Abstract
Reduced upper airway muscle activity during sleep is a key contributor to obstructive sleep apnoea (OSA) pathogenesis. Hypoglossal nerve stimulation (HGNS) activates upper airway dilator muscles, including the genioglossus, and has the potential to reduce OSA severity. The objective of this study was to examine the safety, feasibility, and efficacy of a novel HGNS system (HGNS®, Apnex Medical, Inc., St. Paul, MN) in treating OSA at 12 months following implantation. Thirty-one subjects (35% female, age 52·4±9·4 years) with moderate to severe OSA and unable to tolerate positive airway pressure underwent surgical implantation and activation of the HGNS system in a prospective single-arm interventional trial. Primary outcomes were changes in OSA severity (apnoea-hypopnoea index, AHI, from in-laboratory polysomnogram) and sleep-related quality of life (Functional Outcomes of Sleep Questionnaire, FOSQ). HGNS was used on 86±16% of nights for 5·4±1·4 hours per night. There was a significant improvement (p < 0·001) from baseline to 12 months in AHI (45.4±17·5 to 25·3±20·6 events/h) and FOSQ score (14·2±2·0 to 17·0±2·4) as well as other polysomnogram and symptom measures. Outcomes were stable compared to 6 months following implantation. Three serious device-related adverse events occurred: an infection requiring device removal and two stimulation lead cuff dislodgements requiring replacement. There were no significant adverse events with onset later than 6 months following implantation. HGNS demonstrated favourable safety, feasibility, and efficacy.
INTRODUCTION
Obstructive sleep apnoea (OSA) is characterised by repeated episodes of upper airway obstruction during sleep. This disease is associated with substantial cardiovascular morbidity and mortality, endocrine disturbances, daytime somnolence, decreased quality of life, performance deficits, and motor vehicle crashes. OSA is common, affecting over 100 million individuals worldwide, with increasing prevalence due to obesity and ageing (Young et al., 1993, World Health Organization). Positive airway pressure is the first-line treatment because it eliminates disordered breathing events (Gay et al., 2006); however, at least 30–40% of patients have low adherence (Kribbs et al., 1993, Weaver and Grunstein, 2008). Reported outcomes for treatment alternatives are often based on short-term (6 months or less) assessments, leaving open the important question of longer-term outcomes.
A number of factors contribute to OSA pathogenesis, including decreased tone during sleep in the upper airway dilator muscles, especially the genioglossus (White, 2005). This notion has led to investigations of electrical stimulation of genioglossus using intramuscular or transcutaneous electrodes. While these studies demonstrated improvements in airway patency and OSA severity (Kezirian et al.), muscle stimulation disrupted sleep because of sensory phenomena. As a result direct electrical stimulation of the motor nerve innervating the genioglossus muscle, the hypoglossal nerve (HGN), has been explored as an alternative (Goding et al., 1998), (Eisele et al., 1997, Schwartz et al., 2001). Schwartz et al. showed the benefit of this approach, demonstrating that chronic HGN stimulation in OSA patients decreased the frequency of obstructed breathing events without arousals from sleep (Schwartz et al., 2001). Despite this early promise, a number of device technical failures (Schwartz et al., 2001), primarily electrode breakage and sensor failure, prevented further development.
These technical issues have been addressed in a new generation implantable HGN stimulation therapy system (HGNS®, Apnex Medical, Inc., St. Paul, MN, USA) that has recently been developed to explore further this OSA treatment modality. Early clinical trials in Australia and the United States have demonstrated improvements in airway patency and airflow without causing arousals from sleep (Schwartz et al., 2012) Outcomes at 6 months in an Australian cohort following implantation suggested favourable safety, compliance, and effectiveness.(Eastwood et al., 2011) The present study extends these observations to report the safety, compliance, and efficacy of the HGNS system at 12 months following implantation in the combined Australian and American cohorts. The purpose was to determine whether or not the therapy was associated with sustained benefit, which would be required for this therapeutic approach to be viable.
METHODS
STUDY DESIGN AND PARTICIPANTS
Study methods have been described previously (Eastwood et al., 2011). A single arm, open label study was undertaken at four Australian and four United States clinical trial sites. Inclusion criteria included: moderate to severe OSA; documented failure of positive airway pressure; age 21–70 years; and body mass index (BMI) ≤ 40 kg/m2 (Australia) or ≤ 37 kg/m2 (United States). On the baseline sleep study (polysomnogram), subjects were required to have an AHI of 20–100 events/hour, with at least 15 events/hour occurring in non-REM sleep; after an initial enrollment period (n=5), all subjects were required to have a predominance of hypopneas (≥80%) of the sum of apnea and hypopnea events. Exclusion criteria included: prior upper airway surgery; markedly enlarged tonsils; uncontrolled nasal obstruction; severe retrognathia; > 5% central or mixed apnoeic events; incompletely treated sleep disorders other than OSA; and major disorder of the pulmonary, cardiac, renal, or nervous systems.
HGNS SYSTEM
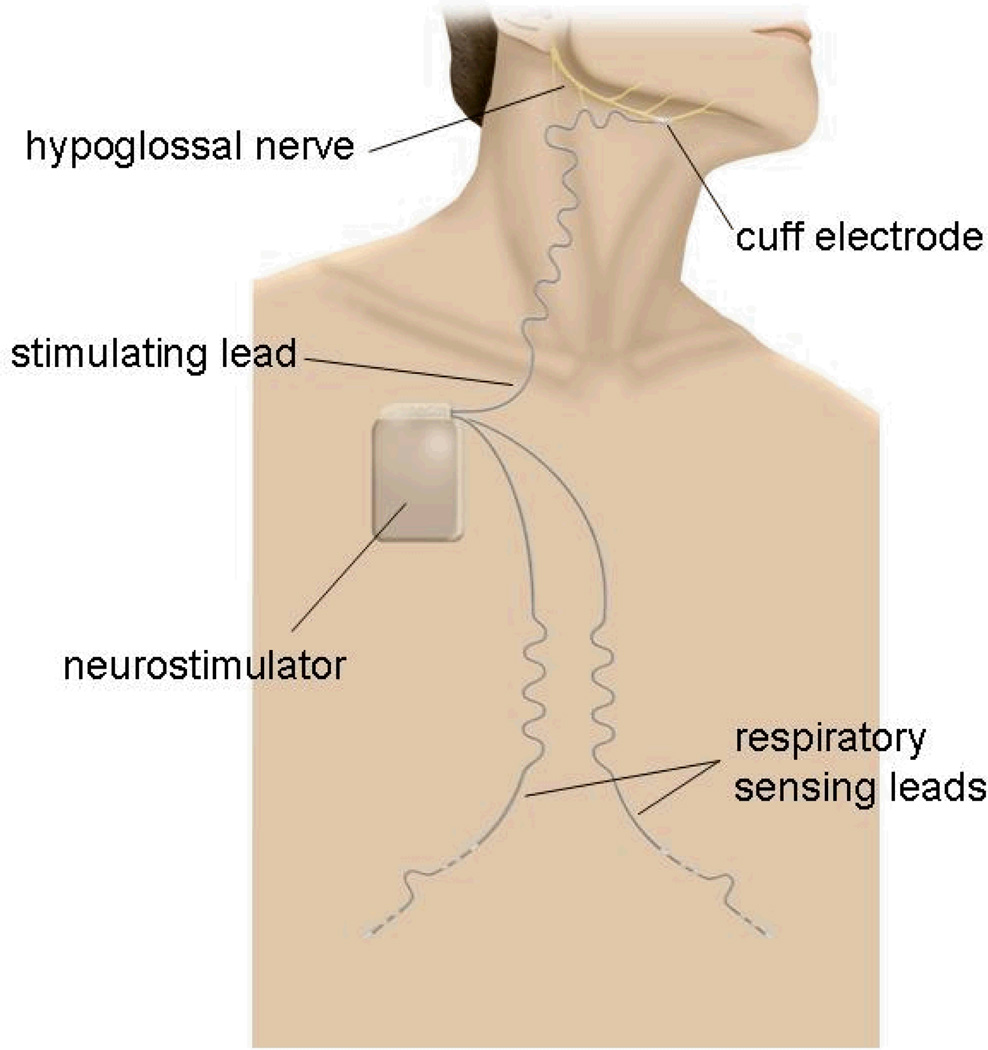
The HGNS system consisted of an implantable neurostimulator connected to a unilateral (generally right-sided) stimulation lead and two respiration sensing leads (Figure 1). The respiration sensing leads were tunneled and placed subcutaneously to monitor respiration from changes in thoracic bioimpedance. A software algorithm controlled the delivery of HGN electrical stimulation so that stimulation began just prior to and continued throughout inspiration but was switched off during expiration. The flexible stimulation lead cuff was designed to distribute the stimulation field uniformly and to limit contact pressure in order to minimise the likelihood of nerve injury. Individualised therapy settings were programmed into the neurostimulator, but participants could control limited aspects (start, stop, and pause) with a handheld controller.
Figure 1.
The implanted components of the hypoglossal nerve stimulating system include a neurostimulator that delivers safe levels of electrical stimulation to one hypoglossal nerve via a stimulation lead having a distal cuff electrode. Respiration sensing leads detect inspiration using bioimpedance, so that the system delivers stimulation synchronous with inspiration.
The HGNS system was implanted under general anaesthesia. Briefly, the cuff of the stimulation lead was placed on the main trunk of the HGN distal to branches innervating the tongue’s retractor muscles to ensure predominant activation of the protrusors. Due to variability in hypoglossal nerve anatomy, final cuff placement was determined by intraoperative response of the upper airway to stimulation, visualized using fluoroscopy. The stimulation lead body was then tunneled deep to the platysma muscle in the neck to the neurostimulator, which was implanted in an ipsilateral infraclavicular subcutaneous pocket. From the pocket, two subcutaneous respiratory sensing leads were placed by tunneling subcutaneously toward the midline and then along each costal margin.
POLYSOMNOGRAMS (SLEEP STUDIES)
All subjects underwent a full-night, in-laboratory polysomnogram at the respective centre prior to implantation (Baseline Night). Data were scored by a central core laboratory (Clinilabs, Inc., New York, NY). The 1999 American Academy of Sleep Medicine apnoea and hypopnoea definitions were used (1999), except that a 4% oxygen desaturation was required for hypopnoeas (i.e. modified Chicago criteria). Sleep staging was consistent with the 2007 American Academy of Sleep Medicine conventions (Iber et al., 2007).
After allowing approximately 1 month following implantation for healing, each subject underwent a titration polysomnogram, during which stimulation settings (e.g., pulse width, frequency, current) were adjusted to levels that consistently abolished inspiratory airflow limitation. Therapy was then commenced nightly at home. Downloadable utilization data were stored in the devices. In-laboratory sleep studies were performed at 3, 6 and 12 months post implantation. Information regarding at-home stimulation utilisation was stored on the device and downloaded.
At baseline and at 6 and 12 months post implantation, subjects completed five questionnaires: the Functional Outcomes of Sleep Questionnaire (FOSQ), assessing the impact of excessive sleepiness on activities of daily living (Weaver et al., 1997); the Epworth Sleepiness Scale (ESS), measuring subjective daytime sleepiness (Johns, 1991); the Calgary Sleep Apnea Quality of Life Index (SAQLI), measuring within-patient change in sleep apnoea-related quality of life in response to therapeutic intervention (Flemons and Reimer, 2002); the Pittsburgh Sleep Quality Index (PSQI), assessing sleep quality and sleep disturbance retrospectively over a one month period (Buysse et al., 1989); and the Beck Depression Inventory (BDI), rating the level of depressive symptoms (Lasa et al., 2000).
EFFECTIVENESS, COMPLIANCE, AND SAFETY ENDPOINTS
The primary effectiveness endpoints were the mean change in AHI and FOSQ total score. Secondary effectiveness endpoints included the mean change for other polysomnographic and symptom measures. The usage endpoints were the proportion of nights with use and nightly hours of use. The primary safety endpoint was the rate of freedom from serious adverse events at implant and at 6 and 12 months post implantation. All adverse events were reported. Adverse events were deemed serious if they resulted in: patient death; life threatening illness or injury; permanent impairment of body structure or function or medical or surgical intervention to prevent this; or inpatient hospitalization or prolongation of existing hospitalization.
STATISTICAL ANALYSIS
There were no human clinical data to perform formal sample size calculations a priori.
Analyses were by an intention to treat analysis, such that all subjects with a post-implant efficacy study were included in the analysis. For subjects without data at the 6 (n=2) and/or 12 (n=3, including 1 due to explant) month time points, the most recent available data were carried forward for statistical analysis. Repeated measures regression models were used to assess statistical differences in outcomes between visits. In cases where the normality assumption was violated (p < 0·05 from a Shapiro-Wilk test for normality of the studentised residuals), nonlinear transformations (including logarithmic) were explored to produce models with improved fits. A linear model was used to compare the 12 month AHI values between those with a BMI ≤ and > 35 kg/m2. P-values were adjusted by the Sidak-Holm method to control for multiple comparisons. Statistical analyses were conducted in SAS version 9·2 (SAS Institute, Cary, NC). Data are presented as mean±SD and/or median and percentiles, where appropriate. P-values < 0·05 were considered statistically significant.
Adverse event profiles for the intraoperative period, perioperative period, and at 6 and 12 months post implantation were calculated to estimate the rate of freedom from incident system- (device or therapy) or procedure-related serious adverse events, using the Kaplan-Meier method of time-to-event analysis.
ETHICAL CONSIDERATIONS
The study protocol was reviewed and approved by the Therapeutic Goods Administration and the Ethics Committees at each participating Australian site. It was also reviewed and approved by the United States Food and Drug Administration and the institutional review board at each participating United States site. Adverse events were adjudicated by an independent Clinical Events Committee. An independent Data Safety Monitoring Committee provided ethical and scientific review of the study. Subjects provided written informed consent prior to their involvement in any study procedure. The trial was registered at ClinicalTrials.gov as NCT01186926 (Australian study) and NCT01211444 (US study).
ROLE OF THE FUNDING SOURCE
The study was funded by Apnex Medical, Inc. (Minneapolis, MN, USA). The academic authors are responsible for study design; the collection, analysis, and interpretation of data; writing of the manuscript; and the decision to submit the paper for publication. Apnex Medical provided assistance with study design and data analysis and reviewed the manuscript prior to submission; however, the academic authors are fully responsible for its contents.
RESULTS
Thirty-two subjects were implanted, but one requested explant (performed without incident) prior to activation and was excluded from further analyses. Of the 31 subjects with device activation, 11 (35%) were female, and age was 52.4±9.4 years. Body mass index was 32.4±3.6 kg/m2. Most (28/31, 90%) were non-Hispanic Caucasian, and one subject each had race/ethnicity of Hispanic Caucasian, Black/African American, and multiracial. At baseline, all subjects had moderate to severe OSA, with reduced deep sleep and REM sleep (Table 1) and related symptoms (Table 2).
Table 1.
Polysomnogram Measures
| Parameter | Baseline | 6 Months | 12 Months*** |
|---|---|---|---|
| AHI (events/h) | 45.4 (17.5) | 20.8 (17.6)* | 25.3 (20.6)* |
| Apnea Index (events/h) | 4.6 (6.3) | 1.5 (2.2)* | 3.2 (5.9)** |
| Hypopnea Index (events/h) | 40.8 (15.3) | 19.4 (16.6)* | 22.1 (17.9)* |
| Arousal Index (events/h) | 44.3 (17.7) | 24.4 (13.2)* | 27.5 (13.4)* |
| Respiratory Arousal Index | 31.4 (18.4) | 11.9 (11.9)* | 14.4 (12.4)* |
| ODI4% Index (events/h) | 20.9 (17.3) | 10.7 (17.1)* | 15.7 (19.6)* |
| Total Sleep Time (min) | 346.4 (71.6) | 355.2 (52.9) | 362.7 (55.9) |
| Sleep Efficiency (%) | 77.2 (12.6) | 82.8 (10.9)** | 82.6 (10.2)** |
| % N1 | 29.3 (11.2) | 20.5 (10.2)* | 21.8 (10.3)* |
| % N2 | 48.8 (7.9) | 52.3 (10.2) | 50.6 (8.4) |
| % N3 | 9.3 (7.7) | 10.9 (8.9) | 11.9 (8.9) |
| % REM | 12.6 (6.5) | 16.1 (5.7)** | 16.4 (5.0)** |
All values are presented as mean (SD). P-values are all for comparison to Baseline, using data only for subjects with data at each respective time point.
P < 0.001
P <0.05 but > 0.001
P values for all comparisons of 12 month vs. 6 month results are >0.10, except for ODI4% Index (p=0.09).
Table 2.
Symptoms and Quality of Life Measures
| Scale | Baseline | 6 month | 12 month*** |
|---|---|---|---|
| FOSQ | 14.2 (2.0) | 16.8 (2.4)* | 17.0 (2.4)* |
| ESS | 12.1 (4.6) | 8.3 (3.6)* | 7.9 (3.8)* |
| SAQLI | 3.1 (1.1) | 4.8 (1.4)* | 4.9 (1.4)* |
| PSQI | 9.9 (3.2) | 8.3 (4.3) | 7.8 (4.3)** |
| BDI | 15.7 (9.0) | 8.5 (7.8)* | 9.1 (8.2)* |
FOSQ, Functional Outcomes of Sleep Questionnaire; ESS, Epworth Sleepiness Scale; SAQLI, Sleep Apnea Quality of Life Index; PSQI, Pittsburgh Sleep Quality Index; BDI, Beck Depression Index.
All values are presented as mean (SD). P-values are all for comparison to Baseline, using data only for subjects with data at each respective time point.
P < 0.001
P <0.05 but > 0.001
P values for all comparisons of 12 month vs. 6 month results are >0.60.
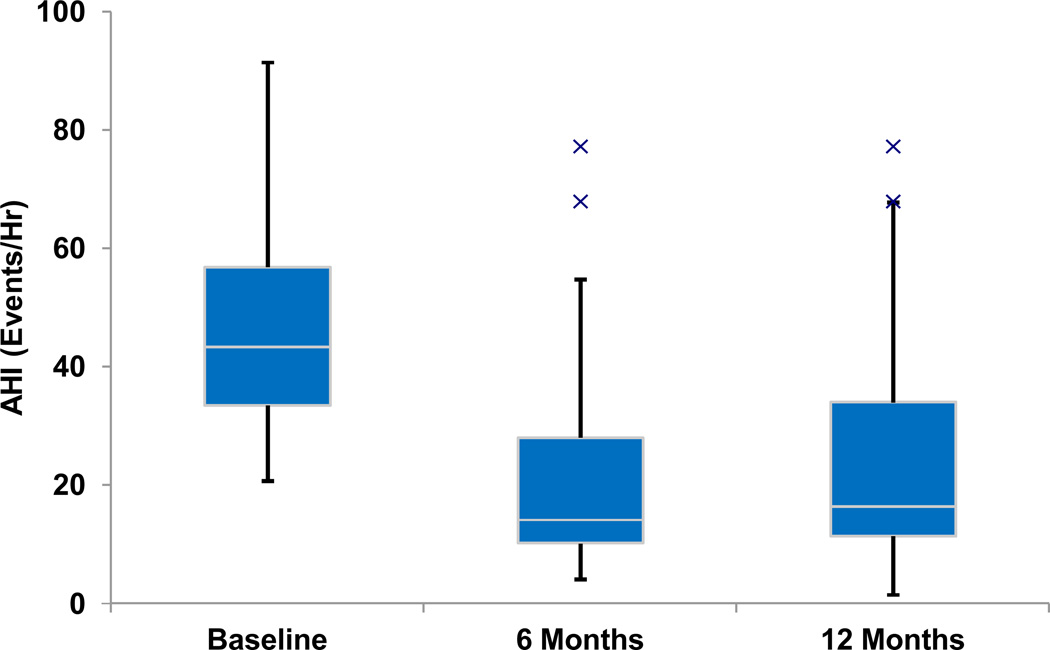
AHI decreased from 45·4±17·5 to 25·3 ± 20·6 events per hour (p < 0.001), and was stable at 12 months relative to 6 months post implant (p = 0·56) (Table 1, Figure 2). There were similar findings between these assessments for other measures of disordered breathing events, arousal index, and sleep architecture (increased REM sleep). Seventeen (55%) participants achieved a response to treatment, using the most common definition for OSA surgical treatment success: an AHI decrease of ≥50% to < 20 events/hour (Sher et al., 1996). Fifteen (48%) had a ≥ 50% AHI decrease to < 15 events hour. Two (6%) had a ≥ 50% AHI decrease to 20–25 events/hour.
Figure 2.
Box and whisker plot of the apnoea-hypopnoea index at baseline and at 6 and 12 months post implantation. The median values are noted by horizontal white lines, and the boxes represent the intraquartile range. The whiskers represent the 1.5x the intraquartile range (or the minimum or maximum value if < 1.5x the intraquartile range). The Xs represent outlier values.
All symptom and quality of life measures improved at 12 months following implantation, with no significant change from outcomes at 6 months (Table 2).
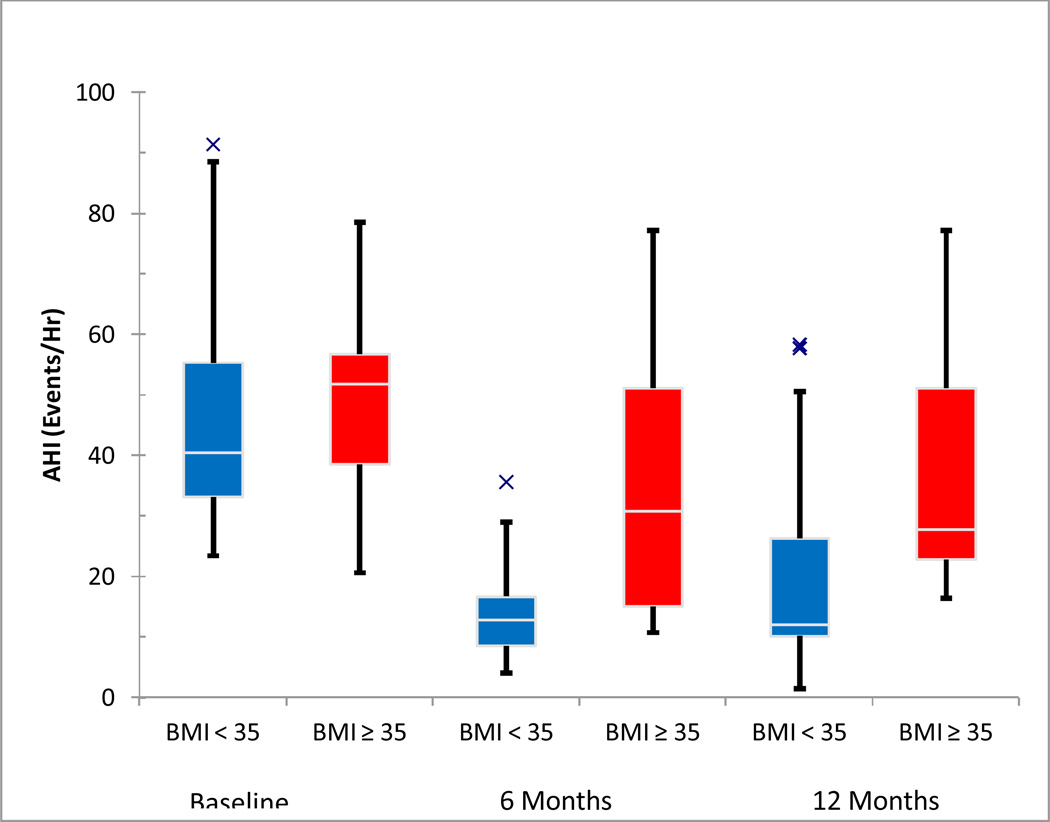
Subjects with BMI ≤35 (68%, 21/31) demonstrated significantly greater AHI reduction and improvement in symptoms at 12 months that was not seen in the subgroup with BMI > 35 kg/m2 (Figure 3). For the subjects with BMI ≤35, AHI decreased from 43·9±17·4 to 18·7 ± 17·0 events per hour (p < 0.001); fourteen (67%) had a 50% AHI decrease to < 15 events hour. There were no statistically significant changes in the group with BMI > 35, although the study was not adequately powered to rule out a change in AHI.
Figure 3.
Box and whisker plot of the apnea-hypopnea index at baseline and at 3, 6, and 12 months post implantation, shown separately for BMI ≤ 35 kg/m2 and BMI > 35 kg/m2. The median values are noted by horizontal white lines, and the boxes represent the intraquartile range. The whiskers represent the 1.5x the intraquartile range (or the minimum or maximum value if < 1.5x the intraquartile range). The Xs represent outlier values.
Following the month allowed postoperatively for healing, subject usage data were available for 266±81 (median 272) nights (range 53–377), excluding days for which therapy was known to be discontinued, often due to lack of objective and/or subjective efficacy. Therapy was used a mean of 86±16% of nights (range 42–100%) for 5·4±1·4 hours per night (range 2·7–8·4 hours).
There were no deaths in the study and no unanticipated adverse device effects. Four devices were explanted (1 with request prior to activation, 2 due to lack of sufficient objective and subjective effectiveness, and 1 due to device infection). Two participants had dislodgement of the stimulation lead cuff within 2 weeks of implantation (without HGN damage) and had replacement surgery accomplished without sequelae. One subject was readmitted to the hospital for psychological disturbance related to a combination of self-discontinuation of anti-depressant medications and prescription of opioids for post-operative pain control (although almost all patients used narcotics in the recovery period, the use of narcotics in this patient was felt to contribute to psychological disturbance). At least 1 adverse event related to the implantation procedure or therapy occurred in 71% (22/31) and 32% (10/31), respectively, but only 3/31 (10%) experienced serious adverse events related specifically to therapy. The most common procedure-related events were numbness/pain at the incision sites (35%, 11/31), and the most common therapy-related events were tongue abrasions (55%, 17/31) caused by movement of the tongue over mandibular dentition. These abrasions were of short duration and self-limited, and they were successfully treated with plastic dental guards. Most adverse events resolved completely, and the rate of freedom from system (device or therapy) or procedure-related adverse events at 12 months was 71% (22/31 subjects). The adverse events persisting at 12 months were incisional numbness/pain (8/31 subjects, 26%) and intermittent tongue soreness (3/31, 10%). Only 1 adverse event had onset later than 6 months following implantation: incisional numbness that resolved spontaneously after 2 days.
DISCUSSION
The HGNS system is a safe, feasible, and effective treatment for individuals with moderate to severe OSA who are unable to use positive airway pressure therapy. At 12 months following implantation, there were substantial improvements in objective measures of OSA severity and sleep disturbance as well as subjective metrics of daytime functioning, demonstrating that prior preliminary results (Eastwood et al., 2011) were robust over time in a larger sample. Furthermore, these improvements in symptom and quality of life measures were not only statistically significant but also clinically meaningful, based on previous studies using these instruments. The subjective measures mirror the objective improvements in sleep architecture seen on polysomnography of improved sleep architecture. Subjects with BMI > 35 kg/m2 appeared to respond less favourably, perhaps because of fat deposition within tissues surrounding the upper airway (Nashi et al., 2007, Schwab et al., 2003). These findings are broadly consistent with previous studies of this and earlier technologies (Eisele et al., 1997, Schwartz et al., 2001, Schwartz et al., 2012, Goding et al., 2012, Van de Heyning et al., 2012).
Therapy usage was high, comparing favorably with positive airway pressure adherence (Kribbs et al., 1993). The HGNS system was safe, with few serious adverse events, resolution of most adverse events over time, and only 1 minor adverse event with onset later than 6 months after therapy initiation.
The feasibility of chronic HGNS as a potential OSA therapy has been described previously by Schwartz et al. (Schwartz et al., 2001). The system used in their study was safe but had technical problems (Eisele et al., 2003, Goding et al., 1998, Schwartz et al., 2001). Numerous design differences were incorporated into the present HGNS device to overcome these problems. Principal among these were the development of a cuff electrode designed to surround the HGN branch safely and securely and the development of a bioimpedance-based respiratory sensing system.
Outcome evaluation in most surgical trials is limited to 6 months following surgery. Longer-term assessments are essential and clinically meaningful, and the stability of the results is clinically meaningful. Two studies have reported 12 month outcomes in hypoglossal nerve stimulation, both in populations with lower body mass index. Mwenge et al. reported similar changes in AHI and subjective outcomes in a smaller, single-center trial utilizing a different technology (Mwenge et al. 2013). At a recent scientific meeting (Sleep 2013), Strollo et al. recently presented similar AHI changes in a multi-center, pivotal trial of yet another hypoglossal nerve stimulation technology, and full presentation of those results in publication is forthcoming. The reproducibility of these findings suggests that hypoglossal nerve stimulation may be a viable treatment option in OSA, but because hypoglossal nerve stimulation does not alleviate OSA in all subjects, there is a potential for improvement in treatment selection or additional benefit from adjunctive interventions.
Acknowledgements
EJK was supported by a career development award from the National Center for Research Resources (NCRR) of the National Institutes of Health and a Triological Society Research Career Development Award of the American Laryngological, Rhinological, and Otological Society. JW is supported by a NHMRC Practitioner Fellowship No. 632910. ARS is supported by the Heart, Lung and Blood Institute of the National Institutes of Health under grant HL50381. PRE is supported by a National Health & Medical Research Council (Australia) Senior Research Fellowship No. 513704. The authors acknowledge the assistance of Mr. Chris Mullin from The Integra Group with the statistical analyses. The authors note with sadness the untimely death of their collaborator Dr. Sam Robinson and dedicate this manuscript to him.
Footnotes
Conflicts of interest
EJK: Apnex Medical (Medical Advisory Board, consultant); ReVENT Medical (Medical Advisory Board); ArthroCare (consultant); Medtronic (consultant); Pavad Medical (consultant). GSG: Apnex Medical (consultant). AM: has consulting/research income from Philips, Apnex, Apnicure, Pfizer, SHC, and SGS but has relinquished all outside personal income since May 2012. GZ: grants/research support (Abbott, Actelion, Ancile, Apnex, Arena, Astra-Zeneca, Aventis, Banyu, Biomarin, BMS, Catalyst, Cephalon Inc., CHDI, Elan, Epix, Eisal, Elminda, Evotec, Forest, Galderma, GlaxoSmithKline, Gilead, H. Lundbeck A/s, King, Merck and Co., National Institutes of Health, Neurim, Neurocrine Biosciences, Naurex, Neurogen, Novo Nordisk, Organon, Orphan Medical, Otsuka, Pfizer, Predix, Respironics, Saladax, Sanofi-Aventis, Sanofi-Synthelabo, Schering-Plough, Sepracor, Shire, Somaxon, Takeda Pharmaceuticals North America, Targacept, Teva, Thymon, Transcept, UCB Pharma, Ultragenyx, Predix, Venda, Wyeth-Ayerst Research; consultant: Acorda, Actelion, Alexza, Arena, Aventis, Biovail, Boehringer-Ingelheim, Cephalon, Elan, Eli Lilly, Evotec, Forest, GlaxoSmithKline, Jazz, King Pharmaceuticals, Ligand, McNeil, Merck, Neurocrine Biosciences, Organon, Pfizer, Purdue, Renovis, Sanofi-Aventis, Select Comfort, Sepracor, Shire, Somnus, Takeda Pharmaceuticals, Vela, Wyeth; honoraria: Neurocrine Biosciences, King Pharmaceuticals, McNeil, Sanofi-Aventis, Sanofi-Synthelabo, Sepracor, Takeda Pha rmaceuticals, Vela Pharmaceuticals, Wyeth-Ayerst Research; ownership, directorship: Clinilabs Inc., Clinilabs IPA, Clinilabs Physician Services. PLS: Apnex Medical (Medical Advisory Board). ARS: Apnex Medical (Scientific Advisory Board). TH: Apnex Medical (Medical Advisory Board, compensated surgical proctor). SYP: Apnex Medical (compensated surgical proctor). CEP: Apnex Medical (consultant). CI: Apnex Medical (salary support). PRE: Apnex Medical (Medical Advisory Board, consultant). DRH: Apnex Medical (Medical Advisory Board). MB: Fisher & Paykel (attendance at Clinical Research Forum), Bird Pty Ltd (salary support for research assistant for investigator driven research project), ResMed (provision of equipment for investigator driven research project),sponsored clinical trials with no direct monies received (Actelion, Boehringer-Ingelheim, Apnex Medical, GlaxoSmithKline, Novartis, Hunter Immunology, Pearl Therapeutics, Sanofi-Aventis).
Author Contributions: EJK: study design, literature search, data analysis and interpretation, writing, final approval. GSG: study design, data collection, manuscript review. AM: study design, data synthesis, review of raw data, data interpretation, study site coordination, and manuscript review. FOD: data collection and interpretation, manuscript review. GZ: data collection. JRW: study design, data collection and interpretation, manuscript review. PGC: data collection, manuscript review. PLS: developed concept for neural stimulation, monitored selection of study subjects, reviewed raw data. ARS: study design, data monitoring and analysis, and formulation of study findings and conclusions. JHW: data collection, analysis, and interpretation; manuscript review. KJM: data collection, analysis, and interpretation; manuscript review. TH: study design and data collection. SYP: data collection. DMC: subject recruitment, data acquisition, data analysis, and manuscript review and approval. MCC: data collection, manuscript review. CEP: data collection, manuscript review. CI: data collection, data interpretation. PRE: study design, data collection, data analysis and interpretation, manuscript writing and review. DRH: study design, data collection, data interpretation, manuscript writing. MB: data collection, data analysis, manuscript writing.
REFERENCES
- Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research: the Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–689. [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Eastwood PR, Barnes M, Walsh JH, et al. Treating obstructive sleep apnea with hypoglossal nerve stimulation. Sleep. 2011;34:1479–1486. doi: 10.5665/sleep.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisele DW, Schwartz AR, Smith PL. Tongue neuromuscular and direct hypoglossal nerve stimulation for obstructive sleep apnea. Otolaryngol Clin North Am. 2003;36:501–510. doi: 10.1016/s0030-6665(02)00178-0. [DOI] [PubMed] [Google Scholar]
- Eisele DW, Smith PL, Alam DS, Schwartz AR. Direct hypoglossal nerve stimulation in obstructive sleep apnea. Arch Otolaryngol Head Neck Surg. 1997;123:57–61. doi: 10.1001/archotol.1997.01900010067009. [DOI] [PubMed] [Google Scholar]
- Flemons WW, Reimer MA. Measurement properties of the Calgary sleep apnea quality of life index. Am J Respir Crit Care Med. 2002;165:159–164. doi: 10.1164/ajrccm.165.2.2010008. [DOI] [PubMed] [Google Scholar]
- Gay P, Weaver T, Loube D, Iber C. Evaluation of positive airway pressure treatment for sleep related breathing disorders in adults. Sleep. 2006;29:381–401. doi: 10.1093/sleep/29.3.381. [DOI] [PubMed] [Google Scholar]
- Goding GS, Jr, Eisele DW, Testerman R, Smith PL, Roertgen K, Schwartz AR. Relief of upper airway obstruction with hypoglossal nerve stimulation in the canine. Laryngoscope. 1998;108:162–169. doi: 10.1097/00005537-199802000-00003. [DOI] [PubMed] [Google Scholar]
- Goding GS, Jr, Tesfayesus W, Kezirian EJ. Hypoglossal nerve stimulation and airway changes under fluoroscopy. Otolaryngol Head Neck Surg. 2012;146:1017–1022. doi: 10.1177/0194599812436472. [DOI] [PubMed] [Google Scholar]
- Iber C, Ancoli-Israel S, Chesson A, Quan S. The AASM manual for the scoring of sleep and associated events: rules, terminology, and technical specifications. 1st edition. Westchester, IL: American Academy of Sleep Medicine; 2007. for the American Academy of Sleep Medicine. [Google Scholar]
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- Kezirian EJ, Boudewyns A, Eisele DW, et al. Electrical stimulation of the hypoglossal nerve in the treatment of obstructive sleep apnea. Sleep Med Rev. 2010;14:299–305. doi: 10.1016/j.smrv.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Kribbs NB, Pack AI, Kline LR, et al. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;147:887–895. doi: 10.1164/ajrccm/147.4.887. [DOI] [PubMed] [Google Scholar]
- Lasa L, Ayuso-Mateos JL, Vazquez-Barquero JL, Diez-Manrique FJ, Dowrick CF. The use of the Beck Depression Inventory to screen for depression in the general population: a preliminary analysis. J Affect Disord. 2000;57:261–265. doi: 10.1016/s0165-0327(99)00088-9. [DOI] [PubMed] [Google Scholar]
- Mwenge GB, Rombaux P, Dury M, Lengele B, Rodenstein D. Targeted hypoglossal neurostimulation for obstructive sleep apnoea: a 1-year pilot study. Eur Respir J. 41:360–367. doi: 10.1183/09031936.00042412. [DOI] [PubMed] [Google Scholar]
- Nashi N, Kang S, Barkdull GC, Lucas J, Davidson TM. Lingual fat at autopsy. Laryngoscope. 2007;117:1467–1473. doi: 10.1097/MLG.0b013e318068b566. [DOI] [PubMed] [Google Scholar]
- Schwab RJ, Pasirstein M, Pierson R, et al. Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am J Respir Crit Care Med. 2003;168:522–530. doi: 10.1164/rccm.200208-866OC. [DOI] [PubMed] [Google Scholar]
- Schwartz AR, Barnes M, Hillman D, et al. Acute upper airway responses to hypoglossal nerve stimulation during sleep in obstructive sleep apnea. Am J Respir Crit Care Med. 2012;185:420–426. doi: 10.1164/rccm.201109-1614OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz AR, Bennett ML, Smith PL, et al. Therapeutic electrical stimulation of the hypoglossal nerve in obstructive sleep apnea. Arch Otolaryngol Head Neck Surg. 2001;127:1216–1223. doi: 10.1001/archotol.127.10.1216. [DOI] [PubMed] [Google Scholar]
- Sher AE, Schechtman KB, Piccirillo JF. The efficacy of surgical modifications of the upper airway in adults with obstructive sleep apnea syndrome. Sleep. 1996;19:156–177. doi: 10.1093/sleep/19.2.156. [DOI] [PubMed] [Google Scholar]
- Strollo PJ, Soose RJ, Strohl KP. Safety and efficacy of upper airway stimulation in treatment of obstructive sleep apnea. Sleep 2013 Program. 92 [Google Scholar]
- Van De Heyning PH, Badr MS, Baskin JZ, et al. Implanted upper airway stimulation device for obstructive sleep apnea. Laryngoscope. 2012;122:1626–1633. doi: 10.1002/lary.23301. [DOI] [PubMed] [Google Scholar]
- Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5:173–178. doi: 10.1513/pats.200708-119MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver TE, Laizner AM, Evans LK, et al. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep. 1997;20:835–843. [PubMed] [Google Scholar]
- White DP. Pathogenesis of obstructive and central sleep apnea. Am J Respir Crit Care Med. 2005;172:1363–1370. doi: 10.1164/rccm.200412-1631SO. [DOI] [PubMed] [Google Scholar]
- Worldhealthorganization. [Accessed May 28, 2012];Chronic respiratory diseases. www.who.int/entity/gard/publications/chronic_respiratory_diseases.pdf.
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]