Abstract
Neural responses are modulated by brain state, which varies with arousal, attention, and behavior. In mice, running and whisking desynchronize the cortex and enhance sensory responses, but the quiescent periods between bouts of exploratory behaviors have not been well-studied. We found that these periods of “quiet wakefulness” were characterized by state fluctuations on a timescale of 1–2 seconds. Small fluctuations in pupil diameter tracked these state transitions in multiple cortical areas. During dilation, the intracellular membrane potential was desynchronized, sensory responses were enhanced, and population activity was less correlated. In contrast, constriction was characterized by increased low-frequency oscillations and higher ensemble correlations. Specific subtypes of cortical interneurons were differentially activated during dilation and constriction, consistent with their participation in the observed state changes. Pupillometry has been used to index attention and mental effort in humans, but the intracellular dynamics and differences in population activity underlying this phenomenon were previously unknown.
Introduction
Responses to external stimuli are strongly modulated by the brain’s internal dynamics, which are organized around characteristic states that vary with arousal, attention, and behavioral context (Harris and Thiele, 2011; Iriki et al., 1996; Kahneman, 1973; Lee and Dan, 2012). Across multiple species, more active states are associated with cortical desynchronization, a reduction in the amplitude of low-frequency oscillations measured in EEG, LFP, or intracellular recordings. For example, primate cortex is more desynchronized during attentive states (Gould et al., 2011; Grent-’t-Jong et al., 2011; Rohenkohl and Nobre, 2011), and in REM sleep compared to deeper sleep stages (Colten and Altevogt, 2006).
Non-primate mammals also display a spectrum of cortical dynamics during waking periods, from more synchronized to more desynchronized states; and this internal variability modulates responses to external stimuli (Hei et al., 2014; Zhuang et al., 2014). Specifically, recent work in awake mice has revealed that the cortex is desynchronized during bouts of exploratory behavior such as whisking (Crochet and Petersen, 2006; Poulet and Petersen, 2008) and running (Bennett et al., 2013; Niell and Stryker, 2010; Polack et al., 2013), compared to stationary periods. In mouse primary visual cortex (V1), this desynchronization is coupled with an enhancement of sensory responses (Fu et al., 2014; Froudarakis et al., 2014) and a reduction in detection thresholds (Bennett et al., 2013), and similar effects are seen in primary somatosensory cortex (area S1; Zagha et al., 2013).
Between bouts of activity, there are longer epochs of “quiet wakefulness”, periods of behavioral quiescence that have not been well-studied. While previous reports have emphasized the average increase in low-frequency synchronous activity during quiet wakefulness, we observed second-to-second state fluctuations during these periods in both V1 and S1. Fast state fluctuations during quiet wakefulness were closely tracked by changes in pupil diameter. During dilation, we found that the cortex was desynchronized and more responsive to external stimuli, compared to constriction, when low-frequency oscillations were enhanced and ensemble correlations were increased. (In this study we always use “dilation” and “constriction” to refer to active dilating and constricting, and not the state of being dilated or constricted.) Furthermore, vasoactive intestinal peptide-expressing (VIP+) GABAergic interneurons and somatostatin-expressing (SOM+) interneurons were differentially modulated during dilation and constriction. These interneuron subtypes have recently been shown to participate in a canonical local circuit essential for the enhancement of visual responses during running (Fu et al., 2014), and our results suggest that this mechanism may be recapitulated in the state fluctuations that occur during quiet wakefulness.
Results
Exploratory behaviors are accompanied by cortical desynchronization and pupil dilation
We performed whole-cell patch clamp recordings of layer 2/3 cortical neurons in awake mice (n=111 total neurons from 38 animals) while monitoring treadmill motion, whisking behavior, eye movements, and pupil diameter (Figure 1A,B). All analyses in Figures 1–3 were of spontaneous recordings, in order to avoid any confounding effects of visual stimuli. Periods of running, whisking and eye movements usually occurred together (Figures 1C, S1; Movie S1), consistent with the idea that these behaviors are manifestations of a common exploratory state (Grant et al., 2012). Approximately 90% of these epochs were accompanied by an increase in pupil size (Figure 1B,D; mean increase in pupil diameter during running periods 202±11 μm, mean ± SEM; see Methods).
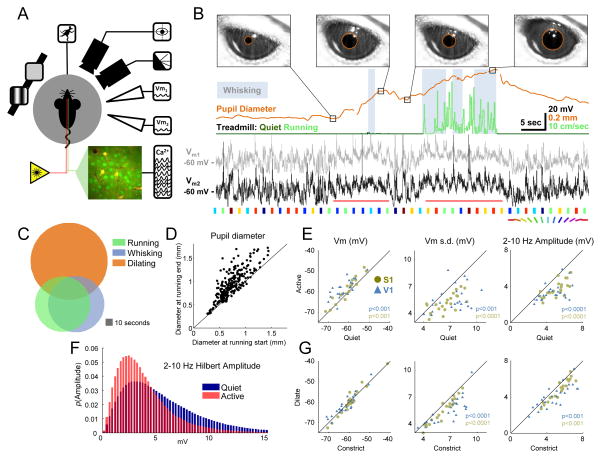
Figure 1.
Pupil diameter, locomotion and whisking correlate with subthreshold measures of cortical state. (A) Schematic of simultaneous recordings showing mouse on spherical treadmill, with eye and whisker cameras, single or dual patch pipettes, calcium imaging, and visual stimuli. (B) Example treadmill activity (running periods in light green, see Supplemental Experimental Procedures), pupil diameter (orange), whisking (gray background, see Supplemental Experimental Procedures), and Vm from two simultaneously patched cells (black and gray; depolarization around whisking and running epochs indicated with horizontal red lines). Colored patches below the voltage traces indicate presentations of oriented drifting gratings (Figure 4 only). Images of the eye are shown at the time points indicated in the pupil diameter trace, with pupil detection indicated by orange circles. Gaps in the pupil trace are due to blinks. (C) Overlap of running, whisking and pupil dilation episodes. (D) Pupil size before and after running epochs. (E) Changes in subthreshold membrane potential between quiet wakefulness and activity (running and whisking) without visual stimulation. (F) Distribution of low frequency amplitude during active behavior compared to quiet wakefulness (G) Changes in subthreshold membrane potential during dilating and constricting epochs of quiet wakefulness, also without visual stimuli. See also Figures S1 and S3 and Movie S1.
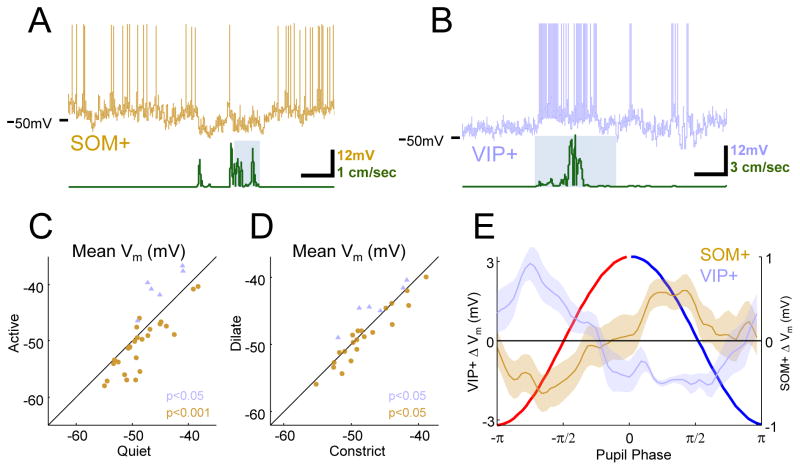
Figure 3.
VIP+ cells are excited and SOM+ cells are inhibited during active behaviors and during dilation. (A) Example SOM+ cell (brown) and treadmill (green) trace with whisking epoch as grey background. SOM+ cells are hyperpolarized during locomotion. (B) Example VIP+ cell (blue) and treadmill (green) trace with whisking epoch as grey background. VIP+ activity is dramatically enhanced during running. (C) Summary of inhibition of SOM+ and excitation of VIP+ during exploratory behaviors compared to quiet wakefulness. (D) In quiet wakefulness, SOM+ cells are inhibited and VIP+ cells are excited during dilation compared to constriction. (E) Phase-binned change in SOM+ and VIP+ Vm showing time course of signature opposition of VIP+ and SOM+ activity over dilating and constricting periods.
Consistent with previous studies (Bennett et al., 2013; Crochet and Petersen, 2006; Niell and Stryker, 2010; Polack et al., 2013; Poulet and Petersen, 2008; Zagha et al., 2013), we found that during exploratory behaviors (running and/or whisking), Vm in both S1 and V1 was desynchronized (red horizontal lines under example V1 recordings in Figure 1B). Relative to quiet wakefulness, the membrane potential (Vm) in these periods was depolarized (S1: 1.9±0.4 mV, P<0.001; V1: 3.6±0.7 mV, P<0.001), less variable (change in Vm standard deviation, S1: −1.4±0.2 mV, P<0.0001; V1: −1.7±0.4 mV, P<0.001), and low-frequency oscillations were reduced (Hilbert amplitude of 2–10 Hz band-pass filtered Vm; S1: −1.4±0.2 mV, P<0.0001; V1: −1.3±0.2 mV, P<0.0001; Figure 1E; mean difference ± SEM; Wilcoxon signed-rank test for all comparisons). High-frequency oscillations in the gamma range were also enhanced during activity compared to quiet wakefulness (Figure S1B), consistent with previous reports (Niell and Stryker, 2010).
During quiet epochs, spontaneous pupil fluctuations are a sensitive index of Vm desynchronization
The large overlap in the amount of synchronous activity during active and quiet periods (Figure 1F) and the reliable increase in pupil size during the desynchronized periods associated with running and whisking led us to ask whether pupil dilation might also be associated with a desynchronized state during quiet wakefulness. To study quiet wakefulness, we excluded periods of running and whisking, and also saccades or blinks, which occurred rarely outside of active behaviors (less than one saccade per minute and less than one blink every five minutes; Figure S1A). We found that during quiet wakefulness, Vm variability was reduced during dilation vs. constriction (change in Vm standard deviation, S1: −0.6±0.08 mV, P<0.0001; V1: −1.2±0.2 mV, P<0.0001). This effect could be explained primarily by a reduction in low-frequency oscillations (2–10 Hz Hilbert amplitude; S1: −0.56±0.09 mV, P<0.001; V1: −0.8±0.2 mV, P<0.001; Figure 1G; mean difference ± SEM; Wilcoxon signed-rank test for all comparisons). A modest increase in Vm oscillations at higher (gamma) frequencies was also observed during dilation compared to constriction (Figure S1C). However, in contrast with the effects of running and whisking, mean Vm was not significantly different for dilation vs. constriction.
Pupil fluctuations were much smaller and faster during quiet wakefulness than around bouts of exploratory behavior (Figure 2A–C, Movies S1 and S2). The duration of individual dilation and constriction periods during quiet wakefulness varied, although in general dilation was faster than constriction (mean dilating duration 1.6 seconds, mean constricting duration 2.0 seconds; Figure S2). In order to characterize the time course of the change in synchronous activity relative to pupil fluctuations during quiet wakefulness, we binned the instantaneous 2–10 Hz Hilbert amplitude by the phase of the pupil trace at each time point, aligning multiple cycles of dilation and constriction to one canonical cycle. We found that the amplitude of low-frequency Vm oscillations reached a minimum towards the middle of the dilating phase and peaked during constriction (Figure 2D; plots are mean ± SEM across cells). Overall, the average intracellular membrane potential was more desynchronized during dilation in both areas S1 and V1 (Figure 2E; S1 and V1, P<0.001; paired t-test; bars are mean change during dilation compared to constriction over cells, error bars are SEM; see also Figure S3). This relationship between cortical state and pupil fluctuations persisted even after removing occasional small postural adjustments or whisker twitches from our analysis, and after habituating the mice to the treadmill for thirty minutes per day for five days prior to recording (Movie S1; Figure S4).
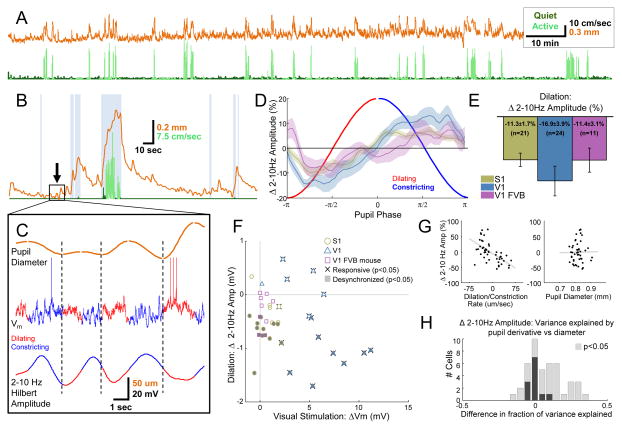
Figure 2.
Pupil diameter correlates with cortical state in the absence of exploratory behavior. (A) Concatenated recordings of treadmill speed (running periods in light green, quiet periods in dark green) and pupil diameter (orange) from a single mouse (total time ~2.4 hours). (B) Pupil diameter (orange) around a single active period (running in light green and whisking in gray background). (C) Zoomed in period of quiet wakefulness from rectangle in (B) showing four sequential cycles of pupil dilation (red) and constriction (dark blue) correlated with low-frequency amplitude (separate cycles of dilation and constriction separated by dashed vertical lines). (D) Vm is desynchronized during dilation and synchronized during constriction of the pupil in S1 (olive), V1 (blue), and V1 of FVB mice (mauve; 64 phase bins from −π to π, plots are mean ± SEM for each bin). (E) Averages over entire dilation and constriction periods for cells in each area (bar plots are mean ± SEM across cells, one-way ANOVA across cell groups was not significant P=0.53). (F) Scatter plot of desynchronization during dilation and visual responsiveness for all cells in each area. Significantly responsive cells are indicated with whiskers, significantly desynchronized cells are indicated with shading. (G) Linear regression of the rate of change (left) and the absolute value of pupil diameter (right) against percent change in 2–10 Hz amplitude for a single cell. (H) Stacked histogram of the difference in total variance in 2–10 Hz amplitude explained by pupil derivative and pupil diameter in a two-way ANOVA for each cell. Cells where either factor was significant (P<.05) are indicated with lighter bars. Overall, variations in cortical state indexed by low-frequency amplitude are more closely tracked by pupil dilation and constriction than by absolute pupil diameter (P<.0001, t-test). See also Figures S2–S4 and Movie S2.
The robust link between pupil dilation and membrane potential desynchronization in area S1 argues against the possibility of a purely visual effect related to increased illumination of the retina. Although a small number of S1 cells (2/17) were weakly visually responsive (responsiveness generously defined as any significant change in Vm from baseline during a visual stimulus; α=0.05, Wilcoxon signed-rank test), the majority of visually unresponsive S1 cells still showed a robust relationship between dilation and Vm desynchronization (10/15; Figure 2F). To further rule out the possibility that changes in the visual input alone were responsible for cortical desynchronization, we patched cells in V1 of eight-week to four-month-old FVB/NJ (FVB) mice. In these mice, the retinal ganglion cell layer undergoes severe degeneration by P21 (Chang et al., 2002). In contrast to wild-type mice where most of the V1 cells were visually responsive (12/13), none of the patched cells (0/9) were visually responsive in FVB mice. Yet the relationship between pupil fluctuations and cortical state was also observed in these cells (Figure 2D, E, F; P<0.05 Wilcoxon signed-rank test; Figure 2E). We did not observe a significant difference in the effect across the three groups of cells (S1, V1, FVB; one-way ANOVA, P=.53)
To examine whether state changes were tracked more closely by dilation and constriction or by pupil diameter, we examined the correlation between Vm synchronization and the derivative and absolute size of the pupil (Figure 2G, H). We found that the amplitude of 2–10 Hz oscillations was more closely tracked by the derivative than diameter of the pupil (mean fraction of variance explained in a two-way ANOVA for each cell by derivative, 0.13; by diameter, 0.05; P<.0001, t-test).
A local interneuron circuit participates in the state changes tracked by pupil fluctuation
Recently, Fu and colleagues found that VIP+ interneurons are activated during running, while SOM+ interneurons are inhibited (Fu et al., 2014):, consistent with several recent studies showing that VIP+ cells directly inhibit SOM+ cells, producing a net disinhibition of nearby pyramidal neurons. Furthermore, Fu et al. demonstrated that this cortical circuit was both necessary and sufficient to produce the enhancement of visual responses observed during running.
We initially sought to replicate the effect of active behavior on these interneuron subtypes with targeted patch recordings of labeled VIP+ (n=6) and SOM+ (n=30) cells in V1. Consistent with Fu et al., 2014, VIP+ cells were robustly depolarized during running (4.6±0.8 mV; P<.05 Wilcoxon signed-rank test; Figure 3B,C). However, the behavior of SOM+ cells was more complex. We found that our SOM+ Cre driver line (SST-IRES-Cre; Taniguchi et al., 2011) labeled a population of neurons that could be grouped into two distinct classes, which we call Type I and Type II. Type I SOM+ cells, which were the majority (n=21/30), exhibited the characteristic low Vm variability described by Petersen and colleagues (Gentet et al., 2012), and these cells were inhibited during active epochs of running and whisking (−2.1±0.47 mV; P<0.001 Wilcoxon signed-rank test; Figure 3A,C; Figure S5). A smaller population of Type II labeled SOM+ cells n=7/30) had intrinsic membrane properties more similar to VIP+ or PV+ cells, and these cells were depolarized during active epochs (3.5±0.9 mV; P<0.01 Wilcoxon signed-rank test; Figure S5). In many cases, we recorded from both types of labeled SOM+ cells in the same animal, and they were completely separable by a number of features, the simplest being the range of voltages spanned by Vm during recordings of spontaneous activity (Figure S5). Both SOM+ Type I and Type II cells were distinct from morphologically identified (spiny) pyramidal cells, which were rarely labeled (n=2/56), presumably due to leaky expression of Cre recombinase (Figure S5). Although the fact that the widely used SST-IRES-Cre line (Taniguchi et al., 2011) labels subpopulations of interneurons with different in vivo functional properties has not been previously reported, a recent in vitro study using these mice found two electrophysiologically distinct populations of labeled cells in almost the same proportions that we observed here (Hu et al., 2013). Based on these findings, we excluded Type II SOM+ cells in subsequent analyses.
Having confirmed the results of Fu et al. with respect to running, we wondered whether the SOM+/VIP+ cortical circuit might also participate in the state changes indexed by dilation and constriction during quiet wakefulness. Indeed, we found that VIP+ cells were relatively depolarized during dilation (2.1±0.6 mV, P<0.05) while SOM+ cells were hyperpolarized (−0.7±0.3mV, P<0.05; Wilcoxon signed-rank test all comparisons; Figure 3D–E). Our results support the view that the SOM+/VIP+ circuit is not only recruited during active behavior, but may also play a role in cortical state changes during quiet wakefulness.
Visual encoding is improved during desynchronized states defined by pupil dilation
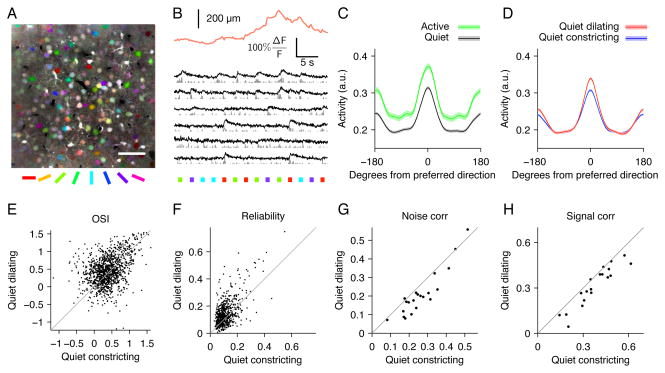
To determine whether stimulus encoding in V1 is improved during the desynchronized state indexed by pupil dilation, we performed two-photon calcium imaging of populations of layer 2/3 cells loaded with the calcium-sensitive fluorescent indicator Oregon Green BAPTA-1AM while presenting drifting oriented gratings (example site, Figure 4A,B; n=34 imaging sites in 6 mice). We analyzed cells that were tuned for orientation (1200/3435 cells significantly tuned; see Methods). During exploratory activity (running and whisking) there was a 20% increase in the mean response to preferred direction (P<10−12) and a 19% increase in the mean response to orthogonal directions (P<10−15; n=516 tuned cells in 14 sites with sufficient numbers of visual responses during both active behavior and quiet wakefulness). These changes resulted in a slight decrease in orientation selectivity, but consistent with previous reports (Niell and Stryker, 2010; Polack et al., 2013), this change was not significant (7% decrease in OSI, P=0.07; Figure 4C, error bands are SEM across cells). During quiet wakefulness, with running, whisking, and saccades removed, dilation was associated with a 9% increase in the mean response to preferred direction (P<10−13) and no change (0.6%, P=.47) in the response to the orthogonal direction, resulting in an enhancement of orientation selectivity (Figure 4D,E; 16% increase in OSI, P<10−6) Note that there was no significant difference in the size of the pupil between dilating and constricting trials (Figure S6). During dilation, responses to drifting gratings were also more reliable (Figure 4F, 28% increase in mean variability explained by stimulus conditions, P<10−15; n=619 cells in 22 sites). Although there are a number of caveats that make it difficult to estimate the absolute magnitude of noise correlations using calcium imaging data (Cotton et al., 2013), we found that the relative magnitude of correlated activity varied significantly between the two states. There was a decrease in both signal and noise correlations during dilation vs. constriction (Figure 4G,H; 20% decrease in mean noise correlations, P<0.0001 and 16% decrease in mean signal correlations, P<0.01; n=21 sites, paired t-test used for all comparisons; see Methods). In summary, we found that responses to drifting gratings were more selective, more reliable, and less correlated during dilation compared to constriction.
Figure 4.
Orientation tuning is enhanced during pupil dilation. (A) Mean fluorescence image colored by orientation preferences of individual pixels; scale bar 50 μm. (B) Example pupil diameter trace (orange) with simultaneous calcium traces from segmented cells (black) and inferred firing rates (gray). Colored squares indicate the direction of drifting gratings. (C) Average tuning curves aligned to cells’ preferred direction for active (running and/or whisking) periods (green) and quiet (black) periods. Peak responses are increased (20%, P<10−12) and orientation selectivity is unchanged (7% decrease, P=.07). Error bands are SEM computed over cells (n=516). (D) Average tuning during pupil dilation (red) and constriction (blue) during quiet periods (excluding running and whisking). Orientation selectivity is increased (16% increase in mean OSI, P<10−6, E) and cells respond more reliably (28% increase in mean binned R2 values of stimulus responses of individual cells, P<10−15, F) during dilation compared to constriction. Across populations of neurons, mean noise correlations (G, P<10−4) and signal correlations (H, P<.01) are reduced during pupil dilation (n=21 sites). Paired t-test for all comparisons. Reliability and correlations are computed on 150 ms bins during stimulus presentations (see Supplemental Experimental Procedures).
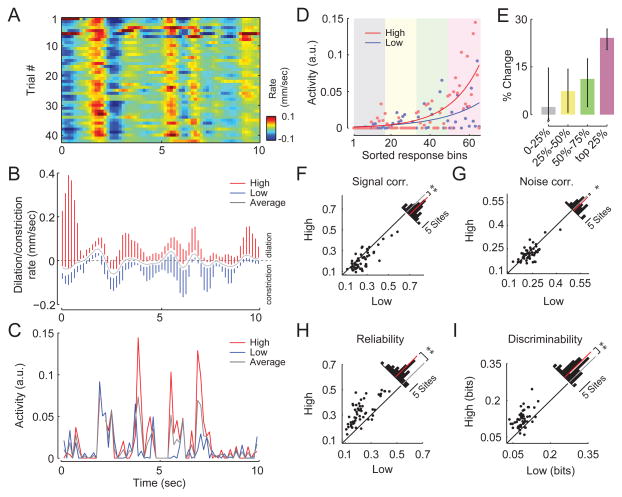
We next asked whether visual encoding of natural stimuli was also enhanced during desynchronized states indexed by pupil dilation. We presented multiple repetitions of short pre-recorded movies from a head-mounted camera of a mouse navigating an enriched environment. We performed calcium imaging in V1 (n=53 imaging sites in 7 mice). In addition to excluding running and whisking periods, we also removed saccades and any epochs where the eye deviated by more than ten degrees from its mean position. Correlating neural activity directly with changes in pupil size was not possible in this experiment because differences in brightness across frames elicited reliable dilations and constrictions of the pupil across multiple repetitions of the movie (r=0.61 correlation of pupil size across trials for one example recording session, Figure 5A). To account for the effects of frame-to-frame changes in luminance, we sorted the responses to each 150 ms segment of the movie by the rate of change of pupil diameter and compared the neural responses for the upper and lower quartiles of the rates of change within each time bin (Figure 5B). Here, we assumed that the average change in pupil size over multiple movie repetitions (gray trace in Figure 5B) was due to luminance changes in the stimulus, while the variability around this average (blue and red vertical lines in Figure 5B) reflected the trial-to-trial differences in cortical state. Thus, for each bin, we compared “high” trials, when the pupil was dilating faster than usual (or constricting slower than usual) to “low” trials, when the pupil was dilating slower (or constricting faster) than the average.
Figure 5.
Encoding of natural images is improved during pupil dilation. (A) Pupil dilation/constriction rates across multiple repetitions of a natural movie. (B) Mean (gray) and range in upper and lower quartiles (red and blue, respectively) of the pupil dilation/constriction rate for multiple presentations of a single movie (150-ms bins). Subsequent analyses compare neural responses in the upper quartile of pupil dilation/constriction rates (“high”) to the lower quartile (“low”). (C) Increase in mean activity during high trials compared to low trials for a single cell. (D) For each cell, movie frames are sorted by the mean neural response, not considering pupil dilation and constriction. Responses in high (red dots) and low (blue dots) conditions are fit with an exponential function (solid red and blue lines) to illustrate the selective increase in response to preferred stimuli. (E) Median change in firing rate in high trials compared to low trials (n=467 neurons, 95% confidence intervals) for least preferred (0–25%), intermediate (25–50% and 50–75%), and most-preferred (75–100%) frames for each cell. Responses to preferred frames are selectively enhanced. Mean signal (F) and noise (G) correlations decreased during the high condition, while reliability (H) and discriminability (I) were enhanced. Insets show histograms of absolute change with red bar indicating the mean difference (n=53 sites, **P<0.001, *P<0.05, t-test).
Visual responses were enhanced during high trials compared to low trials (example cell in Figure 5C). Consistent with the increased OSI we observed for oriented gratings, responses to preferred frames of the movie were selectively enhanced during high trials compared to low trials (Figure 5D,E). Again, the pupil was not larger during high trials than low trials (Figure S6). At the population level, both signal (Figure 5F; P<0.001) and noise (Figure 5G; P<0.05) correlations were reduced, and there was an increase in the reliability (Figure 5H; P<0.001) and discriminability (Figure 5I; P<0.001) of responses during high trials compared to low trials (t-test for all comparisons). In summary, consistent with the enhancement of responses to drifting gratings during dilation, responses to natural movies were more selective, more reliable, and less correlated during high trials.
Discussion
We performed whole-cell patching and two-photon calcium imaging in awake mice (Bennett et al., 2013; Niell and Stryker, 2010; Polack et al., 2013) and focused our analysis on quiet periods between epochs of running and whisking. During these periods, we observed small spontaneous fluctuations in pupil diameter on a timescale of one to two seconds, which tracked changes in intracellular dynamics of L2/3 neurons in both somatosensory (S1) and visual (V1) cortex. Vm was desynchronized while the pupil was dilating and was dominated by low-frequency oscillations while the pupil was constricting. Dilation was accompanied by activation of VIP+ and inhibition of SOM+ interneurons, a phenomenon that has been shown to be essential to the enhanced visual responses observed during running (Fu et al., 2014). Using two-photon calcium imaging, we found that dilation was associated with an enhancement of visual responses and a reduction in both noise and signal correlations across ensembles of neurons for both gratings and natural stimuli.
For almost half a century, pupillometry has been widely used to index covert changes in attention and effort in humans and nonhuman primates (Gilzenrat et al., 2010; Hess and Polt, 1960, 1964; Iriki et al., 1996; Kahneman, 1973; Kahneman and Beatty, 1966; Kristjansson et al., 2009; Onorati et al., 2013; Wierda et al., 2012). Surprising and provocative stimuli produce a transient increase in pupil size (Bradley et al., 2008; Hess and Polt, 1960, 1964; Libby et al., 1973; Preuschoff et al., 2011), and pupil dilation preceding stimulus onset is correlated with faster reaction times in psychophysical tasks (Kristjansson et al., 2009). Our results provide the first evidence of changes in intracellular membrane potential dynamics and neural population activity underlying these psychophysical effects, and emphasize the utility of pupil dilation and constriction as a proxy for non-invasively monitoring internal cortical states.
Previous authors have pointed out the analogy between desynchronization and enhanced sensory encoding during attention in primates, and the changes in cortical state during exploratory behaviors in mice (Harris and Thiele, 2011). Our results suggest that this analogy may be extended further, to encompass fluctuations in brain state in mice in the absence of overt behavioral changes. The ability to leverage mouse genetics to study the mechanisms that produce these state changes will be valuable, as will the ability to examine the potential dysregulation of brain state in mouse models of human disease.
Intracellular signatures of cortical activation were linked to pupil dilation across multiple cortical areas, suggesting that the effects we observe reflect a global change in brain state. Given the close relationship between pupil diameter, locus coeruleus activity and sympathetic tone (Aston-Jones et al., 1999; Bradley et al., 2008; Gilzenrat et al., 2010), our data suggest a role for norepinephrine in the regulation of cortical state. Consistent with this notion, a recent study has implicated norepinephrine in the desynchronization that occurs during running (Polack et al., 2013). However, the potential contribution of other neuromodulators such as acetylcholine (Goard and Dan, 2009; Pinto et al., 2013) is also strongly suggested by the fact that the VIP+ activation during running has been shown to depend on cholinergic input from the diagonal band of Broca (Fu et al., 2014).
Additional mechanisms may also contribute to the state changes we describe here, which differ in at least two important ways from the state associated with exploratory behavior: First, unlike the effects of running, the desynchronization during dilation is not accompanied by a significant depolarization of Vm. Second, we observe a qualitative difference in the effect of state on orientation tuning. During running, visual responses are enhanced at all orientations, while during dilation, responses at preferred orientations are enhanced without changing responses at orthogonal orientations, resulting in an increase in orientation selectivity. Further work will be necessary to uncover the mechanisms underlying these differences.
It is interesting to consider the potential computational role of continuous fluctuations in state during behaviorally quiescent periods. We speculate that during quiet wakefulness, the cortex may rapidly alternate between two distinct modes of information processing: a desynchronized state characterized by improved representation of feed-forward sensory information, and a synchronized state dominated by internally-generated low-frequency network oscillations. Interestingly, theoretical work has suggested that alternating between bottom-up and top-down processing may be an efficient learning mechanism for the cortex to build a representation of the external world (Hinton, 2010).
Experimental Procedures
All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Baylor College of Medicine. Briefly, mice were anesthetized and a 3mm craniotomy was made over the cortex as previously described (Froudarakis et al., 2014). For calcium imaging experiments, OGB1-AM was injected before sealing the craniotomy with a glass coverslip which in some cases was prepared with a small (~500 μm) hole to allow pipette access. Following surgery, the mouse was placed on a treadmill with its head restrained beneath the microscope objective. Locomotion was detected by treadmill movement, and eye and whisker movements were detected optically. Whole-cell in vivo patching was performed using borosilicate patch pipettes (6–10 MΩ) filled with a standard low Cl- internal solution (Jiang et al., 2013) as well as Alexa 488 or 598 for visualization. Visual stimuli were as follows: Figures 1–3, blank screen; Figure 4, full-field square wave gratings (0.04 cycles/degree at 2 Hz, 500 ms trials interleaved with 1 s luminance-matched blanks); Figure 5, natural scene movies collected as previously described (Froudarakis et al., 2014). Calcium imaging was performed using either a standard galvo-galvo (Sutter Instruments) or resonant scanner (Thor Labs) using a Ti-Sapphire laser (Coherent) exciting at either 800 or 1000 nm and equipped with either a 20× (1.0 NA, Olympus) or 25× (1.1 NA, Nikon) objective lens. Imaging data were motion-corrected and cell segmentation was manually supervised. A more detailed description of all experimental procedures can be found online in our Supplemental Experimental Procedures.
Supplementary Material
Figure S1, related to Figure 1. Saccades and power spectra during running and quiet wakefulness. (A) Example traces of simultaneously recorded treadmill speed (running in light green, quiet in dark green), pupil size (orange), whisking periods (gray background) and horizontal (x, black) and vertical (y, gray) eye movements. Detected saccades are shown in dark and light purple for movements in x and y, respectively. Saccades are much more frequent during running. Brief periods of missing data in the pupil traces are due to blinks or frames where the exact diameter and position of the pupil could not be defined due to blurring during a saccade. (B) Ratio of mean Vm power spectra between quiet wakefulness and active periods of whisking and running. Lower-frequency oscillations are enhanced during quiet wakefulness, especially in the 2–10 Hz range; and there is a wide-band increase in gamma frequencies during active behavior. (Vm power spectra for each condition estimated using Welch’s method; mean ± SEM over n=52 cells from both S1 and V1). (C) Ratio of mean power spectra during constriction and dilation in quiet wakefulness. Power in the lower frequencies in the 2–10 Hz range is higher during constriction, and there is a modest wide-band power increase in higher gamma frequencies during dilation (mean ± SEM, n=54 cells).
Figure S2, related to Figure 2. Temporal characteristics of dilating and constricting periods during quiet wakefulness. Dilation and constriction periods were defined by extrema in the low-pass (4th order Butterworth, 1 Hz cutoff) filtered pupil trace. Only periods lasting more than one second with an absolute mean dilation or constriction rate greater than 10 μm/s are included in this figure and all our analyses, as our ability to reliably detect fluctuations smaller than this was limited by the shape and sharpness of the pupil boundary and the camera’s resolution and frame rate. These periods comprised approximately 55% of our recordings during quiet wakefulness, and removing this threshold did not qualitatively change our findings. (A) Histogram of detected period durations. In general, constricting periods (top) were longer in duration (mean: 2.0 s, median 1.8 s) than dilating periods (bottom; mean: 1.6 s, median 1.5 s). (B) Histogram of intervals between sequential detected constricting (top) and dilating (bottom) periods (mean interval between constriction periods: 6.3 s, median: 4.6 s; mean interval between dilation periods: 8.4 s, median: 5.8 s). (C) Histogram of average frequency of detected constricting (top) and dilating (bottom) periods for n=84 mice, including some animals not used elsewhere in this study. On average during quiet wakefulness, constricting periods were detected at approximately 0.15 Hz while dilating periods were detected at approximately 0.09 Hz.
Figure S3, related to Figure 2. Single-cell example of raw data showing state change over multiple cycles of dilation and constriction. (A) Unfiltered continuous trace of pupil diameter over time (top), and filtered trace (bottom). Dilating (red), constricting (blue), and excluded (grey) periods are indicated on filtered trace. (B, top) Change in pupil diameter for multiple consecutive dilation and constriction cycles from trace in (A). (B, bottom) Mean change in pupil diameter averaged over all cycles. (C, top) Corresponding segments of 2–10 Hz Hilbert amplitude. Entire Hilbert amplitude trace is band-pass filtered between 0.1 Hz and 1 Hz, and segments are collected corresponding to each cycle in (B). (C, bottom) Mean 2–10 Hz amplitude change averaged over all cycles. Note the similarity in the mean change in 2–10 Hz amplitude for this single cell example with the phase-binned average in Figure 2D.
Figure S4, related to Figures 1 and 2. Relationship between pupil dilation and Vm dynamics persists when small whisker or treadmill movements are excluded, and is robustly present in mice habituated to the treadmill. (A) Same plot as Figure 2E, after removing all periods of detectable whisker or treadmill movements. Visual responsiveness (X-axis) plotted against desynchronization during dilation (Y-axis) for cells from S1, V1, and V1 in FVB mice. Because some data have been excluded, fewer cells show significant individual effects. (B) Overall, cells are still robustly desynchronized during dilation in S1 (7.3±1.8% reduction in 2–10 Hz Hilbert amplitude during dilation, mean ± SEM, P<.001) and V1 (13.9±3.2% reduction, P<.001, t-test). The effect of dilation was in the same direction for FVB cells, but was no longer significant. (C) Pupil diameter increases significantly during running in mice habituated to head restraint on the treadmill for five days for 30 minutes per day (n=113 running epochs in 3 mice; 102±5 μm, mean change ±SEM, P<.0001, paired t-test). (D) Similar to naïve mice, in habituated mice the amplitude of 2–10 Hz oscillations is reduced during active periods of whisking and running compared to quiet wakefulness (−1.5±.2 mV, P<.001; paired t-test; n=10 cells in 3 mice; experiments performed after habituation on the treadmill for 30 minutes per day x 5 days). (E,F) There is a strong relationship between pupil dilation/constriction and low-frequency oscillations in habituated mice, similar to naïve mice (2–10 Hz Hilbert amplitude reduced during dilation by 27±6%, p<.01, t-test).
Figure S5, related to Figure 3. Two types of SOM+ labeled cells based on electrophysiological properties have different functional properties. (A) Example traces (left) and spike waveform (right) for a pyramidal cell (top, black), a SOM+ labeled “Type II” cell (middle, cyan), and a SOM+ labeled “Type I” cell (bottom, blue). The Type II cell displays large fluctuations in Vm similar to a pyramidal cell, but has a narrow spike waveform. The Type I cell displays comparatively much lower membrane potential variance, and also has a narrow spike waveform. (B) Spike width plotted against the difference between the mean and minimum spontaneous Vm for cells of multiple types. Type I (blue) and Type II (cyan) SOM+ cells form two distinct clusters. Pyramidal cells (black) and VIP+ cells (green) are included for reference. Two SOM+ labeled cells with a pyramidal (spiny) morphology likely due to leakiness in the Cre line are indicated in red. (C) SOM Type I and Type II cells respond differently during active behavior (running and whisking). Type I cells are inhibited, while Type II cells are depolarized by running.
Figure S6, related to Figures 4 and 5. Pupil diameter is not larger during dilating or high trials, when visual responses are enhanced. (A) Pupil diameter is not significantly different for dilating trials compared to constricting trials for analysis in Figure 4. Each dot represents an individual imaging site (n=30 sites). (B) For analysis in Figure 5, pupil diameter is smaller during High trials (defined by dilation/constriction rate for each site), when visual responses are enhanced. Each dot is average over movie bins for each site (n=53 sites, P<0.001).
Pupil fluctuations during quiet and active periods. Fifty seconds of synchronized video from pupil and whisker cameras played at actual speed. Dilating and constricting periods are indicated by a red or blue outline, respectively. The movie begins during a period of quiet wakefulness. In the second half of the recording there are two active periods with locomotion (“RUNNING”) and whisking (“WHISKING”). Smaller detected whisker movements and treadmill movements (“EXCLUDE”) are excluded from the analysis in Figure S3, above.
Vm transitions between synchronized and desynchronized states during quiet wakefulness. Forty seconds of video of the eye, with the simultaneous filtered 2–10Hz Hilbert amplitude of Vm. Dilations and constrictions are indicated by a blue or red outline, respectively, and red or blue segments of the amplitude trace. The low-frequency amplitude tends to peak during pupil constriction. Black vertical line indicates time of current movie frame in Vm trace.
Acknowledgments
This work was supported by grants P30EY002520, T32EY07001, R01DA028525, DP1EY023176, and DP1OD008301 to A.S.T., the McKnight Scholar Award to A.S.T.; the Arnold & Beckman Foundation Young Investigator Award to A.S.T.; grants F30MH095440 and T32GM007330 to C.C.; and grant T32EB006350 to C.C. and D.Y. We thank Xiaolong Jiang for sharing his expertise in in vivo patching, James Cotton and Ryan Ash for helpful comments on the manuscript, and Ben Arenkiel for sharing the SST-IRES-Cre mouse line.
Footnotes
Author Contributions
J.R. collected and analyzed patching and calcium imaging data, performed pupil segmentation in all videos, and helped organize results and prepare the manuscript. M.F. helped build the behavioral apparatus, and collected and analyzed calcium imaging data with natural movie stimuli. C.C. contributed to writing the paper and helped guide analyses. D.Y. created the DataJoint framework for data organization and analyzed calcium imaging data for oriented gratings. G.D. collected data and helped with manuscript revisions. A.S.T. supervised experiments, analysis and preparation of the manuscript. M.F., C.C., and D.Y. contributed equally to this project.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aston-Jones G, Rajkowski J, Cohen J. Role of locus coeruleus in attention and behavioral flexibility. Biological Psychiatry. 1999;46:1309–1320. doi: 10.1016/s0006-3223(99)00140-7. [DOI] [PubMed] [Google Scholar]
- Bennett C, Arroyo S, Hestrin S. Subthreshold Mechanisms Underlying State-Dependent Modulation of Visual Responses. Neuron. 2013;80:350–357. doi: 10.1016/j.neuron.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Miccoli L, Escrig MA, Lang PJ. The pupil as a measure of emotional arousal and autonomic activation. Psychophysiology. 2008;45:602–607. doi: 10.1111/j.1469-8986.2008.00654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang B, Hawes NL, Hurd RE, Davisson MT, Nusinowitz S, Heckenlively JR. Retinal degeneration mutants in the mouse. Vision Res. 2002;42:517–525. doi: 10.1016/s0042-6989(01)00146-8. [DOI] [PubMed] [Google Scholar]
- Cotton RJ, Froudarakis E, Storer P, Saggau P, Tolias AS. Three-dimensional mapping of microcircuit correlation structure. Front Neural Circuits. 2013;7:151. doi: 10.3389/fncir.2013.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crochet S, Petersen CCH. Correlating whisker behavior with membrane potential in barrel cortex of awake mice. Nature Neuroscience. 2006;9:608–610. doi: 10.1038/nn1690. [DOI] [PubMed] [Google Scholar]
- Froudarakis E, Berens P, Ecker AS, Cotton RJ, Sinz FH, Yatsenko D, Saggau P, Bethge M, Tolias AS. Population code in mouse V1 facilitates readout of natural scenes through increased sparseness. Nat Neurosci. 2014;17:851–857. doi: 10.1038/nn.3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Tucciarone Jason M, Espinosa JS, Sheng N, Darcy Daniel P, Nicoll Roger A, Huang ZJ, Stryker Michael P. A Cortical Circuit for Gain Control by Behavioral State. Cell. 2014;156:1139–1152. doi: 10.1016/j.cell.2014.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentet LJ, Kremer Y, Taniguchi H, Huang ZJ, Staiger JF, Petersen CCH. Unique functional properties of somatostatin-expressing GABAergic neurons in mouse barrel cortex. Nature Neuroscience. 2012;15:607–612. doi: 10.1038/nn.3051. [DOI] [PubMed] [Google Scholar]
- Gilzenrat MS, Nieuwenhuis S, Jepma M, Cohen JD. Pupil diameter tracks changes in control state predicted by the adaptive gain theory of locus coeruleus function. Cognitive, Affective, & Behavioral Neuroscience. 2010;10:252–269. doi: 10.3758/CABN.10.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goard M, Dan Y. Basal forebrain activation enhances cortical coding of natural scenes. Nat Neurosci. 2009;12:1444–1449. doi: 10.1038/nn.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould IC, Rushworth MF, Nobre AC. Indexing the graded allocation of visuospatial attention using anticipatory alpha oscillations. J Neurophysiol. 2011;105:1318–1326. doi: 10.1152/jn.00653.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant RA, Mitchinson B, Prescott TJ. The development of whisker control in rats in relation to locomotion. Developmental psychobiology. 2012;54:151–168. doi: 10.1002/dev.20591. [DOI] [PubMed] [Google Scholar]
- Grent-’t-Jong T, Boehler CN, Kenemans JL, Woldorff MG. Differential functional roles of slow-wave and oscillatory-alpha activity in visual sensory cortex during anticipatory visual-spatial attention. Cerebral cortex (New York, NY: 1991) 2011;21:2204–2216. doi: 10.1093/cercor/bhq279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KD, Thiele A. Cortical state and attention. Nature Reviews Neuroscience. 2011;12:509–523. doi: 10.1038/nrn3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hei X, Stoelzel CR, Zhuang J, Bereshpolova Y, Huff JM, Alonso JM, Swadlow HA. Directional selective neurons in the awake LGN: Response properties and modulation by brain state. J Neurophysiol. 2014;112:362–373. doi: 10.1152/jn.00121.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess EH, Polt JM. Pupil size as related to interest value of visual stimuli. Science. 1960;132:349–350. doi: 10.1126/science.132.3423.349. [DOI] [PubMed] [Google Scholar]
- Hess EH, Polt JM. Pupil Size in Relation to Mental Activity during Simple Problem-Solving. Science. 1964;143:1190–1192. doi: 10.1126/science.143.3611.1190. [DOI] [PubMed] [Google Scholar]
- Hinton GE. Learning to represent visual input. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2010;365:177–184. doi: 10.1098/rstb.2009.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Cavendish JZ, Agmon A. Not all that glitters is gold: off-target recombination in the somatostatin-IRES-Cre mouse line labels a subset of fast-spiking interneurons. Front Neural Circuits. 2013;7:195. doi: 10.3389/fncir.2013.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iriki A, Tanaka M, Iwamura Y. Attention-induced neuronal activity in the monkey somatosensory cortex revealed by pupillometrics. Neuroscience Research. 1996;25:173–181. doi: 10.1016/0168-0102(96)01043-7. [DOI] [PubMed] [Google Scholar]
- Jiang X, Wang G, Lee AJ, Stornetta RL, Zhu JJ. The organization of two new cortical interneuronal circuits. Nat Neurosci. 2013;16:210–218. doi: 10.1038/nn.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahneman D. Attention and Effort. Englewood Cliffs, NJ: Prentice-Hall Inc; 1973. [Google Scholar]
- Kahneman D, Beatty J. Pupil diameter and load on memory. Science. 1966;154:1583–1585. doi: 10.1126/science.154.3756.1583. [DOI] [PubMed] [Google Scholar]
- Kristjansson SD, Stern JA, Brown TB, Rohrbaugh JW. Detecting phasic lapses in alertness using pupillometric measures. Applied ergonomics. 2009;40:978–986. doi: 10.1016/j.apergo.2009.04.007. [DOI] [PubMed] [Google Scholar]
- Lee SH, Dan Y. Neuromodulation of Brain States. Neuron. 2012;76:209–222. doi: 10.1016/j.neuron.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby WL, Jr, Lacey BC, Lacey JI. Pupillary and cardiac activity during visual attention. Psychophysiology. 1973;10:270–294. doi: 10.1111/j.1469-8986.1973.tb00526.x. [DOI] [PubMed] [Google Scholar]
- Niell CM, Stryker MP. Modulation of visual responses by behavioral state in mouse visual cortex. Neuron. 2010;65:472–479. doi: 10.1016/j.neuron.2010.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onorati F, Barbieri R, Mauri M, Russo V, Mainardi L. Characterization of affective states by pupillary dynamics and autonomic correlates. Frontiers in Neuroengineering. 2013;6:9. doi: 10.3389/fneng.2013.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto L, Goard MJ, Estandian D, Xu M, Kwan AC, Lee SH, Harrison TC, Feng G, Dan Y. Fast modulation of visual perception by basal forebrain cholinergic neurons. Nat Neurosci. 2013;16:1857–1863. doi: 10.1038/nn.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack PO, Friedman J, Golshani P. Cellular mechanisms of brain state-dependent gain modulation in visual cortex. Nature Neuroscience. 2013;16:1331–1339. doi: 10.1038/nn.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulet JFA, Petersen CCH. Internal brain state regulates membrane potential synchrony in barrel cortex of behaving mice. Nature. 2008;454:881–885. doi: 10.1038/nature07150. [DOI] [PubMed] [Google Scholar]
- Preuschoff K, t Hart BM, Einhauser W. Pupil Dilation Signals Surprise: Evidence for Noradrenaline’s Role in Decision Making. Frontiers in neuroscience. 2011;5:115. doi: 10.3389/fnins.2011.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohenkohl G, Nobre AC. alpha oscillations related to anticipatory attention follow temporal expectations. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:14076–14084. doi: 10.1523/JNEUROSCI.3387-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi H, He M, Wu P, Kim S, Paik R, Sugino K, Kvitsiani D, Fu Y, Lu J, Lin Y, et al. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron. 2011;71:995–1013. doi: 10.1016/j.neuron.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierda SM, Rijn Hv, Taatgen NA, Martens S. Pupil dilation deconvolution reveals the dynamics of attention at high temporal resolution. Proceedings of the National Academy of Sciences. 2012;109:8456–8460. doi: 10.1073/pnas.1201858109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagha E, Casale AE, Sachdev RN, McGinley MJ, McCormick DA. Motor cortex feedback influences sensory processing by modulating network state. Neuron. 2013;79:567–578. doi: 10.1016/j.neuron.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang J, Bereshpolova Y, Stoelzel CR, Huff JM, Hei X, Alonso JM, Swadlow HA. Brain state effects on layer 4 of the awake visual cortex. The Journal of Neuroscience. 2014;34:3888–3900. doi: 10.1523/JNEUROSCI.4969-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1, related to Figure 1. Saccades and power spectra during running and quiet wakefulness. (A) Example traces of simultaneously recorded treadmill speed (running in light green, quiet in dark green), pupil size (orange), whisking periods (gray background) and horizontal (x, black) and vertical (y, gray) eye movements. Detected saccades are shown in dark and light purple for movements in x and y, respectively. Saccades are much more frequent during running. Brief periods of missing data in the pupil traces are due to blinks or frames where the exact diameter and position of the pupil could not be defined due to blurring during a saccade. (B) Ratio of mean Vm power spectra between quiet wakefulness and active periods of whisking and running. Lower-frequency oscillations are enhanced during quiet wakefulness, especially in the 2–10 Hz range; and there is a wide-band increase in gamma frequencies during active behavior. (Vm power spectra for each condition estimated using Welch’s method; mean ± SEM over n=52 cells from both S1 and V1). (C) Ratio of mean power spectra during constriction and dilation in quiet wakefulness. Power in the lower frequencies in the 2–10 Hz range is higher during constriction, and there is a modest wide-band power increase in higher gamma frequencies during dilation (mean ± SEM, n=54 cells).
Figure S2, related to Figure 2. Temporal characteristics of dilating and constricting periods during quiet wakefulness. Dilation and constriction periods were defined by extrema in the low-pass (4th order Butterworth, 1 Hz cutoff) filtered pupil trace. Only periods lasting more than one second with an absolute mean dilation or constriction rate greater than 10 μm/s are included in this figure and all our analyses, as our ability to reliably detect fluctuations smaller than this was limited by the shape and sharpness of the pupil boundary and the camera’s resolution and frame rate. These periods comprised approximately 55% of our recordings during quiet wakefulness, and removing this threshold did not qualitatively change our findings. (A) Histogram of detected period durations. In general, constricting periods (top) were longer in duration (mean: 2.0 s, median 1.8 s) than dilating periods (bottom; mean: 1.6 s, median 1.5 s). (B) Histogram of intervals between sequential detected constricting (top) and dilating (bottom) periods (mean interval between constriction periods: 6.3 s, median: 4.6 s; mean interval between dilation periods: 8.4 s, median: 5.8 s). (C) Histogram of average frequency of detected constricting (top) and dilating (bottom) periods for n=84 mice, including some animals not used elsewhere in this study. On average during quiet wakefulness, constricting periods were detected at approximately 0.15 Hz while dilating periods were detected at approximately 0.09 Hz.
Figure S3, related to Figure 2. Single-cell example of raw data showing state change over multiple cycles of dilation and constriction. (A) Unfiltered continuous trace of pupil diameter over time (top), and filtered trace (bottom). Dilating (red), constricting (blue), and excluded (grey) periods are indicated on filtered trace. (B, top) Change in pupil diameter for multiple consecutive dilation and constriction cycles from trace in (A). (B, bottom) Mean change in pupil diameter averaged over all cycles. (C, top) Corresponding segments of 2–10 Hz Hilbert amplitude. Entire Hilbert amplitude trace is band-pass filtered between 0.1 Hz and 1 Hz, and segments are collected corresponding to each cycle in (B). (C, bottom) Mean 2–10 Hz amplitude change averaged over all cycles. Note the similarity in the mean change in 2–10 Hz amplitude for this single cell example with the phase-binned average in Figure 2D.
Figure S4, related to Figures 1 and 2. Relationship between pupil dilation and Vm dynamics persists when small whisker or treadmill movements are excluded, and is robustly present in mice habituated to the treadmill. (A) Same plot as Figure 2E, after removing all periods of detectable whisker or treadmill movements. Visual responsiveness (X-axis) plotted against desynchronization during dilation (Y-axis) for cells from S1, V1, and V1 in FVB mice. Because some data have been excluded, fewer cells show significant individual effects. (B) Overall, cells are still robustly desynchronized during dilation in S1 (7.3±1.8% reduction in 2–10 Hz Hilbert amplitude during dilation, mean ± SEM, P<.001) and V1 (13.9±3.2% reduction, P<.001, t-test). The effect of dilation was in the same direction for FVB cells, but was no longer significant. (C) Pupil diameter increases significantly during running in mice habituated to head restraint on the treadmill for five days for 30 minutes per day (n=113 running epochs in 3 mice; 102±5 μm, mean change ±SEM, P<.0001, paired t-test). (D) Similar to naïve mice, in habituated mice the amplitude of 2–10 Hz oscillations is reduced during active periods of whisking and running compared to quiet wakefulness (−1.5±.2 mV, P<.001; paired t-test; n=10 cells in 3 mice; experiments performed after habituation on the treadmill for 30 minutes per day x 5 days). (E,F) There is a strong relationship between pupil dilation/constriction and low-frequency oscillations in habituated mice, similar to naïve mice (2–10 Hz Hilbert amplitude reduced during dilation by 27±6%, p<.01, t-test).
Figure S5, related to Figure 3. Two types of SOM+ labeled cells based on electrophysiological properties have different functional properties. (A) Example traces (left) and spike waveform (right) for a pyramidal cell (top, black), a SOM+ labeled “Type II” cell (middle, cyan), and a SOM+ labeled “Type I” cell (bottom, blue). The Type II cell displays large fluctuations in Vm similar to a pyramidal cell, but has a narrow spike waveform. The Type I cell displays comparatively much lower membrane potential variance, and also has a narrow spike waveform. (B) Spike width plotted against the difference between the mean and minimum spontaneous Vm for cells of multiple types. Type I (blue) and Type II (cyan) SOM+ cells form two distinct clusters. Pyramidal cells (black) and VIP+ cells (green) are included for reference. Two SOM+ labeled cells with a pyramidal (spiny) morphology likely due to leakiness in the Cre line are indicated in red. (C) SOM Type I and Type II cells respond differently during active behavior (running and whisking). Type I cells are inhibited, while Type II cells are depolarized by running.
Figure S6, related to Figures 4 and 5. Pupil diameter is not larger during dilating or high trials, when visual responses are enhanced. (A) Pupil diameter is not significantly different for dilating trials compared to constricting trials for analysis in Figure 4. Each dot represents an individual imaging site (n=30 sites). (B) For analysis in Figure 5, pupil diameter is smaller during High trials (defined by dilation/constriction rate for each site), when visual responses are enhanced. Each dot is average over movie bins for each site (n=53 sites, P<0.001).
Pupil fluctuations during quiet and active periods. Fifty seconds of synchronized video from pupil and whisker cameras played at actual speed. Dilating and constricting periods are indicated by a red or blue outline, respectively. The movie begins during a period of quiet wakefulness. In the second half of the recording there are two active periods with locomotion (“RUNNING”) and whisking (“WHISKING”). Smaller detected whisker movements and treadmill movements (“EXCLUDE”) are excluded from the analysis in Figure S3, above.
Vm transitions between synchronized and desynchronized states during quiet wakefulness. Forty seconds of video of the eye, with the simultaneous filtered 2–10Hz Hilbert amplitude of Vm. Dilations and constrictions are indicated by a blue or red outline, respectively, and red or blue segments of the amplitude trace. The low-frequency amplitude tends to peak during pupil constriction. Black vertical line indicates time of current movie frame in Vm trace.