Abstract
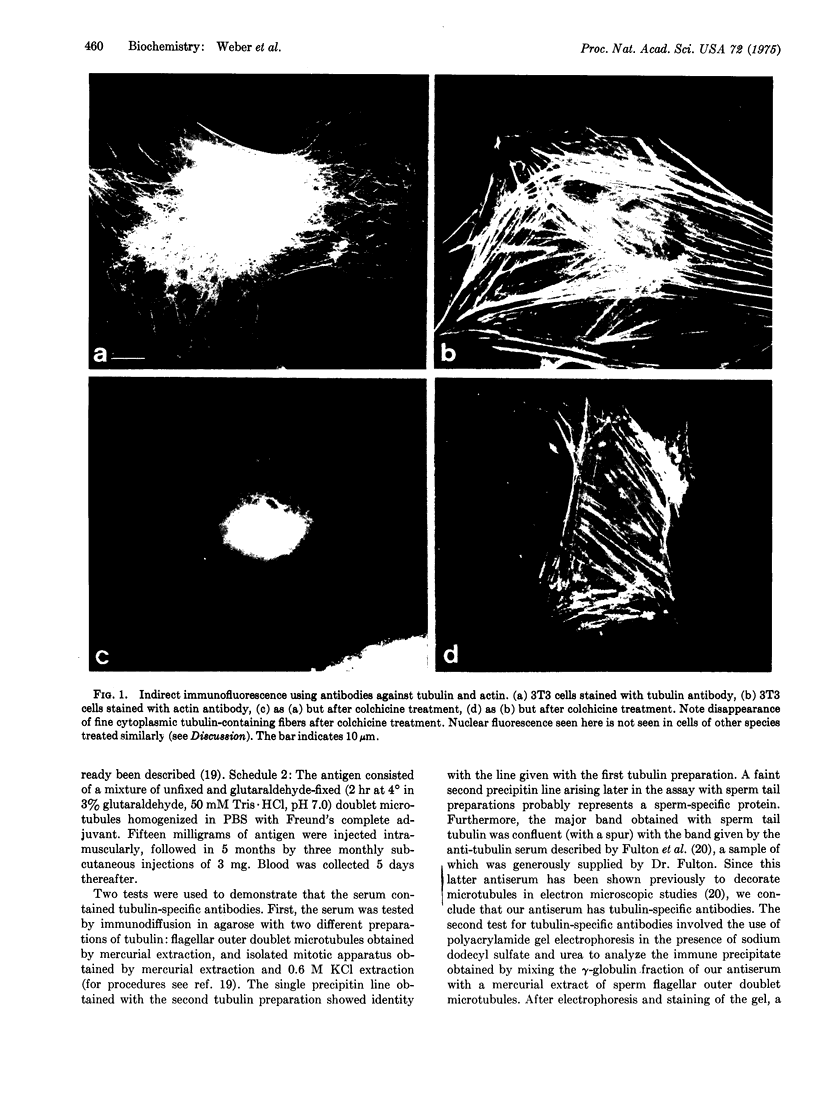
Cytoplasmic microtubules in tissue culture cells can be directly visualized by immunofluorescence microscopy. Antibody against tubulin from the outer doublets of sea urchin sperm flagella decorates a network of fine cytoplasmic fibers in a variety of cell lines of human, monkey, rat, mouse, and chicken origin. These fibers are separate and of uniform thickness and are seen throughout the cytoplasm. The fibers disappear either in a medium containing colchicine or after subjection of the cells to low temperature. The same treatments do not destroy the microfilamentous structures that are visualized by means of antibody against actin. When tryspin-treated enucleated cells are replated and then stained with antibody against tubulin, the fibers can be seen to traverse the entire enucleated cytoplasm.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behnke O., Forer A. Evidence for four classes of microtubules in individual cells. J Cell Sci. 1967 Jun;2(2):169–192. doi: 10.1242/jcs.2.2.169. [DOI] [PubMed] [Google Scholar]
- Bryan J. Vinblastine and microtubules. II. Characterization of two protein subunits from the isolated crystals. J Mol Biol. 1972 Apr 28;66(1):157–168. doi: 10.1016/s0022-2836(72)80013-5. [DOI] [PubMed] [Google Scholar]
- Buckley I. K., Porter K. R. Cytoplasmic fibrils in living cultured cells. A light and electron microscope study. Protoplasma. 1967;64(4):349–380. doi: 10.1007/BF01666538. [DOI] [PubMed] [Google Scholar]
- Dales S. Concerning the universality of a microtubule antigen in animal cells. J Cell Biol. 1972 Mar;52(3):748–754. doi: 10.1083/jcb.52.3.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton C., Kane R. E., Stephens R. E. Serological similarity of flagellar and mitotic microtubules. J Cell Biol. 1971 Sep;50(3):762–773. doi: 10.1083/jcb.50.3.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R. D., Pollack R., Hopkins N. H. Preservation of normal behavior by enucleated cells in culture. Proc Natl Acad Sci U S A. 1973 Mar;70(3):750–754. doi: 10.1073/pnas.70.3.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H., Bischoff R., Holtzer H. Formation of arrowhead complexes with heavy meromyosin in a variety of cell types. J Cell Biol. 1969 Nov;43(2):312–328. [PMC free article] [PubMed] [Google Scholar]
- Lazarides E., Weber K. Actin antibody: the specific visualization of actin filaments in non-muscle cells. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2268–2272. doi: 10.1073/pnas.71.6.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luduena R. F., Woodward D. O. Isolation and partial characterization of alpha and beta-tubulin from outer doublets of sea-urchin sperm and microtubules of chick-embryo brain. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3594–3598. doi: 10.1073/pnas.70.12.3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagayama A., Dales S. Rapid purification and the immunological specificity of mammalian microtubular paracrystals possessing an ATPase activity. Proc Natl Acad Sci U S A. 1970 Jun;66(2):464–471. doi: 10.1073/pnas.66.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmsted J. B., Borisy G. G. Microtubules. Annu Rev Biochem. 1973;42:507–540. doi: 10.1146/annurev.bi.42.070173.002451. [DOI] [PubMed] [Google Scholar]
- Pollard T. D., Weihing R. R. Actin and myosin and cell movement. CRC Crit Rev Biochem. 1974 Jan;2(1):1–65. doi: 10.3109/10409237409105443. [DOI] [PubMed] [Google Scholar]
- STRAUSS A. J., KEMP P. G., Jr, VANNIER W. E., GOODMAN H. C. PURIFICATION OF HUMAN SERUM GAMMA-GLOBULIN FOR IMMUNOLOGIC STUDIES: GAMMA-GLOBULIN FRAGMENTATION AFTER SULFATE PRECIPITATION AND PROLONGED DIALYSIS. J Immunol. 1964 Jul;93:24–34. [PubMed] [Google Scholar]
- Shelanski M. L., Gaskin F., Cantor C. R. Microtubule assembly in the absence of added nucleotides. Proc Natl Acad Sci U S A. 1973 Mar;70(3):765–768. doi: 10.1073/pnas.70.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R. E., Renaud F. L., Gibbons I. R., Stevens R. E. Guanine nucleotide associated with the protein of the outer fibers of flagella and cilia. Science. 1967 Jun 23;156(3782):1606–1608. doi: 10.1126/science.156.3782.1606. [DOI] [PubMed] [Google Scholar]
- TODARO G. J., GREEN H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963 May;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney L. G., Porter K. R. Studies on the microtubules in heliozoa. II. The effect of low temperature on these structures in the formation and maintenance of the axopodia. J Cell Biol. 1967 Jul;34(1):327–343. doi: 10.1083/jcb.34.1.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Groeschel-Stewart U. Antibody to myosin: the specific visualization of myosin-containing filaments in nonmuscle cells. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4561–4564. doi: 10.1073/pnas.71.11.4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Lazarides E., Goldman R. D., Vogel A., Pollack R. Localization and distribution of actin fibers in normal transformed and revertant cells. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):363–369. doi: 10.1101/sqb.1974.039.01.047. [DOI] [PubMed] [Google Scholar]
- Weisenberg R. C. Microtubule formation in vitro in solutions containing low calcium concentrations. Science. 1972 Sep 22;177(4054):1104–1105. doi: 10.1126/science.177.4054.1104. [DOI] [PubMed] [Google Scholar]