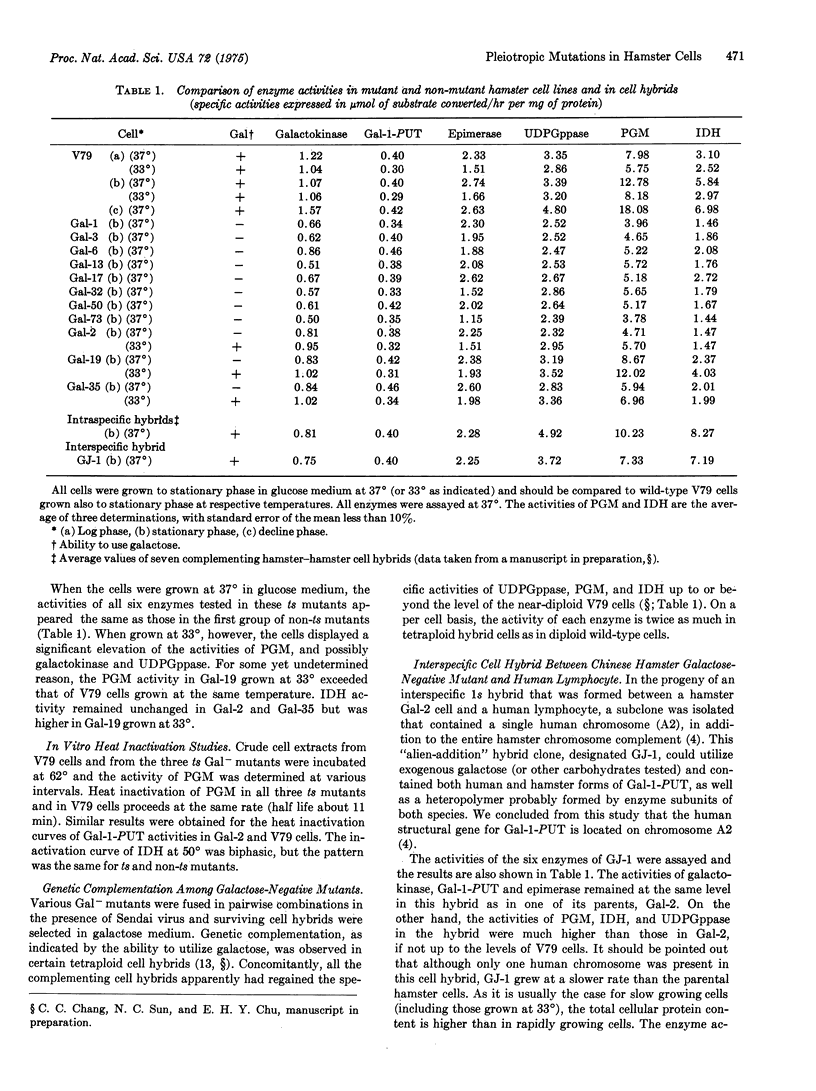
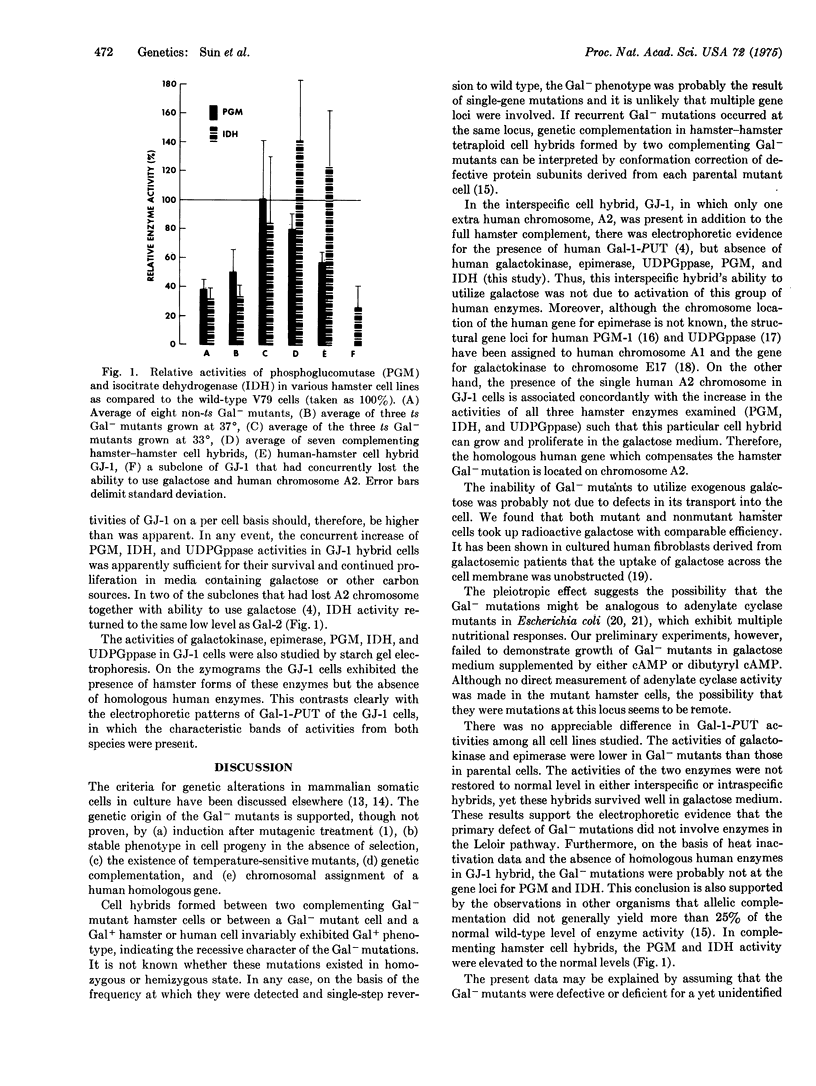
Abstract
Sixty-seven galactose negative (Gal minus) clones were established from survivors after treatment of Chinese hamster lung (V79) cells with 5-bromodeoxyuridine and black light. The mutational origin of these clonal isolates was inferred on the basis of the previously demonstrated mutagenic action of the combined treatment, persistence of the Gal minus phenotype in cell progeny in the absence of selection, conditional lethality of three isolates, interallelic complementation, and assignment of a homologous human gene to chromosome A2. In contrast to the parental cells, Gal minus mutants could not utilize exogenous galactose, mannose, fructose, galactose 1-phosphate, glucose 1-phosphate, or glucose 6-phosphate. Permeation of galactose into mutant cells appeared unimpaired. In intra- and interspecific cell hybrids, the mutation causing the Gal minus phenotype behaved like a recessive character. The pleiotropic nutritional response was due not to deficiency of any one specific enzyme in the Leloir pathway but to significant reduction in activities of phosphoglucomutase, NADP-dependent isocitrate dehydrogenase, and perhaps other enzymes. In temperature-sensitive Gal minus mutants grown at permissive temperature or in complementing intra- and interspecific cell hybrids, the activities of these enzymes were restored to normal levels, accompanied by a regained ability to use the particular hexoses or hexose monophosphates. We postulate that the change from Gal + to Gal minus phenotype in hamster cells could be due to mutations at a regulatory gene locus, or at a yet unknown locus with enzymic defect that causes secondary metabolic imbalances.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brickman E., Soll L., Beckwith J. Genetic characterization of mutations which affect catabolite-sensitive operons in Escherichia coli, including deletions of the gene for adenyl cyclase. J Bacteriol. 1973 Nov;116(2):582–587. doi: 10.1128/jb.116.2.582-587.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britten R. J., Davidson E. H. Gene regulation for higher cells: a theory. Science. 1969 Jul 25;165(3891):349–357. doi: 10.1126/science.165.3891.349. [DOI] [PubMed] [Google Scholar]
- Brown S. W., Chandra H. S. Inactivation system of the mammalian X chromosome. Proc Natl Acad Sci U S A. 1973 Jan;70(1):195–199. doi: 10.1073/pnas.70.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUATRECASAS P., SEGAL S. MAMMALIAN GALACTOKINASE. DEVELOPMENTAL AND ADAPTIVE CHARACTERISTICS IN THE RAT LIVER. J Biol Chem. 1965 Jun;240:2382–2388. [PubMed] [Google Scholar]
- Chu E. H., Brimer P., Jacobson K. B., Merriam E. V. Mammalian cell genetics. I. Selection and characterization of mutations auxotrophic for L-glutamine or resistant to 8-azaguanine in Chinese hamster cells in vitro. Genetics. 1969 Jun;62(2):359–377. doi: 10.1093/genetics/62.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu E. H., Sun N. C., Chang C. C. Induction of auxotrophic mutations by treatment of Chinese hamster cells with 5-bromodeoxyuridine and black light. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3459–3463. doi: 10.1073/pnas.69.11.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croce C. M., Bakay B., Nyhan W. L., Koprowski H. Reexpression of the rat hypoxanthine phosphoribosyltransferase gene in rat-human hybrids. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2590–2594. doi: 10.1073/pnas.70.9.2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. One-step growth curve of Western equine encephalomyelitis virus on chicken embryo cells grown in vitro and analysis of virus yields from single cells. J Exp Med. 1954 Feb;99(2):183–199. doi: 10.1084/jem.99.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSimone J., Linde M., Tashian R. E. Evidence for linkage of carbonic anhydrase isozyme genes in the pig-tailed macaque, Macaca nemestrina. Nat New Biol. 1973 Mar 14;242(115):55–56. doi: 10.1038/newbio242055a0. [DOI] [PubMed] [Google Scholar]
- Douglas G. R., McAlpine P. J., Hamerton J. L. Regional localization of loci for human PGM and 6PGD on human chromosome one by use of hybrids of Chinese hamster-human somatic cells. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2737–2740. doi: 10.1073/pnas.70.10.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- FUDENBERG H. H., HIRSCHHORN K. AGAMMAGLOBULINEMIA: THE FUNDAMENTAL DEFECT. Science. 1964 Aug 7;145(3632):611–612. doi: 10.1126/science.145.3632.611. [DOI] [PubMed] [Google Scholar]
- Hutton J. J., Roderick T. H. Linkage analyses using biochemical variants in mice. 3. Linkage relationships of eleven biochemical markers. Biochem Genet. 1970 Apr;4(2):339–350. doi: 10.1007/BF00485782. [DOI] [PubMed] [Google Scholar]
- Kao F. T., Puck T. T. Genetics of somatic mammalian cells: demonstration of a human esterase activator gene linked to the adeB gene. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3273–3277. doi: 10.1073/pnas.69.11.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball R. F., Perdue S. W., Chu E. H., Ortiz J. R. Microphotometric and autoradiographic studies on the cell cycle and cell size during growth and decline of Chinese hamster cell cultures. Exp Cell Res. 1971 May;66(1):17–32. doi: 10.1016/s0014-4827(71)80007-1. [DOI] [PubMed] [Google Scholar]
- Lucchesi J. C. Dosage compensation in Drosophila. Annu Rev Genet. 1973;7:225–237. doi: 10.1146/annurev.ge.07.120173.001301. [DOI] [PubMed] [Google Scholar]
- Mayes J. S., Miller L. R. The metabolism of galactose by galactosemic fibroblasts in vitro. Biochim Biophys Acta. 1973 Jun 20;313(1):9–16. doi: 10.1016/0304-4165(73)90184-0. [DOI] [PubMed] [Google Scholar]
- Pastan I., Perlman R. Cyclic adenosine monophosphate in bacteria. Science. 1970 Jul 24;169(3943):339–344. doi: 10.1126/science.169.3943.339. [DOI] [PubMed] [Google Scholar]
- Perlman R. L., Pastan I. Pleiotropic deficiency of carbohydrate utilization in an adenyl cyclase deficient mutant of Escherichia coli. Biochem Biophys Res Commun. 1969 Sep 24;37(1):151–157. doi: 10.1016/0006-291x(69)90893-6. [DOI] [PubMed] [Google Scholar]
- Schwartz D. Genetic control of alcohol dehydrogenase--a competition model for regulation of gene action. Genetics. 1971 Mar;67(3):411–425. doi: 10.1093/genetics/67.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shows T. B., Ruddle F. H. Function of the lactate dehydrogenase B gene in mouse erythrocytes: evidence for control by a regulatory gene. Proc Natl Acad Sci U S A. 1968 Oct;61(2):574–581. doi: 10.1073/pnas.61.2.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun N. C., Chang C. C., Chu E. H. Chromosome assignment of the human gene for galactose-1-phosphate uridyltransferase. Proc Natl Acad Sci U S A. 1974 Feb;71(2):404–407. doi: 10.1073/pnas.71.2.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson L. H., Baker R. M. Isolation of mutants of cultured mammalian cells. Methods Cell Biol. 1973;6:209–281. doi: 10.1016/s0091-679x(08)60052-7. [DOI] [PubMed] [Google Scholar]


