox C. F. Isolation and mapping of phosphotransferase mutants in Escherichia coli. J Bacteriol. 1970 Nov;104(2):793–797. doi: 10.1128/jb.104.2.793-797.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenci T., Kornberg H. L. Role of fructose-1,6-diphosphatase in fructose utilization by Escherichia coli. FEBS Lett. 1971 May 20;14(5):360–363. doi: 10.1016/0014-5793(71)80301-0. [DOI] [PubMed] [Google Scholar]
- Ganesan A. K., Rotman B. Transport systems for galactose and galactosides in Escherichia coli. I. Genetic determination and regulation of the methyl-galactoside permease. J Mol Biol. 1966 Mar;16(1):42–50. doi: 10.1016/s0022-2836(66)80261-9. [DOI] [PubMed] [Google Scholar]
- Hazelbauer G. L., Adler J. Role of the galactose binding protein in chemotaxis of Escherichia coli toward galactose. Nat New Biol. 1971 Mar 24;230(12):101–104. doi: 10.1038/newbio230101a0. [DOI] [PubMed] [Google Scholar]
- Hirota Y. THE EFFECT OF ACRIDINE DYES ON MATING TYPE FACTORS IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1960 Jan;46(1):57–64. doi: 10.1073/pnas.46.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg R. W., Englesberg E. L-arabinose binding protein from Escherichia coli B-r. J Bacteriol. 1969 Oct;100(1):423–432. doi: 10.1128/jb.100.1.423-432.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Malamy M. H. Frameshift mutations in the lactose operon of E. coli. Cold Spring Harb Symp Quant Biol. 1966;31:189–201. doi: 10.1101/sqb.1966.031.01.027. [DOI] [PubMed] [Google Scholar]
- Ordal G. W., Adler J. Isolation and complementation of mutants in galactose taxis and transport. J Bacteriol. 1974 Feb;117(2):509–516. doi: 10.1128/jb.117.2.509-516.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordal G. W., Adler J. Properties of mutants in galactose taxis and transport. J Bacteriol. 1974 Feb;117(2):517–526. doi: 10.1128/jb.117.2.517-526.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxender D. L. Membrane transport. Annu Rev Biochem. 1972;41(10):777–814. doi: 10.1146/annurev.bi.41.070172.004021. [DOI] [PubMed] [Google Scholar]
- Parsons R. G., Hogg R. W. A comparison of the L-arabinose- and D-galactose-binding proteins of Escherichia coli B-r. J Biol Chem. 1974 Jun 10;249(11):3608–3614. [PubMed] [Google Scholar]
- Robbins A. R., Rotman B. Inhibition of methylgalactoside transport in Escherichia coli upon the cessation of unsaturated fatty acid biosynthesis. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2125–2129. doi: 10.1073/pnas.69.8.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotman B., Ellis J. H., Jr Antibody-mediated modification of the binding properties of a protein related to galactose transport. J Bacteriol. 1972 Sep;111(3):791–796. doi: 10.1128/jb.111.3.791-796.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotman B., Ganesan A. K., Guzman R. Transport systems for galactose and galactosides in Escherichia coli. II. Substrate and inducer specificities. J Mol Biol. 1968 Sep 14;36(2):247–260. doi: 10.1016/0022-2836(68)90379-3. [DOI] [PubMed] [Google Scholar]
- SWANSTROM M., ADAMS M. H. Agar layer method for production of high titer phage stocks. Proc Soc Exp Biol Med. 1951 Nov;78(2):372–375. doi: 10.3181/00379727-78-19076. [DOI] [PubMed] [Google Scholar]
- Wilson T. H., Kashket E. R. Isolation and properties of thiogalactoside transacetylase-negative mutants of Escherichia coli. Biochim Biophys Acta. 1969 Apr;173(3):501–508. doi: 10.1016/0005-2736(69)90014-5. [DOI] [PubMed] [Google Scholar]


