Summary
DNA assembler enables design and rapid construction of biochemical pathways in a one-step fashion by exploitation of the in vivo homologous recombination mechanism in Saccharomyces cerevisiae. It has many applications in pathway engineering, metabolic engineering, combinatorial biology, and synthetic biology. Here we use the zeaxanthin biosynthetic pathway as an example to describe the key steps in the construction of pathways containing multiple genes using the DNA assembler approach. Methods for the construction of the clones, S. cerevisiae transformation, and zeaxanthin production and detection are shown.
Keywords: DNA assembler, In vivo homologous recombination, Pathway engineering, Synthetic biology, Metabolic engineering, Zeaxanthin biosynthesis
1. Introduction
Methods that enable design and rapid construction of biochemical pathways will be invaluable in pathway engineering, metabolic engineering, combinatorial biology and synthetic biology (1–6). In all these studies, the conventional multi-step, sequential-cloning method, including primer design, PCR amplification, restriction digestion, in vitro ligation and transformation, is typically involved and multiple plasmids are often required (7, 8). This method is not only time-consuming and inefficient but also relies on unique restriction sites that become limited for large recombinant DNA molecules.
Thanks to its high efficiency and ease to work with, in vivo homologous recombination in yeast has been widely used for gene cloning, plasmid construction and library creation (9–12). Recently, we developed a new method, called “DNA assembler”, which enables design and rapid construction of large biochemical pathways in a one-step fashion by exploitation of the in vivo homologous recombination mechanism in S. cerevisiae (13). This method is highly efficient and circumvents the potential problems associated with the conventional cloning methods, representing a versatile approach for the construction of biochemical pathways for synthetic biology, metabolic engineering, and functional genomics studies. Here we use the zeaxanthin biosynthetic pathway as an example to illustrate the experiment procedures.
As shown in Fig. 1, for each individual gene in the zeaxanthin pathway, an expression cassette including a promoter, a structural gene, and a terminator is PCR-amplified and assembled using overlap extension PCR (OE-PCR) (14). The 5′ end of the first gene expression cassette is designed to overlap with a vector, while the 3′ end is designed to overlap with the second cassette. Each successive cassette is designed to overlap with the two flanking ones, and the 3′ end of the last cassette overlaps with the vector. All overlaps are designed to be at least 50 bp for efficient in vivo homologous recombination (see Note 1). The resulting multiple expression cassettes are co-transformed into S. cerevisiae with the linearized vector through electroporation, which allows the entire pathway to be assembled into a vector. Restriction digestion is subsequently used to verify the correctly assembled pathway, after which the cells carrying the correct construct are checked for zeaxanthin production.
Figure 1.
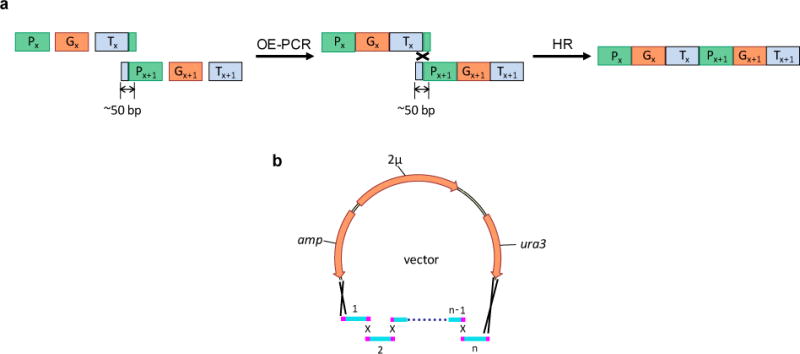
(a) Preparation of each gene expression cassette using OE-PCR. Promoters (Px, Px+1), genes (Gx, Gx+1), and terminators (Tx, Tx+1) are individually PCR-amplified and joined together through OE-PCR. The resulting two cassettes are fused through the in vivo homologous recombination (HR) process. To generate an overlap of approximately 50 bp, the reverse primer used to amplify Tx contains a sequence of the first 20–25 nucleotides of Px+1, and the forward primer used to amplify Px+1 contains a sequence of the last 20–25 nucleotides of Tx. (b) One-step method for assembly of a biochemical pathway using in vivo homologous recombination in S. cerevisiae.
2. Materials
Prepare all solutions using ultrapure water, prepared by purifying deionized water to attain a sensitivity of 18.2 mΩ cm at 25°C. Prepare and store all reagents at room temperature unless indicated otherwise.
2.1. DNA preparation
pRS416 (New England Biolabs, Beverly, MA, USA).
pRS416m: pRS416 with a HisG sequence and a Delta2 (15) sequence that flank the multiple cloning site and serves as the vector for assembly of the zeaxanthin pathway (Fig. 2a) (see Note 2).
pCAR-ΔCrtX: It contains the genes crtE, crtB, crtI, crtY and crtZ from Erwinia uredovora for zeaxanthin biosynthesis (Prof. E.T. Wurtzel, City University of New York, NY, USA) (16–18).
0.5 M EDTA (ethylenediamine tetraacetic acid) solution pH 8.0: For a 500 mL of stock solution of 0.5 M EDTA, weigh out 93.05 g of EDTA disodium salt (MW = 372.2) and dissolve it in 400 mL of deionized water. Adjust to pH 8.0 with NaOH and correct the final volume to 500 mL. EDTA will not dissolve completely in water unless the pH is adjusted to about 8.0.
Concentrated stock solution of TAE (50x): Weigh 242 g of Tris base (MW = 121.14) and dissolve it in approximately 750 mL of deionized water. Carefully add 57.1 mL of glacial acid and 100 mL of 0.5 M EDTA, and adjust the solution to a final volume of 1 L. This stock solution can be stored at room temperature. The pH of this buffer is not adjusted and should be about 8.5.
Working solution of TAE buffer (1x): Dilute the stock solution by 50 fold with deionized water. Final solute concentrations are 40 mM Tris acetate and 1 mM EDTA.
0.7% Agarose gel in 1xTAE buffer: Add 0.7 g of agarose into 100 mL of 1xTAE buffer and microwave until agarose is completely melted. Cool the solution to approximately 70–80°C. Add 5 μL of ethidium bromide into the solution and mix well. Pour 25–30 mL of solution onto an agarose gel rack with appropriate 2-well or 8-well combs.
QIAquick Gel Extraction Kit (QIAGEN, Valencia, CA, USA).
QIAprep Miniprep Kit (QIAGEN, Valencia, CA, USA).
DNA polymerase: Any polymerase with high fidelity can be used.
Failsafe PCR 2xPremix G: containing dNTP and PCR reaction buffer (EPICENTRE Biotechnologies, Madison, WI, USA).
BamHI restriction enzyme.
3 M sodium acetate pH 5.0: Weigh 12.3 g of sodium acetate (MW=82.03) and dissolve it into 50 mL of deionized water. Adjust to pH 5.0 by HCl.
10 mg/mL glycogen: Dissolve 10 mg of glycogen in 1 mL of deionized water.
NanoDrop2000c: used to measure the concentration of DNA and check the OD600 of the cells (Thermo Scientific, Wilmington, DE, USA).
Benchtop centrifuges to separate cells and supernatant.
Molecular imager gel doc: used to check DNA on the agarose gel (Bio-Rad, Hercules, CA, USA).
Figure 2.
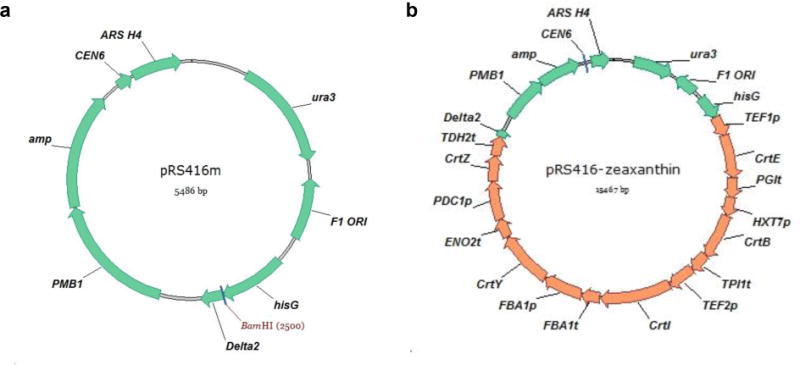
(a) The vector map of pRS416m. (b) The vector map of the construct pRS416m-zeaxanthin. CEN6: centromere; ARS H4: automatic replication sequence; ura3: selection marker in S. cerevisiae; amp: selection marker in E. coli; hisG and Delta2: two regions flanking the zeaxanthin biosynthetic pathway; PMB1: E. coli origin of replication. F1 ORI: this region is contained in the original pRS416 vector, but is not required for the construction of the plasmid containing the zeaxanthin biosynthetic pathway.
2.2. Transformation
cerevisiae YSG50 (MATα, ade2-1, ade3Δ22, ura3-1, his3-11, 15, trp1-1, leu2-3, 112 and can1-100): Used as the host for DNA assembly (see Note 3).
YPAD medium: Dissolve 6 g of yeast extract, 12 g of peptone, 12 g of dextrose, and 60 mg of adenine hemisulphate in 600 mL of deionized water. Autoclave at 121°C for 15 min.
Synthetic complete drop-out medium lacking uracil (SC-Ura): Dissolve 3 g of ammonium sulfate, 1 g of yeast nitrogen source without ammonium sulfate and amino acids, 0.5 g of complete synthetic medium minus uracil (CSM-Ura; MP Biomedicals, Solon, OH, USA), 26 mg of adenine hemisulphate, and 12 g of dextrose in 600 mL of deionized water, and adjust pH to 5.6 by NaOH. Autoclave at 121°C for 15 min.
SC-Ura-agar: SC-Ura and 20 g/L g of agar.
1 M sorbitol solution: Dissolve 91.1 g of sorbitol (MW=182.17) in 400 mL of deionized water and adjust to a final volume of 500 mL. Sterilize the solution by filtering it through a filter with a pore size of 0.22 μm.
Gene pulser II and Pulse controller plus: Used to transform plasmids into S. cerevisiae and E. coli through electroporation (Bio-Rad, Hercules, CA, USA).
2.3. Verification of the clones
Zymoprep II yeast plasmid miniprep (Zymo Research, Orange, CA, USA).
ScaI restriction enzyme (see Note 4).
PsiI restriction enzyme (see Note 4).
1 M glucose solution: Dissolve 90 g of D-glucose in 400 mL of deionized water and adjust to a final volume of 500 mL. Filter-sterilize it.
SOC: Add 20 g of Bacto-tryptone, 5 g of yeast extract, 0.5 g of NaCl, 186.4 mg of KCl into 980 mL of deionized water. Adjust pH to 7.0 with NaOH. Autoclave at 121°C for 15 min. After the solution cools down to 70–80°C, add 20 mL of sterile 1 M glucose.
100 mg/mL ampicillin stock solution: Dissolve 1 g of ampicillin powder in 10 mL deionized water and filter-sterilize it.
LB broth: Add 10 g of bacto-tryptone, 5 g of yeast extract, 10 g of NaCl into 1 L of deionized water. Autoclave at 121°C for 15 min.
LB agar: LB and 20 g/L agar.
LB-Amp+ agar plates: Autoclave LB-agar and when the solution cools down to 70–80°C, add 1 mL of 100 mg/mL ampicillin to 1 L of LB-agar. Pour 20–25 mL into each Petri dish.
2.4. Detection of zeaxanthin
0.1% trifluoroacetic acid (TFA) buffer: Add 1 mL of neat TFA into 999 mL of deionized water.
10 μg/mL zeaxanthin standard solution: Dissolve 1 mg of zeaxanthin (Sigma, St Louis, MO, USA) in 100 mL of methanol.
French pressure cell press: Used to lyse the yeast cells.
Rotavapor R-205: Used to evaporate the solvent.
High performance liquid chromatography (HPLC) equipment.
ZORBAX SB-C18 column (Agilent Technologies, Palo Alto, CA, USA).
3. Methods
3.1. DNA preparation
Amplify the genes crtE, crtB, crtI, crtY and crtZ from the plasmid pCAR-ΔCrtX and amplify the corresponding promoter and terminator from the genomic DNA of S. cerevisiae using the primers listed in Table 1. Set up the reaction mixtures as follows: 50 μL of FailSafe PCR 2xPreMix G, 2.5 μL of forward primer (20 pmol/μL), 2.5 μL of reverse primer (20 pmol/μL), 1 μL of template (10–50 ng of S. cerevisiae genomic DNA or the plasmid pCAR-ΔCrtX), 1 μL of DNA polymerase, and 43 μL of ddH2O in a total volume of 100 μL.
PCR condition: Fully denature at 98°C for 30 s, followed by 25 cycles of 98°C for 10 s, 58°C for 30 s and 72°C for 1 min, with a final extension at 72°C for 10 minutes.
Load the 100 μL of PCR products onto 0.7% agarose gels and perform electrophoresis at 120 V for 20 minutes.
Gel-purify PCR products using the QIAquick Gel Extraction Kit.
Check the concentrations of the purified products using NanoDrop.
Perform OE-PCR to generate each gene expression cassette (see Note 5). Set up the first-step reaction mixture as follows: 10 μL of FailSafe PCR 2xPreMix G, 100 ng of promoter fragment, 100 ng of crt gene fragment, 100 ng of terminator fragment, and 0.2 μL of DNA polymerase. Add ddH2O to a final volume of 20 μL.
Reaction condition: Fully denature at 98°C for 30 s, followed by 10 cycles of 98°C for 10 s, 58°C for 30 s and 72°C for 1 min, with a final extension at 72°C for 10 minutes.
Set up the second-step reaction mixture as follows: 50 μL of FailSafe PCR 2xPreMix G, 10 μL of first-step reaction mixture, 1 μL of DNA polymerase, 2.5 μL of forward primer (20 pmol/μL), 2.5 μL of reverse primer (20 pmol/μL), and 34 μL ddH2O in a total volume of 100 μL.
Reaction condition: Fully denature at 98°C for 30 s, followed by 25 cycles of 98°C for 10 s, 58°C for 30 s and 72°C for 1 min, with a final extension at 72°C for 10 minutes.
Digest pRS416m by BamHI at 37°C for 3 hours. Digestion condition: 5 μL of 10x buffer, 0.5 μL of 100×BSA, 3 μg of pRS416m, and 30 units of BamHI. Add ddH2O to a final volume of 50 μL.
Load the PCR and digestion products onto 0.7% agarose gels and perform electrophoresis at 120 V for 20–30 minutes.
Gel-purify the PCR and digestion products using the QIAquick Gel Extraction Kit.
Check the concentrations of the purified products using NanoDrop.
Take 200–300 ng of each fragment, mix in a tube and calculate the final volume.
Add 10% v/v 3 M sodium acetate and 2% v/v 10 mg/mL glycogen (e.g. if you have 100 μL of mixture, add 10 μL of sodium acetate and 2 μL of glycogen) and mix well.
Add 2× v/v 100% ethanol (e.g. if the final volume is about 110 μL, add 220 μL ethanol) and mix well.
Store the DNA mixture at −80°C for at least an hour.
Centrifuge at 4°C, 13,200 rpm for 20 minutes. Usually the precipitated DNA can be seen at the bottom of the tube.
Remove the supernatant completely (do not touch the DNA).
Add 500 μL of 70% ethanol to wash the DNA pellet, and centrifuge at room temperature, 13,200 rpm for 3 minutes.
Remove the ethanol completely and air dry the pellet for 1–2 minutes (do not over-dry it).
Resuspend the DNA pellet by 4 μL of ddH2O. Now the DNA is ready for transformation (see Note 6).
Table 1.
The primers used in assembling the zeaxanthin pathway in pRS416m.
| Name | Sequence |
|---|---|
| hisG-f | GGCCAGTGAGCGCGCGTAATACGACTCACTATAGGCGCGCCTGCGTGAAGTCGAAG |
| hisG-r | GGAGTAGAAACATTTTGAAGCTATTTCCAGTCAATCAGGGTATTG |
| TEF1p-f | CTTCAATACCCTGATTGACTGGAAATAGCTTCAAAATGTTTCTACTC |
| TEF1p-r | GAACGTGTTTTTTTGCGCAGACCGTCATTTTGTAATTAAAACTTAGATTAG |
| CrtE-f | CTAATCTAAGTTTTAATTACAAAATGACGGTCTGCGCAAAAAAACACGTTC |
| CrtE-r | GGTATATATTTAAGAGCGATTTGTTTAACTGACGGCAGCG |
| PGIt-f | CGCTGCCGTCAGTTAAACAAATCGCTCTTAAATATATACC |
| PGIt-r | CCGAAATTGTTCCTACGAGAAGTGGTATACTGGAGGCTTCATGAGTTATG |
| HXT7p-f | CATAACTCATGAAGCCTCCAGTATACCACTTCTCGTAGGAACAATTTCGG |
| HXT7p-r | GAGTAACGACGGATTATTCATTTTTTGATTAAAATTAAAAAAAC |
| CrtB-f | GTTTTTTTAATTTTAATCAAAAAATGAATAATCCGTCGTTACTC |
| CrtB-r | GATAATATTTTTATATAATTATATTAATCCTAGAGCGGGCGCTGCCAGAG |
| TPI1t-f | CTCTGGCAGCGCCCGCTCTAGGATTAATATAATTATATAAAAATATTATC |
| TPI1t-r | CTATATGTAAGTATACGGCCCTATATAACAGTTGAAATTTGG |
| TEF2p-f | CCAAATTTCAACTGTTATATAGGGCCGTATACTTACATATAG |
| TEF2p-r | CACCAATTACCGTAGTTGGTTTCATGTTTAGTTAATTATAGTTCGTTG |
| CrtI-f | CAACGAACTATAATTAACTAAACATGAAACCAACTACGGTAATTGGTG |
| CrtI-r | CTCATTAAAAAACTATATCAATTAATTTGAATTAACTCATATCAGATCCTCCAGCATC |
| FBA1t-f | GATGCTGGAGGATCTGATATGAGTTAATTCAAATTAATTGATATAGTTTTTTAATGAG |
| FBA1t-r | GTTCAAGCCAGCGGTGCCAGTTGGAGTAAGCTACTATGAAAGACTTTAC |
| FBA1p-f | GTAAAGTCTTTCATAGTAGCTTACTCCAACTGGCACCGCTGGCTTGAAC |
| FBA1p-r | CAGATCATAATGCGGTTGCATTTTGAATATGTATTACTTGGTTATGG |
| CrtY-f | CCATAACCAAGTAATACATATTCAAAATGCAACCGCATTATGATCTG |
| CrtY-r | CTAATAATTCTTAGTTAAAAGCACTTTAACGATGAGTCGTCATAATGG |
| ENO2t-f | CCATTATGACGACTCATCGTTAAAGTGCTTTTAACTAAGAATTATTAG |
| ENO2t-r | GGAACATATGCTCACCCAGTCGCATGAGGTATCATCTCCATCTCCCATATG |
| PDC1p-f | CATATGGGAGATGGAGATGATACCTCATGCGACTGGGTGAGCATATGTTCC |
| PDC1p-r | GGCATTCCAAATCCACAACATTTTGATTGATTTGACTGTG |
| CrtZ-f | CACAGTCAAATCAATCAAAATGTTGTGGATTTGGAATGCC |
| CrtZ-r | CATTAAAGTAACTTAAGGAGTTAAATTTACTTCCCGGATGCGGGCTC |
| TDH2t-f | GAGCCCGCATCCGGGAAGTAAATTTAACTCCTTAAGTTACTTTAATG |
| TDH2t-r | GATCCGTTAGACGTTTCAGCTTCCAGCGAAAAGCCAATTAGTGTGATAC |
| Delta2-f | GTATCACACTAATTGGCTTTTCGCTGGAAGCTGAAACGTCTAACGGATC |
| Delta2-r | TTACGCCAAGCGCGCAATTAACCCTCACTAAAGGCGCGCCGAGAACTTCTAGTATATTC |
3.2. Transformation
Inoculate a single colony of YSG50 into 3 mL of YPAD medium and grow overnight in a shaker at 30°C and 250 rpm.
Measure the OD600 of the seed culture and inoculate the appropriate amount to 50 mL of fresh YPAD medium to obtain an OD600 of 0.2 (e.g. if the overnight culture has an OD600 of 10, then add 1 mL into 50 mL of fresh YPAD medium).
Continue growing the 50 mL of culture for approximately 4 hours to obtain an OD600 of 0.8 (see Note 7).
Spin down the yeast cells at 4°C, 4,000 rpm for 10 minutes and remove the spent medium.
Use 50 mL of ice cold ddH2O to wash the cells once and centrifuge again.
Discard water, add 1 mL of ice-cold ddH2O to resuspend the cells and move them to a sterile Eppendorf tube.
Spin down the cells using a bench top centrifuge for 30 s at 4°C, 7,000 rpm.
Remove water and use 1 mL of 1 M ice-cold sorbitol to wash the cells once (now the cells look slightly yellow). Centrifuge again and remove the sorbitol.
Resuspend the cells in 250–300 μL of chilled 1 M sorbitol and distribute them into 50 μL of aliquots.
Now each 50 μL of cells is ready for electroporation (see Note 8). Mix the 4 μL of DNA with 50 μL of yeast cells and put the mixture into a chilled electroporation cuvette.
Electroporate the cells at 1.5 kV, and quickly add 1 mL of pre-warmed (30°C) YPAD medium to resuspend cells (see Note 9).
Grow in a shaker at 30°C, 250 rpm for 1 hour.
Spin down the cells in a sterile tube at 13,200 rpm for 30 s and remove the YPAD medium.
Use 1 mL of room temperature sorbitol solution to wash the cells two to three times and finally resuspend the cells in 1 mL sorbitol.
Spread 100 μL of resuspended cells onto SC-Ura plates.
Incubate the plates at 30°C for 2–3 days until colonies appear.
3.3. Verification of the correctly assembled zeaxanthin pathway
Randomly pick 10 colonies from the SC-Ura plate and inoculate each colony into 1.5 mL of SC-Ura liquid medium. Grow at 30°C for 1.5 days (see Note 10).
Purify yeast plasmid DNA from each 1.5 mL of culture using the Zymoprep II kit.
Mix 2 μL of isolated plasmid with 50 μL of E. coli BW25141 cells and put the mixture into a chilled electroporation cuvette (see Note 11).
Electroporate the cells at 2.5 kV, and quickly add 1 mL of SOC medium to resuspend cells (see Note 9).
Grow in a shaker at 37°C, 250 rpm for 1 hour.
Spin down the cells, remove 800 μL of SOC medium, resuspend the pellet with the remaining 200 μL of SOC medium and spread the cells on a LB-Amp+ plate.
Incubate the plates at 37°C for 16–18 hours until colonies appear (see Note 12).
Inoculate a single colony from each plate to 5 mL of LB supplemented with 100 μg/mL ampicillin, and grow at 37°C for 12–16 hours.
Purify E. coli plasmids from each 5 mL of culture using the QIAgen Miniprep kit.
Check the plasmid concentrations by NanoDrop.
Verify the correctly assembled pathway through two separate restriction digestion reactions (see Note 4).
Digestion condition by ScaI at 37°C for 3 hours: 1.5 μL of 10x buffer, 0.15 μL of 100× BSA, 300 ng of plasmid, and 5 units of ScaI. Add ddH2O to a final volume of 15 μL. Expected bands: 1750 bp, 2131 bp, 2628 bp, 3223 bp, 5735 bp.
Digestion condition by PsiI at 37°C for 3 hours: 1.5 μL of 10x buffer, 0.15 μL of 100× BSA, 300 ng of plasmid, and 5 units of PsiI. Add ddH2O to a final volume of 15 μL. Expected bands: 215 bp, 1389 bp, 1689 bp, 1782 bp, 2425 bp, 2752 bp, 5215 bp.
3.4. Detection of zeaxanthin
Inoculate a single colony carrying the zeaxanthin biosynthetic pathway into 3 mL of SC-Ura liquid medium and grow at 30°C, 250 rpm for 1.5 days.
Inoculate 2.5 mL of seed culture into 250 mL of fresh SC-Ura medium and continue growing at 30°C, 250 rpm for 4 days.
Cells are collected by centrifugation at 4,000 rpm, resuspended with 5 mL of acetone and lysed by French press at 10,000 psi.
Supernatants are collected after centrifugation at 13,200 rpm for 3 minutes and evaporated to dryness using rotavapor.
After resuspension in 0.5–1 mL of methanol, 100 μL of sample is loaded onto the Agilent ZORBAX SB-C18 column and analyzed at 450 nm by HPLC with a 0.5 mL/min flow rate as follows: buffer A: H2O with 0.1% TFA, buffer B: 100% CH3OH; 0–3 minutes, 60% CH3OH; 3–15 minutes, linear gradient from 60% CH3OH to 100% CH3OH; 15–17 minutes, 100% CH3OH; 17–20 minutes, linear gradient from 100% CH3OH to 60% CH3OH. Authentic zeaxanthin is used as standard, which was eluted at 19.9 minutes.
Footnotes
If a larger biochemical pathway needs to be assembled, increasing the length of the overlaps between the adjacent fragments is necessary. For example, to assemble a pathway with a size of ~25 kb, a longer overlap (e.g. 125 bp) could ensure high assembly efficiency (> 50%), while low efficiency (10–20%) is obtained if the length of the overlaps is only 50 bp (13).
The hisG and delta2 sequences are not essential for assembling a pathway. As long as the 5′ end of the first promoter and the 3′ end of the last terminator contain overlaps (at least 50 bp) with the vector backbone, the pathway can be assembled on a plasmid. Similarly, any linearized S. cerevisiae-E. coli shuttle vector containing an ura3 gene as a selection marker can be used as the vector backbone.
S. cerevisiae YSG50 is used as the host for DNA assembly. However, any S. cerevisiae strain with a non-functional ura3 gene can be used as a host.
In order to verify the correctly assembled constructs through restriction digestion, one or two enzymes that cut the expected construct multiple times are chosen. Usually, two to three groups of digestion need to be set up in order to ensure the correct assembly. For a plasmid with a size of 15–20 kb, such as pRS416-zeaxanthin, find one or two enzymes which cut the DNA molecule 5–9 times. Try to avoid using enzyme digestion that will result in multiple fragments with similar sizes.
In the construction of the first gene expression cassette and the last gene cassette, the hisG sequence and the delta2 sequence are also included. Therefore, for these two reactions, four pieces of fragments are spliced together.
The fragment mixture can be maintained at −20°C for several months.
Normally, the doubling time for a S. cerevisiae laboratory strain is approximately two hours.
Unlike E. coli, yeast competent cells need to be freshly prepared each time.
For an efficient electroporation, a time constant of 5.0–5.2 ms should be obtained.
Assembly efficiency is defined as the percentage of the correct clones among the transformants appearing on the plate. Usually, ten colonies are picked, and an average efficiency of 80% can be obtained for assembly of the zeaxanthin pathway.
E. coli strain BW25141 is used for plasmid enrichment and verification. However, any E. coli strain suitable for DNA cloning, such as DH5α and JM109, can be used.
The number of obtained E. coli transformants could vary from a few to several thousands. This is mainly due to the low quality of the isolated yeast plasmids. However, as long as colonies appear, experiments can proceed.
References
- 1.Hjersted JL, Henson MA, Mahadevan R. Genome-scale analysis of Saccharomyces cerevisiae metabolism and ethanol production in fed-batch culture. Biotechnol Bioeng. 2007;97:1190–204. doi: 10.1002/bit.21332. [DOI] [PubMed] [Google Scholar]
- 2.Keasling JD. Synthetic biology for synthetic chemistry. ACS Chem Biol. 2008;3:64–76. doi: 10.1021/cb7002434. [DOI] [PubMed] [Google Scholar]
- 3.Menzella HG, Reid R, Carney JR, Chandran SS, Reisinger SJ, Patel KG, Hopwood DA, Santi DV. Combinatorial polyketide biosynthesis by de novo design and rearrangement of modular polyketide synthase genes. Nat Biotechnol. 2005;23:1171–1176. doi: 10.1038/nbt1128. [DOI] [PubMed] [Google Scholar]
- 4.Pitera DJ, Paddon CJ, Newman JD, Keasling JD. Balancing a heterologous mevalonate pathway for improved isoprenoid production in Escherichia coli. Metab Eng. 2007;9:193–207. doi: 10.1016/j.ymben.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Ro DK, Paradise EM, Ouellet M, Fisher KJ, Newman KL, Ndungu JM, Ho KA, Eachus RA, Ham TS, Kirby J, Chang MC, Withers ST, Shiba Y, Sarpong R, Keasling JD. Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature. 2006;440:940–943. doi: 10.1038/nature04640. [DOI] [PubMed] [Google Scholar]
- 6.Szczebara FM, Chandelier C, Villeret C, Masurel A, Bourot S, Duport C, Blanchard S, Groisillier A, Testet E, Costaglioli P, Cauet G, Degryse E, Balbuena D, Winter J, Achstetter T, Spagnoli R, Pompon D, Dumas B. Total biosynthesis of hydrocortisone from a simple carbon source in yeast. Nat Biotechnol. 2003;21:143–149. doi: 10.1038/nbt775. [DOI] [PubMed] [Google Scholar]
- 7.Dejong JM, Liu Y, Bollon AP, Long RM, Jennewein S, Williams D, Croteau RB. Genetic engineering of taxol biosynthetic genes in Saccharomyces cerevisiae. Biotechnol Bioeng. 2006;93:212–224. doi: 10.1002/bit.20694. [DOI] [PubMed] [Google Scholar]
- 8.Yan Y, Kohli A, Koffas MA. Biosynthesis of natural flavanones in Saccharomyces cerevisiae. Appl Environ Microbiol. 2005;71:5610–5613. doi: 10.1128/AEM.71.9.5610-5613.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gunyuzlu PL, Hollis GF, Toyn JH. Plasmid construction by linker-assisted homologous recombination in yeast. Biotechniques. 2001;31:1246–1250. doi: 10.2144/01316bm03. [DOI] [PubMed] [Google Scholar]
- 10.Ma H, Kunes S, Schatz PJ, Botstein D. Plasmid construction by homologous recombination in yeast. Gene. 1987;58:201–16. doi: 10.1016/0378-1119(87)90376-3. [DOI] [PubMed] [Google Scholar]
- 11.Oldenburg KR, Vo KT, Michaelis S, Paddon C. Recombination-mediated PCR-directed plasmid construction in vivo in yeast. Nucleic Acids Res. 1997;25:451–2. doi: 10.1093/nar/25.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raymond CK, Pownder TA, Sexson SL. General method for plasmid construction using homologous recombination. Biotechniques. 1999;26:134–141. doi: 10.2144/99261rr02. [DOI] [PubMed] [Google Scholar]
- 13.Shao Z, Zhao H, Zhao H. DNA assembler, an in vivo genetic method for rapid construction of biochemical pathways. Nucleic Acids Res. 2009;37:e16. doi: 10.1093/nar/gkn991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horton RM, Hunt HD, Ho SN, Pullen JK, Pease LR. Engineering hybrid genes without the use of restriction enzymes – Gene-splicing by overlap extension. Gene. 1989;77:61–8. doi: 10.1016/0378-1119(89)90359-4. [DOI] [PubMed] [Google Scholar]
- 15.Lee FW, Da Silva NA. Sequential delta-integration for the regulated insertion of cloned genes in Saccharomyces cerevisiae. Biotechnol Prog. 1997;13:368–73. doi: 10.1021/bp970055d. [DOI] [PubMed] [Google Scholar]
- 16.Chemler JA, Yan Y, Koffas MA. Biosynthesis of isoprenoids, polyunsaturated fatty acids and flavonoids in Saccharomyces cerevisiae. Microb Cell Fact. 2006;5:20. doi: 10.1186/1475-2859-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Misawa N, Nakagawa M, Kobayashi K, Yamano S, Izawa Y, Nakamura K, Harashima K. Elucidation of the Erwinia uredovora carotenoid biosynthetic pathway by functional analysis of gene products expressed in Escherichia coli. J Bacteriol. 1990;172:6704–12. doi: 10.1128/jb.172.12.6704-6712.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Misawa N, Shimada H. Metabolic engineering for the production of carotenoids in non-carotenogenic bacteria and yeasts. J Biotechnol. 1997;59:169–81. doi: 10.1016/s0168-1656(97)00154-5. [DOI] [PubMed] [Google Scholar]


