Abstract
Agave tequilana Weber var. ‘Azul’ is grown for the production of tequila, inulin and syrup. Diverse bacteria inhabit plant tissues and play a crucial role for plant health and growth. In this study culturable endophytic bacteria were extracted from leaf bases of 100 healthy Agave tequilana plants. In plant tissue bacteria occurred at mean population densities of 3 million CFU/g of fresh plant tissue. Three hundred endophytic strains were isolated and 16s rDNA sequences grouped the bacteria into eight different taxa that shared high homology with other known sequences. Bacterial endophytes were identified as Acinectobacter sp., A. baumanii, A. bereziniae, Cronobacter sakazakii, Enterobacter hormaechei, Bacillus sp. Klebsiella oxytoca, Pseudomonas sp., Enterococcus casseliflavus, Leuconostoc mesenteroides subsp. mesenteroides and Gluconobacter oxydans. Isolates were confirmed to be plant growth promoting bacteria (PGPB) by their capacities for nitrogen fixation, auxin production, phosphate solubilization, or antagonism against Fusarium oxysporum AC132. E. casseliflavus JM47 and K. oxytoca JM26 secreted the highest concentrations of IAA. The endophyte Acinectobacter sp. JM58 exhibited the maximum values for nitrogen fixation and phosphate solubilization index (PSI). Inhibition of fungi was found in Pseudomonas sp. JM9p and K. oxytoca JM26. Bacterial endophytes show promise for use as bio-inoculants for agave cultivation. Use of endophytes to enhance cultivation of agave may be particularly important for plants produced by micropropagation techniques, where native endophytes may have been lost.
Keywords: Agave tequilana, endophytic, Fusarium oxysporum, pathogen inhibition
Introduction
Agaves are succulent plants growing primarily in both arid and semiarid lands of North America, with the center of distribution in Mexico (García-Mendoza, 2007). The ‘blue agave’ (Agave tequilana Weber var. ‘Azul’) is the only variety legally permitted by the Mexican government for production of tequila. Recently in the Mexican state of Jalisco, blue agave has also been employed commercially for manufacture of inulin and agave syrup (López and Urías-Silvas, 2007). Because of its economic importance, the blue agave plant has been vegetatively propagated for the last 200 years. In this process, shoots (termed ‘hijuelos’) from rhizomes of parental blue agave plants are used to clonally produce new plants. Hijuelos are cultivated to reach physiological maturity after six to eight years. At maturity, inulin content of the stem and aggregate of leaf bases, termed ‘piña’, is highest (Ogura and Kojima, 2007). During the years prior to harvesting, agave plants are vulnerable to pathogens and adverse environmental conditions. In 2010, the fungus Fusarium oxysporum, causal agent of vascular wilt of blue agave, destroyed 35% of the blue agave harvest in Mexico, generating substantial economic losses for farmers and increasing application of fungicides to control the disease (Gómez-Ortiz et al., 2011; Ávila-Miranda et al., 2012; Vega-Ramos et al., 2013). Biological control of plant pathogens using antagonistic bacteria is a promising strategy and has attracted considerable attention as an option to reduce the use of agrochemicals. Biocontrol using endophytic bacteria may represent an attractive alternative to enhance growth and reduce disease in blue agave cultivation. This study was inspired by our early findings that healthy piñas are inhabited by up to three million cfu/gram of cultivable bacteria. Recently, evidence has increased that many plants live in association with non-pathogenic bacteria (Jiménez-Hidalgo et al., 2004; De Lima et al., 2011; De Oliveira-Costa et al., 2012; Dudeja et al., 2012). In addition, some endophytic bacteria have been shown to function in host defensive or nutritional enhancement roles (Andreote et al., 2009; Bulgarelli et al., 2013; Lahrmann et al., 2013). In blue agave, previous research has examined pathogens (Jiménez-Hidalgo et al., 2004; Ayala-Escobar and Yañez-Morales, 2005; Martinez-Ramirez et al., 2006; Vega-Ramos et al., 2013), but endophytic bacterial symbionts have not been evaluated. The objectives of this study were identify the cultivable endophytic bacterial community in blue agave plants, and characterize their beneficial capacities for growth promotion and antagonistic activity toward a pathogenic strain of Fusarium oxysporum.
Materials and Methods
Agave plants collection
Agave piñas were obtained from 6.5-years-old blue agave plants (cultivated from 2004 to 2011 June) at the ‘Las Majadas’ experimental plantation (21°30′45″ N, 104°37′16″ W), Nayarit State, México, where four hundred plants were grown in 10 furrows (40 plants/furrow).
Sampling of agave “piñas” and microbial determinations
Ten plants were selected randomly from each furrow (F1–F10), and the leaves were removed to obtain 100 piñas. Within 8 h, the harvested piñas were disinfected with a bleach solution (sodium hypochlorite 200 ppm for 15 m), and washed three times with sterile distilled water. Approximately 100 g of tissue were collected from leaf bases of each piña. Samples were blended using an industrial blender (Inmeza, Jal., México) and subsequently the liquid component was extracted by means of a manual orange juice extractor purchased in a local market.
One mL of juice was successively diluted to 10−3, 10−4, and 10−5 in 0.85% saline solution; 1 mL of each dilution was plated by triplicate on trypticase soy agar (TSA) (BD Bioxon, standard agar (STD) (BD Bioxon, México, México) and MRS agar (Difco, México, México) supplemented with 25% sucrose (MRSS). The plates were incubated at 30 °C for 3 days, counting and collecting colonies every 24 h. Each colony was re-inoculated on a plate to purify it further. The isolated strains were characterized by colony morphology and Gram stain.
Genomic DNA extraction, Amplified Ribosomal DNA Restriction Analysis (ARDRA) and identification of isolated strains
Bacterial DNA was extracted by recovering plate biomass using the microbial genomic DNA isolation kit (MoBio. Carlsbad, CA. USA). One μL of each extracted microbial DNA was used as template for 16S rDNA amplification using the primers set fd1 (5′AGAGTTTGATCCT GGCTCAG-3′) and rd1 (5′AAGGAGGTGATCCAGCC-3′) (Weisburg et al., 1991), which yielded a 1.5 kb product. Amplified 16S rDNA fragments were ARDRA characterized using the restriction enzyme HaeIII (Fermentas, BioAdvanced Systems, México, México). The restriction patterns observed were compared and grouped into 11 different restriction site profiles described previously (Escalante et al., 2004). A strain of L. mesenteroides CDBB-68 (Instituto Politécnico Nacional, México) was used as control. Each strain represented by a unique restriction pattern was sequenced by the Taq FS Dye Terminator Cycle Fluorescence-Based Sequencing method, using a Perkin Elmer/Applied Biosystems model 377-18 sequencer. Resulting DNA sequences were submitted to the non-redundant nucleotide database at GenBank using the BLAST program to determine isolate identity. Multiple alignment of 16S rDNA isolate sequences and reference 16S rDNA sequences retrieved from the GenBank database were performed using the ClustalW application in the MEGA version 5 program. The sequences of cultured strains were submitted to the GenBank database with the consecutive accession numbers: KC461186 through KC461198.
Acetylene Reduction Assays (ARA)
Nitrogen fixation of bacterial isolates was determined following a standard assay described previously by Turner and Gibson (1980). Three replicates of vials inoculated with pure cultures, and control treatment using sterile culture media were injected with 0.2 mL of pure acetylene into the head space (10% of vial volume), and incubated at 30 °C for 24 h. Samples were taken at 24 h and 48 h following inoculation. Acetylene reduction was detected by gas chromatographic analysis of samples using a Gas Chromatograph Autosystem (Perkin-Elmer Inc) fitted with a Porapack N column (Alltech Associates, Il, USA), and a hydrogen flame ionization detector (FID).
Assay of Indole Acetic Acid (IAA) production
IAA production was determined according to a modified method of Sawar and Kremer (1995), with 5 mM L-triptophan supplementation. The isolates were incubated at 30 °C for 24 h in dishes with 50% TSA. A suspension of each strain was prepared to OD600 = 0.1, from which 100 mL were added to a vial containing 5 mL of half-strength tripticase soy broth medium supplemented with 50 mM tryptophan, and subsequently incubated for 96 h at 30 °C. The bacterial cells were then removed from the culture medium by centrifuging at 6,000 rpm for 15 min at room temperature. The supernatant was mixed vigorously with 4 mL of Salkowski’s reagent, and left undisturbed at room temperature for 1 h before absorbance at 540 nm was measured. IAA concentration in each culture medium was determined by comparing with a standard curve of 1, 10, and 100 ppm of IAA (Sigma-Aldrich, México).
Determination of Phosphate-Solubilization Index (PSI)
Phosphate-solubilization was assayed as described by Nautiyal (1999) using National Botanical Research Institute’s phosphate (NBRIP) medium supplemented with 1.5% Bacto-agar (Difco Laboratories USA). Sterilized NBRIP media was poured into sterilized Petri plates, after solidification of the media a pin point inoculation was made and plates were incubated at 30 °C for 7 days. Then the ability of the isolates to solubilize the insoluble phosphate was assessed by determination of PSI: the ratio of the total diameter (colony + halozone) and the colony diameter (Premono et al., 1996).
Pathogen antagonism assays
Fusarium oxysporum strain AC132, previously reported as a pathogenic strain (Vega-Ramos et al., 2013), was used for antagonism assays. Endophytic bacteria were incubated on TSA for 24h at 30 °C. F. oxysporum AC 132 was grown on potato dextrose agar (PDA, Difco, México) on a Petri dish at 30 °C in the dark. For assays mycelial discs (8 mm in diam) were cut from margins of 7-day-old colonies.
A mycelial disc on PDA medium without bacteria was maintained as a control. A loop of the selected bacterial strain was streaked on a PDA plate (8 cm Lid Petri dish) 3 cm from the plate center. A fungal plug (0.7 cm diameter) was subsequently cut from the periphery of an actively growing culture, and placed at the plate center. The plates were incubated at 25 ± 2 °C in the dark. The fungal growth radius forward to the bacterial line (A), lateral growth radius (B), backward growth radius (C), and growth radius of the control plate (D) were measured after five, eight and twelve days of incubation. Each treatment was conducted in five replicates. The inhibition percentages at forward (If), lateral (Il), and backward (Ib) were calculated as follows:
Where 0.35 is the radius of fungal plug.
Statistical analysis
All statistical analyses were performed with Statgraphics Centurion XVI.1 (Statpoint Technologies, INC., Virginia USA). The data were analyzed by one-way analysis of variance (ANOVA). Mean separations were performed by Fisher Least Significance Difference (LSD) test with a confidence level of 95%.
Results and Discussion
Bacterial Isolation and identification
We found cultivable endophytic bacteria in 100 healthy plants ranging from 4 × 104 to 3 × 106 cfu/g-tissue in TSA culture medium; and ranging from 2 × 104 to 1.3 × 106 cfu/g-tissue in MRSS culture medium. We obtained a total of 350 bacterial isolates, and 65 representative strains were selected from colonies grown on TSA and MRSS media based on differences in colony morphology and Gram stain. The ARDRA (Amplified Ribosomal DNA Restriction Analysis) characterization of 16S rDNA was performed using in all strains selected. Eleven different restriction patterns were obtained following enzymatic digestion of the 16S rDNA gene. Strains showing the same restriction pattern were counted, and frequencies (%) were calculated (Table 1).
Table 1.
Molecular identification of endophytic strains.
| Strain | Classa | Bacterial species | Genbank accession num. | Frequencyc (%) |
|---|---|---|---|---|
| JM12 | γ-proteobacteria | Acinectobacter baumanii | KC461187 | 40 |
| JM8 | γ-proteobacteria | Acinectobacter bereziniae | KC461186 | 11.7 |
| JM58 | γ-proteobacteria | Acinectobacter sp | KC461191 | 3.3 |
| JM5p | γ-proteobacteria | Cronobacter sakazakii | KC461197 | 15 |
| JM52 | γ-proteobacteria | Enterobacter hormaechei | KC461190 | 11.7 |
| JM26 | γ-proteobacteria | Klebsiella oxytoca | KC461192 | 5 |
| JM9p | γ-proteobacteria | Pseudomonas sp. | KC461198 | 3.3 |
| JM2 | α-proteobacteria | Gluconobacter oxydans | KC461196 | b |
| JM25 | Bacilli | Bacillus sp. | KC461194 | 8.3 |
| JM47 | Bacilli | Enterococcus casseliflavus | KC461188 | 1.7 |
| JM3 | Bacilli | Leuconostoc mesenteroides subsp. Mesenteroides | KC461195 | b |
Bergey’s manual 2004: www.bergeys.org.
Strains isolated in MRSS médium.
Based on the ARDRA patterns of 60 isolates.
Through 16S rDNA sequence analysis, eleven endophytic bacteria were confirmed to be Acinetobacter baumanii, A. bereziniae, Acinetobacter sp., Cronobacter sakazakii, Bacillus sp., Enterobacter hormaechei, Klebsiella oxytoca, Pseudomonas sp., Enterococcus casseliflavus Leuconostoc mesenteroides subsp. mesenteroides, and Gluconobacter oxydans (Table 1). The MRS medium suplemented with sucrose (25%) was selective for Leuconostoc mesenteroides subsp. mesenteroides and Gluconobacter oxydans. Only L. mesenteroides strains developed a mucoid colony. Although L. mesenteroides was previously found in a traditional Mexican beverage named “Pulque”, produced from several species of Agave (Sánchez-Marroquín et al., 1967; Escalante et al., 2008), this is the first report of its occurrence as an endophyte of blue agave. Another study from our group show that L. mesenteroides is a widely distributed endophyte in whole tissues at all grown stages of A. tequilana plants (unpublished results). The genus Acinetobacter exhibited the highest frequency (52%), and together with Cronobacter, Bacillus, Enterobacter, and Klebsiella, these genera represented 95% of all isolates.
Plant growth promotion properties were assessed including nitrogen fixation, indoleacetic acid (IAA) production and phosphate solubilization (Table 2). Only G. oxydans JM2 strain was not tested due to the difficulty in maintaining the strain active under laboratory conditions. All isolates were capable of nitrogen fixation as shown in Table 2. The highest relative ratio was displayed by Acinetobacter sp. JM58, followed by C. sakazakii and L. mesenteroides. These results clearly demonstrate the presence of diazotrophic endophytic bacteria within leaf bases of A. tequilana. It has been reported that diazotrophic endophytes promote growth of host plants. (Sachdev et al., 2008; Doty et al., 2009; López et al., 2011; Bulgarelli et al., 2013). Screening for IAA production revealed positive results for all isolates (Table 2). Among all these isolates the highest production of IAA was shown in Enterococcus casseliflavus JM47 (16.7 ppm) and K. oxytoca JM26 (14.4 ppm). Interestingly, no previous reports of E. casseliflavus as an IAA-producing bacterium has been published. Publications recording K. oxytoca as a producer of IAA (Jha and Kumar; 2007; Liu et al., 2013), as well as the other isolates tested (Rokhbakhsh-Zamin et al., 2011; López et al., 2011) are found. For phosphate solubilization index (PSI), eight isolates showed the appearance of well-developed clearing zones after 7 days of incubation. Acinectobacter sp. and A. baumannii showed maximum solubilization index: 9.72.6 and 8.9 ± 0.2 respectively. The PSI was measured every 24h during seven days and we observed a rapid colony growth and formation of clear halos for Pseudomonas sp. during the experiment, Nevertheless, its PSI was the lowest (2.5) of all PS bacteria (Table 2). This low level of PS may be due to the high motility and rapid growth of Pseudomonas sp., thus resulting in formation of a thin colony. E. casseliflavus JM47 and C. sakazakii JM5p also solubilized phosphate efficiently. Many plant endophytes possess phosphate solubilizing capacity. This capability is probably not important for plant growth, but rather may be useful during growth in soils (De Lima et al., 2011). The capacity for phosphate solubilization suggests that the possessing microbe originated in soils or in rhizospheres of plants.
Table 2.
Plant-growth promoting activity of endophytic strains.
| Plant-growth promoting activitya (SD) | ||||
|---|---|---|---|---|
|
|
||||
| Strain | Bacterial species | % of N2 fixationb | IAAc production (ppm) | Phosphate solubilization indexd |
| JM12 | Acinectobacter baumanii | 0.16 (0.04) | 4.1 (0.07) | 8.9 (0.2) |
| JM8 | Acinectobacter bereziniae | 0.30 (0.11) | 3.0 (0.06) | 4.7 (0.4) |
| JM58 | Acinectobacter sp | 1.31 (0.36) | 5.7 (0.62) | 9.7 (2.6) |
| JM5p | Cronobacter sakazakii | 1.07 (0.61) | 3.1 (0.15) | 4.3 (0.2) |
| JM52 | Enterobacter hormaechei | 0.08 (0.00) | 7.2 (1.01) | 3.8 (0.8) |
| JM26 | Klebsiella oxytoca | 0.13 (0.08) | 14.4 (0.56) | 3.4 (0.5) |
| JM9p | Pseudomonas sp. | 0.16 (0.04) | 2.8 (0.44) | 2.5 (0.04) |
| JM25 | Bacillus sp. | 0.14 (0.04) | 1.2 (0.17) | 0 |
| JM47 | Enterococcus casseliflavus | 0.50 (0.06) | 16.7 (1.04) | 6.3 (0.2) |
| JM3 | Leuconostoc mesenteroides subsp. Mesenteroides | 0.53 (0.16) | 1.7 (0.01) | 0 |
Tested by triplicate. Standard deviation is shown in parenthesis.
Calculated as the ratio ethylene/acetylene* 100 in peak area.
Indol Acetic Acid in parts per million.
PSI: (Colony + halo diameter)/Colony diameter.
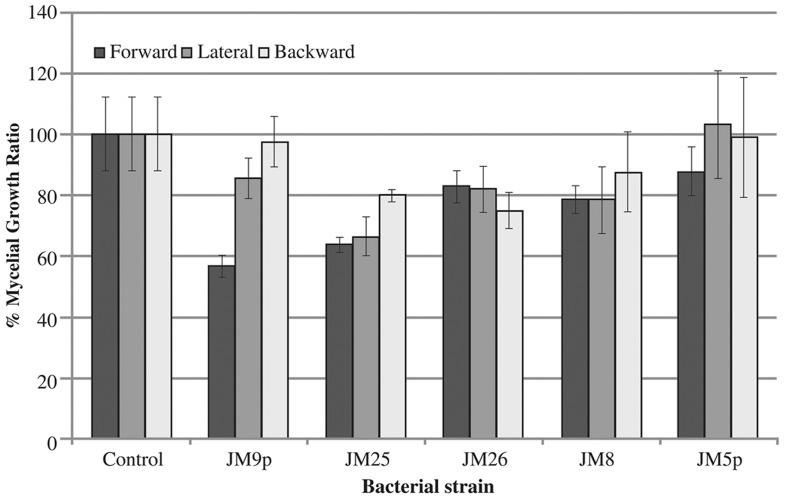
The fungus F. oxysporum causes diseases in a wide variety of plants including Agave (Gómez-Ortiz et al., 2011; Ávila-Miranda et al., 2012; Fortunato et al., 2013; Sivanantham et al., 2013). We tested bacterial isolates against a pathogenic strain F. oxysporum AC132 isolated previously from A. tequilana (Vega-Ramos et al., 2013). Pseudomonas sp. JM9p, Bacillus sp. JM25, K. oxytoca and A. bereziniae exhibited antagonism against F. oxysporum as shown in Figure 1. Bacillus sp. significantly inhibited forward (36%), lateral (34%) and backward (20%) mycelia growth in comparison to controls. Pseudomonas sp. JM9p showed the strongest inhibition against mycelial forward growth (44%); K. oxytoca JM26 and Bacillus sp. JM25 significantly inhibited backward mycelial growth at 25% and 20% respectively.
Figure 1.
Quantification of F. oxysporum AC132 forward, lateral and backward mycelial growth ratio in the presence of five different strains of endophytic bacterial isolates: JM9p (Pseudomonas sp), JM 25 (Bacillus sp.), JM26 (K. oxytoca), JM8 (A. bereziniae) and JM5p (C. sakazakii). Vertical bars represent the SD of the mean of five replications.
The results obtained suggest that mycelial inhibition is due to secretion of inhibitory molecules in all strains tested, and in addition, inhibition of lateral and backward growth against K. oxytoca JM26 and Bacillus sp. JM25 might be due to volatile compounds secreted by the bacterial strains, since the bacterial plug was settled in the opposite side of the plate where no direct contact between the bacterium and F. oxysporum AC132 was possible. This idea is consistent with previous studies in which volatile compounds secreted by endophytes are involved in growth inhibition of fungal pathogens (Edward et al., 2013; Tenorio-Salgado et al., 2013), but further studies must be conducted to corroborate this idea.
The endophytic bacteria examined in our study had plant growth promoting traits and suppressed plant pathogens. This suggests their potential for use as bioinoculants for A. tequilana growth. It is a reasonable hypothesis that bacteria are maintained as endophytes in Agave leaf bases because they are beneficial to plants in multiple ways, perhaps enhancing the capacity of plants to grow and survive. In this respect endophytic bacteria in agave may protect hosts from biotic and abiotic stresses and provide nutrients that are difficult to obtain otherwise.
Recently, aseptically micropropagated blue agave seedlings have been widely exploited for agave cultivation. This reduces contamination with phytopathogens that are currently of increased prevalence in agave plantations. However, aseptic micropropagation also removes beneficial endophytes, which might be inherited through rhizomes in traditional cultivation using “hijuelos”. It is also possible that micropropagation techniques may reduce levels of endophytic populations with the consequent outcome that plants are less resistant to biotic and abiotic stress and more dependent on applications of agrichemical. Further studies are needed to evaluate whether loss of endophytes is occurring during blue agave micropropagation and to evaluate ways to restore endophytes into micropropagated plant materials if they have been lost.
Conclusion
The present study is the first report of endophytic bacteria populations isolated from healthy plant tissues of A. tequilana grown in Jalisco, México. The eleven bacteria isolated were shown to have plant growth promotion capabilities. Also, two isolates may be considered to have promise as biocontrol agents. Diseases caused by fungi and bacteria diminish the commercial production of A. tequilana and large quantities of both chemical fertilizers and pesticides are currently in use to cultivate blue agave. Some of these isolates reported here have potential use as biofertilizers or bioenhancers in agave cultivation. Application of microbial endophytes in cultivation of blue agave could reduce or eliminate applications of agrichemical currently used to produce this crop.
Acknowledgments
We would like to thank Alvaro Muñoz-Juárez, Mónica Limón-Coro, and Juan Gregorio Valenzuela-Del Real for their excellent technical assistance. We appreciate Dr. J. Xavier Uvalle-Bueno and MC. Karla L. Vega-Ramos from Azul Agricultura y Servicios S.A. de C.V. for kindly providing us the F. oxysporum AC132 strain. This work was supported by CONACYT-FOMIXJAL-2009-05-122513. JCM-R thanks to CONACYT for PhD fellowship # 84019. Also we thank to Dr. James F. White from Rutgers University for providing valuable suggestions and English revision of manuscript.
References
- Andreote FD, Azevedo JL, Araújo WL. Assessing the diversity of bacterial communities associated with plants. Braz J Microbiol. 2009;40:417–432. doi: 10.1590/S1517-83822009000300001. [DOI] [PMC free article] [PubMed] [Google Scholar]; Andreote FD, Azevedo JL, Araújo WL (2009) Assessing the diversity of bacterial communities associated with plants. Braz J Microbiol 40:417–432. [DOI] [PMC free article] [PubMed]
- Ávila-Miranda ME, León-Campos C, Peña-Cabriales JJ, Rodríguez-Mendiola MA, Mancilla-Margalli NA, González-Pérez F, Arias-Castro C. Genetic diversity and vegetative compatibility groups in Fusarium oxysporum cause of wilt symptoms in agave (Agave tequilana Weber var. azul) Gayana Bot. 2012;69:40–48. [Google Scholar]; Ávila-Miranda ME, León-Campos C, Peña-Cabriales JJ, Rodríguez-Mendiola MA, Mancilla-Margalli NA, González-Pérez F, Arias-Castro C (2012) Genetic diversity and vegetative compatibility groups in Fusarium oxysporum cause of wilt symptoms in agave (Agave tequilana Weber var. azul). Gayana Bot 69:40–48.
- Ayala-Escobar V, Yañez-Morales MJ. Cercospora agavicola a new foliar pathogen of Agave tequilana var. Azul from Mexico. Mycotaxon. 2005;93:115–121. [Google Scholar]; Ayala-Escobar V and Yañez-Morales MJ (2005) Cercospora agavicola a new foliar pathogen of Agave tequilana var. Azul from Mexico. Mycotaxon 93:115–121.
- Bulgarelli D, Schlaeppi K, Spaepen S, Ver Loren van Themaat E, Schulze-Lefert P. Structure and functions of the bacterial microbiota of plants. Annu Rev Plant Biol. 2013;64:807–38. doi: 10.1146/annurev-arplant-050312-120106. [DOI] [PubMed] [Google Scholar]; Bulgarelli D, Schlaeppi K, Spaepen S, Ver Loren van Themaat E, Schulze-Lefert P (2013) Structure and functions of the bacterial microbiota of plants. Annu Rev Plant Biol 64:807–38. [DOI] [PubMed]
- De Lima AS, Xavier TF, De Lima CE, De Paula Oliveira J, Mergulhão AC, Figueiredo FM. Triple inoculation with Bradyrhizobium, Glomus and Paenibacillus on cowpea (Vigna unguiculata [L.] walp.) development. Braz J Microbiol. 2011;42:919–926. doi: 10.1590/S1517-838220110003000010. [DOI] [PMC free article] [PubMed] [Google Scholar]; De Lima AS, Xavier TF, De Lima CE, De Paula Oliveira J, Mergulhão AC, Figueiredo FM (2011) Triple inoculation with Bradyrhizobium, Glomus and Paenibacillus on cowpea (Vigna unguiculata [L.] walp.) development. Braz J Microbiol 42:919–926. [DOI] [PMC free article] [PubMed]
- De Oliveira-Costa LE, De Queiroz MV, Borges AC, De Moraes CA, De Araújo EF. Isolation and characterization of endophytic bacteria isolated from the leaves of the common bean (Phaseolus vulgaris) Braz J Microbiol. 2012;43:1562–1575. doi: 10.1590/S1517-838220120004000041. [DOI] [PMC free article] [PubMed] [Google Scholar]; De Oliveira-Costa LE, De Queiroz MV, Borges AC, De Moraes CA, De Araújo EF (2012) Isolation and characterization of endophytic bacteria isolated from the leaves of the common bean (Phaseolus vulgaris). Braz J Microbiol 43:1562–1575. [DOI] [PMC free article] [PubMed]
- Doty SL, Oakley B, Xin G, Kang JW, Singleton G, Khan Z, Vajzovic A, Staley JT. Diazotrophic endophytes of native black cottonwood and willow. Symbiosis. 2009;47:23–33. [Google Scholar]; Doty SL, Oakley B, Xin G, Kang JW, Singleton G, Khan Z, Vajzovic A, and Staley JT (2009) Diazotrophic endophytes of native black cottonwood and willow. Symbiosis 47:23–33.
- Dudeja SS, Giri R, Saini R, Suneja-Madan P, Kothe E. Interaction of endophytic microbes with legumes. J Basic Microbiol. 2012;52:248–260. doi: 10.1002/jobm.201100063. [DOI] [PubMed] [Google Scholar]; Dudeja SS, Giri R, Saini R, Suneja-Madan P, and Kothe E (2012) Interaction of endophytic microbes with legumes. J Basic Microbiol 52:248–260. [DOI] [PubMed]
- Edward EJ, King WS, Teck SLC, Jiwan M, Aziz ZFA, Kundat FR, et al. Antagonistic activities of endophytic bacteria against Fusarium wilt of black pepper (Piper nigrum) J Agric Biol. 2013;15:291–296. [Google Scholar]; Edward EJ, King WS, Teck SLC, Jiwan M, Aziz ZFA, Kundat FR, et al. (2013) Antagonistic activities of endophytic bacteria against Fusarium wilt of black pepper (Piper nigrum). J Agric Biol 15:291–296.
- Escalante A, Giles GM, Hernández G, González F, Córdova M, López MA, Gosset G, Bolívar F. Analysis of bacterial community during the fermentation of pulque, a traditional Mexican alcoholic beverage, using a polyphasic approach. Int J Food Microbiol. 2008;124:126–134. doi: 10.1016/j.ijfoodmicro.2008.03.003. [DOI] [PubMed] [Google Scholar]; Escalante A, Giles GM, Hernández G, González F, Córdova M, López MA, Gosset G, Bolívar F (2008) Analysis of bacterial community during the fermentation of pulque, a traditional Mexican alcoholic beverage, using a polyphasic approach. Int J Food Microbiol 124:126–134. [DOI] [PubMed]
- Escalante A, Rodríguez ME, Martínez A, López-Munguía A, Bolivar F, Goseet G. Characterization of bacterial diversity in pulque, a traditional Mexican alcoholic fermented beverage, as determined by 16S rDNA analysis. FEMS Microbiol Lett. 2004;235:273–279. doi: 10.1016/j.femsle.2004.04.045. [DOI] [PubMed] [Google Scholar]; Escalante A, Rodríguez ME, Martínez A, López-Munguía A, Bolivar F, and Goseet G (2004) Characterization of bacterial diversity in pulque, a traditional Mexican alcoholic fermented beverage, as determined by 16S rDNA analysis. FEMS Microbiol Lett 235:273–279. [DOI] [PubMed]
- Fortunato AA, da Silva WL, Rodrigues F. Phenylpropanoid pathway is potentiated by silicon in the roots of banana plants during the infection process of Fusarium oxysporum f. sp. cubense. Phytopathology. 2013;104:597–603. doi: 10.1094/PHYTO-07-13-0203-R. [DOI] [PubMed] [Google Scholar]; Fortunato AA, da Silva WL, Rodrigues F (2013) Phenylpropanoid pathway is potentiated by silicon in the roots of banana plants during the infection process of Fusarium oxysporum f. sp. cubense. Phytopathology 104:597–603. [DOI] [PubMed]
- García-Mendoza AJ. Los agaves de México. Ciencias. 2007;87:14–23. [Google Scholar]; García-Mendoza AJ (2007) Los agaves de México. Ciencias 87:14–23.
- Gómez-Ortiz P, Sánchez-Arizpe A, Virgen-Calleros G, Carvajal-Cazola CR, Padrón-Corral E. Incidencia y severidad de la marchitez del Agave tequilana Weber var. azul en la zona sur del Estado de Nayarit, México. Agraria. 2011;8:21–25. [Google Scholar]; Gómez-Ortiz P, Sánchez-Arizpe A, Virgen-Calleros G, Carvajal-Cazola CR, and Padrón-Corral E (2011) Incidencia y severidad de la marchitez del Agave tequilana Weber var. azul en la zona sur del Estado de Nayarit, México. Agraria 8:21–25.
- Jha PN, Kumar AJ. Endophytic colonization of Typha australis by a plant growth-promoting bacterium Klebsiella oxytoca strain GR-3. Appl Microbiol. 2007;103:1311–20. doi: 10.1111/j.1365-2672.2007.03383.x. [DOI] [PubMed] [Google Scholar]; Jha PN and Kumar AJ (2007) Endophytic colonization of Typha australis by a plant growth-promoting bacterium Klebsiella oxytoca strain GR-3. Appl Microbiol 103:1311–20. [DOI] [PubMed]
- Jiménez-Hidalgo I, Virgen G, Martinez D, Vandemark GJ, Alejo J, Olalde V. Identification and characterization of roft bacteria of Agave tequilana Weber var. azul. Eur J Plant Pathol. 2004;110:317–331. [Google Scholar]; Jiménez-Hidalgo I, Virgen G, Martinez D, Vandemark GJ, Alejo J, and Olalde V (2004) Identification and characterization of roft bacteria of Agave tequilana Weber var. azul. Eur J Plant Pathol 110:317–331.
- Lahrmann U, Ding Y, Banhara A, Rath M, Hajirezaei MR, Döhlemann S, et al. Host-related metabolic cues affect colonization strategies of a root endophyte. Proc Natl Acad Sci USA. 2013;110:13965–13970. doi: 10.1073/pnas.1301653110. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lahrmann U, Ding Y, Banhara A, Rath M, Hajirezaei MR, Döhlemann S, et al. (2013) Host-related metabolic cues affect colonization strategies of a root endophyte. Proc Natl Acad Sci USA 110:13965–13970. [DOI] [PMC free article] [PubMed]
- Liu Y, Shi Z, Yao L, Yue H, Li H, Li C. Effect of IAA produced by Klebsiella oxytoca Rs-5 on cotton growth under salt stress. J Gen Appl Microbiol. 2013;59:59–65. doi: 10.2323/jgam.59.59. [DOI] [PubMed] [Google Scholar]; Liu Y, Shi Z, Yao L, Yue H, Li H, Li C (2013) Effect of IAA produced by Klebsiella oxytoca Rs-5 on cotton growth under salt stress. J Gen Appl Microbiol 59:59–65. [DOI] [PubMed]
- López BR, Bashan Y, Bacilio M. Endophytic bacteria of Mammillaria fraileana, an endemic rock-colonizing cactus of the southern Sonoran Desert. Arch Microbiol. 2011;193:527–41. doi: 10.1007/s00203-011-0695-8. [DOI] [PubMed] [Google Scholar]; López BR, Bashan Y, Bacilio M (2011) Endophytic bacteria of Mammillaria fraileana, an endemic rock-colonizing cactus of the southern Sonoran Desert. Arch Microbiol 193:527–41. [DOI] [PubMed]
- López MG, Urías-Silvas JE. Agave fructans as prebiotics. In: Shiomi N, Benkeblia N, Onodera S, editors. Recent Advances in Fructooligosaccharides Research. Research Signpost Publisher; Kerala, India: 2007. pp. 1–12. [Google Scholar]; López MG and Urías-Silvas JE (2007) Agave fructans as prebiotics. Recent Advances in Fructooligosaccharides Research. Shiomi, N., Benkeblia, N. and Onodera, S. (eds). Research Signpost Publisher, Kerala, India pp 1–12.
- Martinez-Ramirez J, Posos-Ponce P, Robles-Gomez J, Beas-Ruvalcaba K, Fucikovsky-Zak L. Base leaf spot and a black rot of Agave caused by Thielaviopsis paradoxa. Phytopathology; Am Phytopathol Soc Ann Meeting; Quebec City, Canada. 2006. p. 74. [Google Scholar]; Martinez-Ramirez J, Posos-Ponce P, Robles-Gomez J, Beas-Ruvalcaba K, and Fucikovsky-Zak L (2006) Base leaf spot and a black rot of Agave caused by Thielaviopsis paradoxa. Am Phytopathol Soc Ann Meeting. Quebec City, Canada. Phytopathology 96:74.
- Nautiyal CS. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol Lett. 1999;170:265–270. doi: 10.1111/j.1574-6968.1999.tb13383.x. [DOI] [PubMed] [Google Scholar]; Nautiyal CS (1999) An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol Lett 170:265–270. [DOI] [PubMed]
- Ogura T, Kojima Y. Introduction to products made of agave and results of research into inulin products and its characterization. J Food and Food Ingredient. 2007;212:872–884. [Google Scholar]; Ogura T and Kojima Y (2007) Introduction to products made of agave and results of research into inulin products and its characterization. J Food and Food Ingredient 212:872–884.
- Premono EM, Moawad AM, Vlek PLG. Effect of phosphate-solubilizing Pseudomonas putida on the growth of maize and its survival in the rhizosphere. Indones J Crop Sci. 1996;11:13–23. [Google Scholar]; Premono EM, Moawad AM, and Vlek PLG (1996) Effect of phosphate-solubilizing Pseudomonas putida on the growth of maize and its survival in the rhizosphere. Indones J Crop Sci 11:13–23.
- Rokhbakhsh-Zamin F, Sachdev DP, Kazemi-Pour N, Engineer A, Pardesi KR, Zinjarde S, et al. Characterization of plant growth promoting traits of Acinetobacter species isolated from rhizosphere of Pennisetum glaucum. J Microbiol Biotechnol. 2011;21:556–566. [PubMed] [Google Scholar]; Rokhbakhsh-Zamin F, Sachdev DP, Kazemi-Pour N, Engineer A, Pardesi KR, Zinjarde S, et al. (2011) Characterization of plant growth promoting traits of Acinetobacter species isolated from rhizosphere of Pennisetum glaucum. J Microbiol Biotechnol 21:556–566. [PubMed]
- Sachdev DP, Chaudhari HG, Dhakephalkar PK, Chopade BA. Isolation of dinitrogen fixing Acinetobacter species from rhizosphere of wheat (Triticum aestivum) during flowering stage and evaluation of other PGP traits. Proceedings of 1st Conference of International Society of Biotechnology (ISBT); Sikkim, India. 2008. Abstract 5. [Google Scholar]; Sachdev DP, Chaudhari HG, Dhakephalkar PK, and Chopade BA (2008) Isolation of dinitrogen fixing Acinetobacter species from rhizosphere of wheat (Triticum aestivum) during flowering stage and evaluation of other PGP traits. In: Proceedings of 1st Conference of International Society of Biotechnology (ISBT) Sikkim, India. Abstract pp 5.
- Sánchez-Marroquín A, Larios C, Vierna L. Estudios sobre la microbiología del pulque XIX. Elaboración de la bebida mediante cultivos puros. Rev Latinoam Microbiol Parasitol. 1967;9:83–85. [PubMed] [Google Scholar]; Sánchez-Marroquín A, Larios C, and Vierna L (1967) Estudios sobre la microbiología del pulque XIX. Elaboración de la bebida mediante cultivos puros. Rev Latinoam Microbiol Parasitol 9:83–85. [PubMed]
- Sawar M, Kremer RJ. Determination of bacterially derived auxins using microplate method. Lett in Appl Microb. 1995;20:282–285. [Google Scholar]; Sawar M and Kremer RJ (1995) Determination of bacterially derived auxins using microplate method. Lett in Appl Microb 20:282–285.
- Sivanantham T, Rasaiyah V, Satkunanathan N, Thavaranjit AC. In vitro screening of antagonistic effect of soil borne bacteria on some selected phytopathogenic fungi. Arch Appl Sci Res. 2013;5:1–4. [Google Scholar]; Sivanantham T, Rasaiyah V, Satkunanathan N, and Thavaranjit AC (2013) In vitro screening of antagonistic effect of soil borne bacteria on some selected phytopathogenic fungi. Arch Appl Sci Res 5:1–4.
- Tenorio-Salgado S, Tinoco R, Vázquez-Duhalt R, Caballero-Mellado J, Perez-Rueda E. Identification of volatile compounds produced by the bacterium Burkholderia tropica that inhibit the growth of fungal pathogens. Bioengineered. 2013;4:236–243. doi: 10.4161/bioe.23808. [DOI] [PMC free article] [PubMed] [Google Scholar]; Tenorio-Salgado S, Tinoco R, Vázquez-Duhalt R, Caballero-Mellado J, Perez-Rueda E (2013) Identification of volatile compounds produced by the bacterium Burkholderia tropica that inhibit the growth of fungal pathogens. Bioengineered 4:236–243. [DOI] [PMC free article] [PubMed]
- Turner GL, Gibson AH. Measurement of nitrogen fixation by indirect means. In: Bergersen FJ, editor. Methods for Evaluating Biological Nitrogen Fixation. Chichester, New York: J Wiley; 1980. pp. 111–138. [Google Scholar]; Turner GL and Gibson AH (1980)Measurement of nitrogen fixation by indirect means. In Methods for Evaluating Biological Nitrogen Fixation. Bergersen, F.J. (ed). Chichester, New York, J Wiley pp 111–138.
- Vega-Ramos KL, Uvalle-Bueno JX, Gómez-Leyva JF. Molecular variability among isolates of Fusarium oxysporum associated with root rot disease of Agave tequilana. Biochemical Genetics. 2013;51:243–255. doi: 10.1007/s10528-012-9559-4. [DOI] [PubMed] [Google Scholar]; Vega-Ramos KL, Uvalle-Bueno JX, and Gómez-Leyva JF (2013) Molecular variability among isolates of Fusarium oxysporum associated with root rot disease of Agave tequilana. Biochemical Genetics 51:243–255. [DOI] [PubMed]
- Weisburg WG, Barns SM, Pelletier DA, Lane DJ. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173:697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]; Weisburg WG, Barns SM, Pelletier DA, and Lane DJ (1991) 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol 173:697–703. [DOI] [PMC free article] [PubMed]