Abstract
Purpose of review
Interleukin-6 (IL-6) has emerged as a cytokine involved in cachexia progression with some cancers. This review will present recent breakthroughs in animal models and humans related to targeting IL-6 as a cancer cachexia therapy.
Recent Findings
IL-6 can target adipose, skeletal muscle, gut, and liver tissue, which can all affect cachectic patient recovery. IL-6 trans-signaling through the soluble IL-6r has the potential to amplify IL-6 signaling in the cachectic patient. In skeletal muscle chronic IL-6 exposure induces proteasome and autophagy protein degradation pathways that lead to wasting. IL-6 is also indirectly associated with AMPK and NF-κB activation. Several mouse cancer models have clearly demonstrated that blocking IL-6 and associated signaling can attenuate cachexia progression. Additionally, pharmaceuticals targeting IL-6 and associated signaling can relieve some cachectic symptoms in cancer patients. Research with cachectic mice has demonstrated that exercise and nutraceutical administration can interact with chronic IL-6 signaling during cachexia progression.
Summary
IL-6 remains a promising therapeutic strategy for attenuating cachexia progression with many types of cancer. However, improvement of this treatment will require a better understanding of the indirect and direct effects of IL-6 as well as its tissue specific actions in the cancer patient.
Keywords: IL-6 therapies, cancer cachexia, skeletal muscle, Liver, Gut
I. Introduction
Cancer cachexia is wasting syndrome that occurs in approximately 80% of cancer patients and is the primary cause of death for 22–30% of all cancer patients [1, 2]. A significant complication related to prevention of cachexia is that cancer patients are not usually diagnosed with cachexia until they have lost more than 5-7% of body mass [3, 4]. Additionally, the radiochemotherapy used to treat cancer can exacerbate cachexia progression in some patients which may ultimately affect patient outcomes [3, 5]. Thus cancer cachexia can severely diminish quality of life in cancer survivors due to a severe loss of skeletal muscle mass [1, 6]. Therefore, the future identification of therapies to treat cachexia will not only involve the attenuation of wasting, but also target muscle mass recovery after wasting has occurred. To this end, nutritional or anabolic treatments have only been partially effective in attenuating cachectic muscle mass loss [7]. Recovery of muscle mass has not been widely examined. Although several systemic hallmarks (anemia, hypogonadism, insulin resistance) are associated with cancer cachexia, chronic inflammation has been widely investigated as an important regulator of wasting [1, 8]. Chronic inflammation in cachectic patients is often a combination of both tumor and host derived factors. A multitude of cytokines including; TNF–α, IL-1β IL-6, glucocorticoids, and myostatin have been implicated in facilitating a cachectic state [1, 2, 9]. Researchers have been examining potential cachexia treatments for some time and extensive research has emerged on the role of individual cytokines for regulating cachexia progression in rodent cancer models. IL-6 has emerged a major player in cancer cachexia progression with its levels correlating to survival time in patients [10]. The purpose of this review is to present recent breakthroughs in animal models and humans related to targeting IL-6 as a therapy for treatment of cancer cachexia.
IL-6 Signaling: Classical IL-6 Signaling
IL-6 is a pleiotropic cytokine essential for wound healing and regeneration in mitotic tissues like skin and liver [11-13]. It is also associated with hypertrophy in post-mitotic tissues, such as muscle [14]. IL-6 function involves leukocyte activation, particularly through priming of macrophages towards an anti–inflammatory phenotype. IL-6 has also been implicated in muscle wasting, tumorigenesis and the production of liver acute phase proteins (APPs) [15, 16]. These paradoxical effects of IL-6 have been attributed to a temporal function, based on acute versus chronic IL-6 exposure [17]. However the specific signaling intermediates responsible for the IL-6 signaling profile are not well understood.
The IL-6 cytokine family shares the secondary receptor glycoprotein 130 (gp130). The IL-6/mIL-6R (membrane IL-6R) complex recruits two gp130 subunits to induce downstream JAK/STAT and ERK signaling. STAT-3 phosphorylation leads to the transcription of SOCS-3 – a negative regulator of IL-6 signaling [18, 19](Fig 1). Classical IL-6 signaling, limited by cellular expression of mIL-6R leads to proliferation, survival, regeneration and acute phase response (APR) in target tissues [20]. However, in chronic conditions like cancer cachexia, prolonged activation of proliferation, survival and APR can lead to tumorigenesis and hypermetabolism, leading to recruitment of the adaptive immune system and soluble IL-6R (sIL-6R) signaling.
Figure 1. IL-6 signaling and its downstream targets in skeletal muscle.
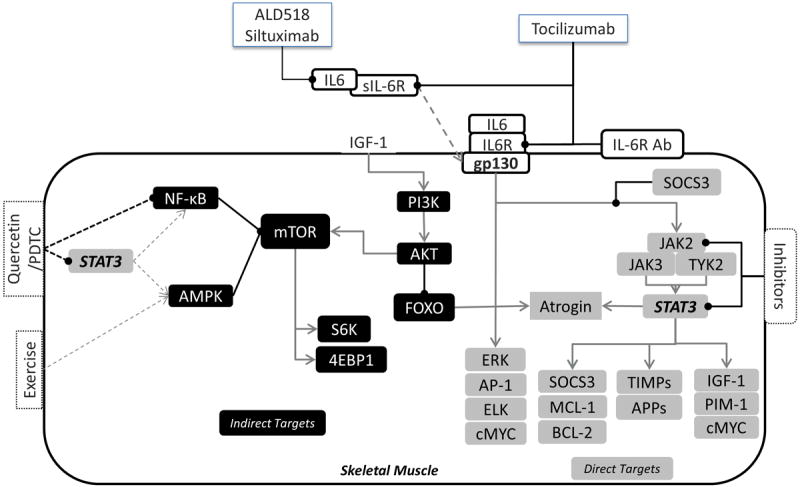
The IL-6/IL-6R complex or IL6/sIL-6R complex can signal through the membrane bound gp130 receptor to activate both the STAT-3 and ERK cascade (Direct targets in gray). Activation of the IL – 6 signaling cascade leads to the activation of various proliferative, anti-inflammatory and anti – apoptotic, and inflammatory genes downstream. Indirect targets (In Black) of IL – 6 include AMPK and NF – kB which are upregulated with IL -6 induced cachexia in vivo, however these cannot be attenuated by IL – 6 inhibitors. Pre – clinical trials have tested antibodies against the IL – 6 receptor and various inhibitors for the JAK/STAT-3 signaling to study the effect of IL – 6 on cachexia progression. Based on these studies currently antibodies against IL -6 (ALD518 and siltuximab) and its receptor (Tocilizumab) are being tested for attenuation of cachexia in patients. The black arrows ending in a circle represent inhibitory pathways. The gray arrow ending in a “V” represent activation pathways
IL-6 Signaling: A Role for Trans Signaling
Coupled to the complexity of IL-6 signaling are its far-reaching effects on tissues lacking the IL-6R [21, 22]. Acute cellular response to IL-6 under non-inflammatory conditions can be limited by the expression level of the mIL-6R [20-22]. Tissue IL-6 sensitivity can, however, be increased by the “trans – IL-6 signaling cascade” [21, 22] mediated by the sIL-6R. The sIL-6R is generated by mIL-6R cleavage, also described as IL-6R shedding, and orchestrated by neutrophils and macrophages[21]. Hence, the trans – IL-6 signaling pathway is associated with pro-inflammatory effects of IL-6 signaling observed under disease and autoimmune conditions [20]. Thus inhibition of the mIL-6R alone is insufficient to block IL-6 signaling and therapies targeting IL-6 regulation of cancer cachexia progression will likely need to account for the differential effects of classical and trans – IL-6 signaling in various tissues[21, 22].
Skeletal Muscle as a Target of IL-6
A large body of work in rodents has demonstrated that muscle wasting with cancer cachexia is a double edge sword that involves protein synthesis suppression and activation of several protein degradation pathways [23-26]. The ApcMin/+ and C26 tumor-implanted mice have an established IL-6 dependent loss of skeletal muscle during cancer cachexia[9, 27, 28]. Muscle wasting in these models corresponds with an increase in muscle STAT-3 and NF-κB signaling that is linked to the induction ubiquitin-proteasome degradation and autophagy. In ApcMin/+ mice, Atrogin1, a muscle specific E3 ligase, is induced with as little as 5% body weight loss; however the classical disuse marker MURF1 is not increased. As the mice transition to a more cachectic state, when levels of plasma IL-6 are chronically high[29], ATP-independent mechanisms, such as autophagy, are also involved in skeletal muscle breakdown. In the cachectic ApcMin/+ mouse both ubiquitin-proteasome and autophagy protein degradation processes can be suppressed by systemic IL-6R antibody (IL-6RAb) administration [29].
Unlike protein degradation, a clear linkage between IL-6 and STAT-3 activation has not been established for the suppression of muscle protein synthesis (MPS). However, IL-6 may indirectly inhibit MPS via IGF-1 suppression and AMPK activation [25, 29, 30]. Cachexia also induces the loss of muscle mitochondrial content [26, 31], which may be related to chronic activation of muscle AMPK signaling and mTOR suppression. Administration of an AMPK inhibitor to C2C12 cells attenuates IL-6 inhibition of mTOR signaling [27]. In the cachectic ApcMin/+ mouse inhibition of IL-6 signaling through systemic administration of IL-6RAb attenuates further body weight and muscle loss without rescuing MPS. Additionally, the activation of mTOR signaling by both glucose and exercise is suppressed in the cachectic ApcMin/+ mouse [23, 26, 27]. However, this may be an indirect effect of IL-6, as IL-6 administration to C2C12 myotubes does not inhibit insulin stimulation of mTOR signaling [27]. Further work is needed to establish if suppressed MPS is a response to elevated systemic inflammation represented by classical inflammatory markers like NF-κB signaling pathways. The simultaneous inhibition of both NF-κB and STAT-3 attenuates MPS suppression in cachectic mice [32].
Unlike rodent models, pathways regulating skeletal muscle protein turnover in the cachectic cancer patient have been difficult to ascertain and are still being established. Some recent studies show that induction of autophagy, independent of proteasome pathways, is sufficient to induce wasting in cachectic patients [33, 34]. Additionally, colon cancer patients with reduced muscle mass have demonstrated a trend for increased protein breakdown and decreased induction of post-prandial MPS [35]. However, male non-small cell lung cancer patients with elevated IL-6 levels were able to improve whole body net protein balance through increased synthesis stimulated by hyperaminoacidemia [36]. There is also evidence against STAT3 and NF-κB signaling being associated with the progression of cancer cachexia in abdominal muscle from cancer patients [37]. Such discrepancies between rodent and human signaling cascades, coupled with genetic polymorphisms in the IL-6/IL-6R genes could limit efficacy of pre-clinical drugs in human trials [37, 38]. Further work is needed to establish if these differences between cancer patients and rodent models are related to muscle phenotype or the type of cancer.
The Liver as a Target of IL-6
The liver governs a host of metabolic and inflammatory processes in the body and is known to hypertrophy, as peripheral tissues atrophy in cachectic patients [1, 7, 29]. Given the hypermetabolic and pro-inflammatory etiology of cachexia, disruption of liver functions could play a role in cachexia progression. Liver expression of TGFβ family transcription factor TSC22D4 correlates with body weight loss and VLDL hypo-secretion in the IL-6 dependent C26 cancer cachexia model [39]. Chronic IL-6 exposure during cachexia is also known to induce anemia and produce APPs, which provides further evidence of liver dysfunction [1, 28]. In fact serum CRP, hemoglobin and immunoglobulin levels are considered while calculating cachexia index in patients [25]. Liver dysfunction related to the disrupted metabolic state with cancer cachexia may also be related to the tumor induced Warburg effect [1, 28]. Interestingly, hepatic tissue is known to have a higher level of IL-6 expression even under normal disease-free conditions. Constitutively induced hepatic IL-6 expression helps maintain the liver dendritic cells in an immature state and tolerate basal endotoxin exposure from the portal blood. Activation of hepatic dendritic cells can lead to immune cell recruitment and fibrosis [40]. However, loss of systemic IL-6 in mice also leads to liver fibrosis that is associated with insulin resistance and obesity with aging and further complicates the effect of IL-6 on the liver [41]. Thus liver function could play an important role in cachexia progression, but since cachexia is characterized as a wasting disorder most of the research has focused on muscle and fat tissue restoration, while the effect of cachexia progression on the liver, which may directly impact muscle and fat mass, is largely unknown.
The Gut as a Target of IL – 6
The small intestine plays an important role in absorption of nutrients from the food and its optimal functioning is essential during the hypermetabolic cachectic state. While altered absorption with cachexia has not been clearly established, Puppa et. al. investigated the role of gut barrier dysfunction in the cachectic ApcMin/+ mouse and found an association between increased plasma IL – 6 levels and gut permeability. This elevated gut permeability with severe cachexia could point to a disruption of epithelial cell tight junction proteins. Since plasma endotoxin levels were found elevated only in the severely cachectic mice, it can be hypothesized that increased gut permeability could lead to seepage of bacterial endotoxin into the blood stream[42]. However, it is not known if treatment with IL-6 inhibitors could attenuate GBD in the cachectic ApcMin/+ mouse. Another emerging research area is the composition of gut microbiota with cancer cachexia [43, 44]. Cachectic mice have suppressed levels of cecal Lactobacillus spp., a bacteria known for its immunomodulatory properties [44]. Restoring the levels of these bacteria led to suppression of systemic levels of IL-6 and MCP -1 and reduced muscle atrophy by inhibition of both proteasome and autophagy pathways in the gastrocnemius muscle [44, 45]. Thus modulating the gut bacterial environment can impact cachexia progression in mice. Further research is warranted to elucidate the interactions between muscle, liver and gut during cachexia progression (Fig 2).
Figure 2. Representative figure demonstrating the systemic effect of IL - 6 dependent cachexia in vivo.
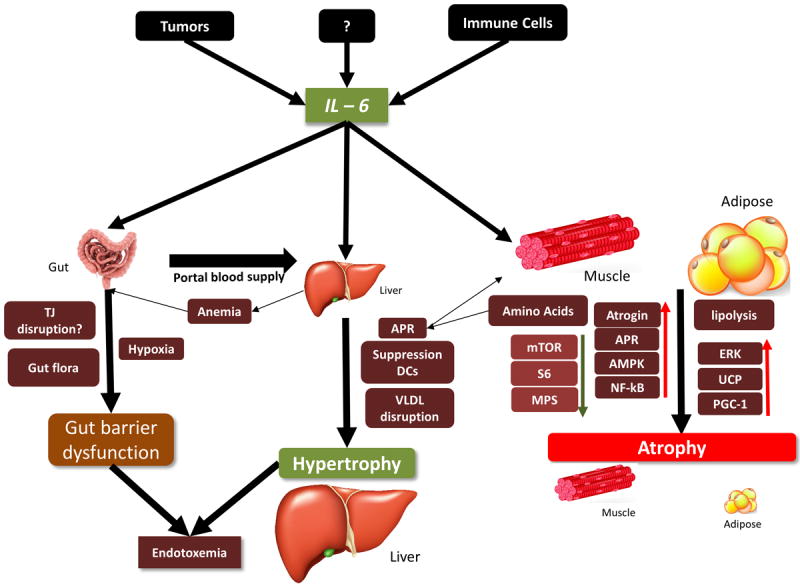
IL - 6 source mainly from the tumor and immune cells like macrophages affects various organs like adipose, muscle liver and gut with contrasting effects. Adipose tissue undergoes rapid lipolysis under chronic IL-6 conditions [6, 13, 19]. Muscle demonstrates activation of inflammatory and degradation pathways with a suppression of protein synthesis signaling. The liver demonstrates hypertrophy, an upregulation of innate inflammatory pathways, with a possible suppression of adaptive responses and disruption in lipid signaling. The gut is affected by chronic IL - 6 exposure with increased gut permeability possible due to a disruption in tight junction proteins and anemia. Both increased gut permeability and liver dysfunction may contribute to endotoxemia seen in the later stages of cachexia. Abbreviations: TJ = Tight junctions, APR = Acute Phase Response, VLDL = Very low density lipoproteins. Images in the figure were downloaded and adapted from an online image library: dreamstime royaltyfree stock photos. http://www.dreamstime.com/photos-images.html.
IL-6 Targeted Therapeutics: Progress in Preclinical Models of Cancer Cachexia
Mouse models of cancer cachexia like the ApcMin/+ and C-26 adenocarcinoma implant models, have demonstrated a clear dependence on IL-6, with IL-6 signaling inhibition being able to attenuate cachexia progression [1, 2]. However, another widely used mouse implant model, Lewis Lung Carcinoma, has been equivocal related to IL-6 dependence [32]. Recent studies demonstrate the importance of downstream targets of JAK/STAT signaling for the regulation of muscle loss [9]. Inhibition of STAT-3 in vitro abolishes IL-6 induced myotube atrophy [9], however, in vivo; studies report an attenuation rather than eradication of muscle wasting. Incomplete inhibition of STAT-3 and/or STAT–3 independent signaling has been implicated in this discrepancy between the in vivo and in vitro results [9]. Until more specific downstream targets of IL-6 induced signaling cascade are identified, inhibition of IL-6R and JAK/STAT-3 intermediates seems to be the best approach to attenuate muscle atrophy during cachexia.
IL-6 dependent models of cancer cachexia are also associated with the induction of signaling pathways like NF-κB, AMPK, and TLR4 that are not directly downstream of IL-6 signaling, but are induced as IL-6 levels increase, with cachexia progression. Recently, these indirect pathways have been examined for their effect on IL-6 dependent cachexia. Inhibition of NF-κB along with STAT-3 by Pyrrolidine dithiocarbamate (PDTC) is able to attenuate the suppression of protein synthesis in cachectic mouse muscle [23]. Quercetin, a dietary flavanol can suppress systemic inflammation by inhibition of NF-κB in cachexia models[46]. In ApcMin/+ mouse however quercetin attenuated muscle atrophy by lowering plasma IL-6 and muscle STAT-3 activation, independent of muscle NF-κB activation[47]. This suggests that in models of IL-6 induced cachexia; NF- κB activation is additive but not essential to regulate muscle wasting. There may also be a role for indirect IL-6 signaling through muscle AMPK activation, which can suppress muscle mTOR/S6 signaling related to protein synthesis [24, 27]. Interestingly, exercise may be beneficial for countering the effects of IL-6 on muscle wasting[48]. Treadmill exercise ablates IL-6-indued bodyweight and muscle loss in ApcMin/+ mice, even though muscle STAT-3 and NF-κB signaling are activated [17]. There is the possibility that the chronic inflammatory response thought to be detrimental with cachexia could be modulated by altering the indirect effects of IL-6 in various target tissues during cancer cachexia.
L-6 targeted Therapeutics: Clinical Studies
Advanced and terminal cancer patients have exhibited elevated levels of plasma IL-6, which have correlated with body weight loss in these patients and are associated with anemia, anorexia and depression [49]. Clinical trials with drugs such as ghrelin, and thalidomide combined with megasterol acetate do not directly target IL-6, but have demonstrated promise by attenuating some cachectic symptoms in cancer patients, such as appetite, weight gain and a feeling of well-being [50-52]. However, these therapies have the potential to indirectly suppress systemic IL-6 levels or muscle IL-6 production [51, 53]. Recent targeted human therapies for cachexia related to IL-6 have been focused on developing humanized antibodies that could rescue cachexia symptoms. Earlier attempts with IL-6 antibodies have had some success rescuing lean body mass; however its effect on muscle mass is unknown and could be misleading since visceral organs hypertrophy in cachexia [49, 53, 54]. Recent clinical studies against humanized IL-6RAb, toculizumab, have been promising, demonstrating attenuated muscle loss, reduced plasma IL-6 levels and a restoration of plasma albumin levels, without altering tumor proliferation in humans [3, 55, 56]. However, the side effects of these studies need to be evaluated as suppression of IL – 6 can compromise the immune response to infection affecting patient recovery and quality of life[57].
Conclusion
Recent research in animal models and cancer patients has further established IL-6’s involvement in the regulation of cachexia progression with some cancers. Additionally, IL-6 trans-signaling through the soluble IL-6R has the potential to amplify IL-6 signaling in cachectic patients. Chronic IL-6 exposure has also shown to induce wasting in skeletal muscle by both direct and indirect activation of pathways involved in protein turnover, metabolism and inflammation. However, majority of cachexia research in pre-clinical studies is concentrated on the physiology of skeletal muscle while the effect of IL-6 signaling on organs like liver and gut are still being elucidated. IL-6 remains a promising therapeutic strategy for attenuating cachexia progression and has the potential to affect patient’s quality of life.
Key Points.
IL-6 signals through the IL-6/IL-6R/gp130 pathway to activate JAK/STAT-3 and ERK cascades associated with inflammatory and mitotic processes respectively. Prolonged activation of these cascades can however lead to wasting and tumorigenesis observed during cancer cachexia
Trans IL-6 signaling mediated by the soluble IL-6R is associated with the pro-inflammatory effects of IL-6, while the membrane bound receptor is associated with the anti-inflammatory and acute phase response. Cachexia progression is associated with an elevation of both pro-inflammatory and acute phase proteins.
Muscle as a target: Inhibition IL-6/IL-6R/STAT-3 cascade can attenuate muscle degradation pathways but does not affect the IL-6 dependent suppression of muscle protein synthesis in cachectic mice. Cachectic patients however can induce muscle wasting independent of STAT-3.
Liver and Gut as a target: Chronic exposure to IL-6 during cachexia progression leads to anemia and elevated plasma CRP and immunoglobulin levels pointing towards hepatic dysfunction. Elevated plasma IL-6 also affects gut microbiota and possibly elevates intestinal permeability with severe cachexia. Further work is needed to elucidate the role of these visceral organs in cachexia progression.
Therapeutic use: Targeted inhibition of IL-6 signaling cascade in rodent models attenuated muscle atrophy and have shown potential in human studies by increasing muscle mass, survival time and quality of life in cachectic patients However, large scale clinical trials are needed to rule out any side-effects of this treatment.
Acknowledgments
Dr. James Carson is currently being funded by the NCI grant RO1CA121249.
Funding Sources: Dr. James A. Carson is currently funded by the NCI grant RO1-121249.
Footnotes
Conflicts of Interest
There are no conflicts of interest.
The authors would also like to acknowledge Dreamstime.com for providing the gut, liver, fat and muscle cartoon figures depicted in Figure 2. The referral to the images is noted below:
Fat cells: <a href=“http://www.dreamstime.com/royalty-free-stock-image-fat-cells-image40092946#res8244673>”Photo Fat cells</a> - © Designua ∣ Dreamstime.com
Skeletal muscle: <strong> © <a href=‘http://www.dreamstime.com/designua_info’>Designua</a> ∣ <a href=‘http://www.dreamstime.com/’>Dreamstime.com</a></strong>
Liver: <strong> © <a href=‘http://www.dreamstime.com/jaeeho_info’>Jaeeho</a> ∣ <a href=‘http://www.dreamstime.com/’>Dreamstime.com</a></strong>
Gut : <strong> © <a href=‘http://www.dreamstime.com/rajcreationzs_info’>Rajcreationzs</a> ∣ <a href=‘http://www.dreamstime.com/’>Dreamstime.com</a></strong>
References and Recommended Reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
-
*
of special interest
-
**
of outstanding interest
- 1.Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab. 2012 Aug 8;16(2):153–66. doi: 10.1016/j.cmet.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 2.Onesti JK, Guttridge DC. Inflammation Based Regulation of Cancer Cachexia. Biomed Res Int. 2014 doi: 10.1155/2014/168407. 168407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ando K, Takahashi F, Motojima S, et al. Possible role for tocilizumab, an anti-interleukin-6 receptor antibody, in treating cancer cachexia. J Clin Oncol. 2013 Feb 20;31(6):e69–72. doi: 10.1200/JCO.2012.44.2020. [DOI] [PubMed] [Google Scholar]
- 4.Gallagher IJ, Stephens NA, MacDonald AJ, et al. Suppression of skeletal muscle turnover in cancer cachexia: evidence from the transcriptome in sequential human muscle biopsies. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012 May 15;18(10):2817–27. doi: 10.1158/1078-0432.CCR-11-2133. Research Support, Non-U.S Gov’t. [DOI] [PubMed] [Google Scholar]
- 5.Laine A, Iyengar P, Pandita TK. The role of inflammatory pathways in cancer-associated cachexia and radiation resistance. Mol Cancer Res. 2013 Sep;11(9):967–72. doi: 10.1158/1541-7786.MCR-13-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petruzzelli M, Schweiger M, Schreiber R, et al. A Switch from White to Brown Fat Increases Energy Expenditure in Cancer-Associated Cachexia. Cell Metab. 2014 Jul 15; doi: 10.1016/j.cmet.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 7.Fearon K, Arends J, Baracos V. Understanding the mechanisms and treatment options in cancer cachexia. Nat Rev Clin Oncol. 2013 Feb;10(2):90–9. doi: 10.1038/nrclinonc.2012.209. [DOI] [PubMed] [Google Scholar]
- 8.White JP, Puppa MJ, Narsale A, et al. Characterization of the male ApcMin/+ mouse as a hypogonadism model related to cancer cachexia. Biol Open. 2013;2(12):1346–53. doi: 10.1242/bio.20136544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonetto A, Aydogdu T, Jin X, et al. JAK/STAT3 pathway inhibition blocks skeletal muscle wasting downstream of IL-6 and in experimental cancer cachexia. American journal of physiology Endocrinology and metabolism. 2012 Aug 1;303(3):E410–21. doi: 10.1152/ajpendo.00039.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suh SY, Choi YS, Yeom CH, et al. Interleukin-6 but not tumour necrosis factor-alpha predicts survival in patients with advanced cancer. Support Care Cancer. 2013 Nov;21(11):3071–7. doi: 10.1007/s00520-013-1878-4. [DOI] [PubMed] [Google Scholar]
- 11.Sommer J, Engelowski E, Baran P, et al. Interleukin-6, but not the interleukin-6 receptor plays a role in recovery from dextran sodium sulfate-induced colitis. Int J Mol Med. 2014 Sep;34(3):651–60. doi: 10.3892/ijmm.2014.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tachibana S, Zhang X, Ito K, et al. Interleukin-6 is required for cell cycle arrest and activation of DNA repair enzymes after partial hepatectomy in mice. Cell Biosci. 2014;4(1):6. doi: 10.1186/2045-3701-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang Y, Ju D, Zhang M, et al. Interleukin-6 stimulates lipolysis in porcine adipocytes. Endocrine. 2008 Jun;33(3):261–9. doi: 10.1007/s12020-008-9085-7. [DOI] [PubMed] [Google Scholar]
- 14.Begue G, Douillard A, Galbes O, et al. Early activation of rat skeletal muscle IL-6/STAT1/STAT3 dependent gene expression in resistance exercise linked to hypertrophy. PloS one. 2013;8(2):e57141. doi: 10.1371/journal.pone.0057141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang K, Grivennikov SI, Karin M. Implications of anti-cytokine therapy in colorectal cancer and autoimmune diseases. Ann Rheum Dis. 2013 Apr;72(Suppl 2):ii100–3. doi: 10.1136/annrheumdis-2012-202201. [DOI] [PubMed] [Google Scholar]
- 16.Taniguchi K, Karin M. IL-6 and related cytokines as the critical lynchpins between inflammation and cancer. Semin Immunol. 2014 Feb;26(1):54–74. doi: 10.1016/j.smim.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Puppa MJ, W J, Velazquez KT, Baltgalvis KA, Sato S, Baynes JW, Carson JA. The effect of exercise on IL-6 induced cachexia in the Apc min/+ mouse. J Cachexia Saropenia Muscle. 2012;3:117–37. doi: 10.1007/s13539-011-0047-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carow B, Rottenberg ME. SOCS3, a Major Regulator of Infection and Inflammation. Front Immunol. 2014;5:58. doi: 10.3389/fimmu.2014.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White UA, Stephens JM. The gp130 receptor cytokine family: regulators of adipocyte development and function. Curr Pharm Des. 2011;17(4):340–6. doi: 10.2174/138161211795164202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scheller J, Garbers C, Rose-John S. Interleukin-6: from basic biology to selective blockade of pro-inflammatory activities. Semin Immunol. 2014 Feb;26(1):2–12. doi: 10.1016/j.smim.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Wolf J, Rose-John S, Garbers C. Interleukin-6 and its receptors: A highly regulated and dynamic system. Cytokine. 2014 Jun 28; doi: 10.1016/j.cyto.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 22.Scheller J, Chalaris A, Schmidt-Arras D, et al. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochimica et biophysica acta. 2011 May;1813(5):878–88. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 23.Puppa MJ, Murphy EA, Fayad R, et al. Cachectic skeletal muscle response to a novel bout of low-frequency stimulation. J Appl Physiol (1985) 2014 Apr 15;116(8):1078–87. doi: 10.1152/japplphysiol.01270.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Penna F, Bonelli Gabriella, Baccino Francesco M, et al. Chapter Eleven - Mechanism based Therapeutic Approaches to Cachexia. Elsevier; 2013. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki H, Asakawa A, Amitani H, et al. Cancer cachexia--pathophysiology and management. J Gastroenterol. 2013 May;48(5):574–94. doi: 10.1007/s00535-013-0787-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gordon BS, Kelleher AR, Kimball SR. Regulation of muscle protein synthesis and the effects of catabolic states. Int J Biochem Cell Biol. 2013 Oct;45(10):2147–57. doi: 10.1016/j.biocel.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.White JP, Puppa MJ, Gao S, et al. Muscle mTORC1 suppression by IL-6 during cancer cachexia: a role for AMPK. American journal of physiology Endocrinology and metabolism. 2013 May 15;304(10):E1042–52. doi: 10.1152/ajpendo.00410.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonetto A, Aydogdu T, Kunzevitzky N, et al. STAT3 activation in skeletal muscle links muscle wasting and the acute phase response in cancer cachexia. PloS one. 2011;6(7):e22538. doi: 10.1371/journal.pone.0022538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.White JP, Baynes JW, Welle SL, et al. The regulation of skeletal muscle protein turnover during the progression of cancer cachexia in the Apc(Min/+) mouse. PloS one. 2011;6(9):e24650. doi: 10.1371/journal.pone.0024650. Research Support, N.I.H., Extramural. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Egerman MA, Glass DJ. Signaling pathways controlling skeletal muscle mass. Crit Rev Biochem Mol Biol. 2014 Jan-Feb;49(1):59–68. doi: 10.3109/10409238.2013.857291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.White JP, P M, Sato S, Gao S, Price RL, Baynes JW, Kostek MC, Matesic LE, Carson JA. IL-6 regulation on skeletal muscle mitochondrial remodeling during cancer cachexia in the Apc min/+ mouse. Skelet Muscle. 2012;2(14) doi: 10.1186/2044-5040-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Puppa MJ, Gao S, Narsale AA, et al. Skeletal muscle glycoprotein 130’s role in Lewis lung carcinoma-induced cachexia. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2014 Feb;28(2):998–1009. doi: 10.1096/fj.13-240580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tardif N, Klaude M, Lundell L, et al. Autophagic-lysosomal pathway is the main proteolytic system modified in the skeletal muscle of esophageal cancer patients. Am J Clin Nutr. 2013 Dec;98(6):1485–92. doi: 10.3945/ajcn.113.063859. [DOI] [PubMed] [Google Scholar]
- 34.Stephens NA, Gallagher IJ, Rooyackers O, et al. Using transcriptomics to identify and validate novel biomarkers of human skeletal muscle cancer cachexia. Genome Med. 2010;2(1):1. doi: 10.1186/gm122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams JP, Phillips BE, Smith K, et al. Effect of tumor burden and subsequent surgical resection on skeletal muscle mass and protein turnover in colorectal cancer patients. Am J Clin Nutr. 2012 Nov;96(5):1064–70. doi: 10.3945/ajcn.112.045708. [DOI] [PubMed] [Google Scholar]
- 36.Winter A, MacAdams J, Chevalier S. Normal protein anabolic response to hyperaminoacidemia in insulin-resistant patients with lung cancer cachexia. Clin Nutr. 2012 Oct;31(5):765–73. doi: 10.1016/j.clnu.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 37.Johns N, Hatakeyama S, Stephens NA, et al. Clinical classification of cancer cachexia: phenotypic correlates in human skeletal muscle. PloS one. 2014;9(1):e83618. doi: 10.1371/journal.pone.0083618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruzzo A, Catalano V, Canestrari E, et al. Genetic modulation of the interleukin 6 (IL-6) system in patients with advanced gastric cancer: a background for an alternative target therapy. BMC Cancer. 2014;14:357. doi: 10.1186/1471-2407-14-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones A, Friedrich K, Rohm M, et al. TSC22D4 is a molecular output of hepatic wasting metabolism. EMBO Mol Med. 2013 Feb;5(2):294–308. doi: 10.1002/emmm.201201869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sumpter TL, Dangi A, Matta BM, et al. Hepatic stellate cells undermine the allostimulatory function of liver myeloid dendritic cells via STAT3-dependent induction of IDO. J Immunol. 2012 Oct 15;189(8):3848–58. doi: 10.4049/jimmunol.1200819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cressman DE, Greenbaum LE, DeAngelis RA, et al. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science. 1996 Nov 22;274(5291):1379–83. doi: 10.1126/science.274.5291.1379. [DOI] [PubMed] [Google Scholar]
- 42.Puppa MJ, W J, Sato S, Cairns M, Baynes JW, Carson JA. Gut barrier dysfunction in the Apc min/+ mouse model of colon cancer cachexia. Biochim Biophys Acta. 2012;1812(12):1601–6. doi: 10.1016/j.bbadis.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mi L, Lin J, Zheng H, et al. Bacterial translocation contributes to cachexia from locally advanced gastric cancer. Hepatogastroenterology. 2012 Oct;59(119):2348–51. doi: 10.5754/hge11810. [DOI] [PubMed] [Google Scholar]
- 44.Bindels LB, Delzenne NM. Muscle wasting: the gut microbiota as a new therapeutic target? Int J Biochem Cell Biol. 2013 Oct;45(10):2186–90. doi: 10.1016/j.biocel.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 45.Bindels LB, Beck R, Schakman O, et al. Restoring specific lactobacilli levels decreases inflammation and muscle atrophy markers in an acute leukemia mouse model. PloS one. 2012;7(6):e37971. doi: 10.1371/journal.pone.0037971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Srivastava DS, Dhaulakhandi DB. Role of NF-KB in Loss of Skeletal Muscle Mass in Cancer Cachexia and its Therapeutic Targets. American Journal of Cancer Biology. 2013;1(2):8–23. [Google Scholar]
- 47.Velazquez KT, Enos RT, Narsale AA, et al. Quercetin supplementation attenuates the progression of cancer cachexia in ApcMin/+ mice. J Nutr. 2014 Jun;144(6):868–75. doi: 10.3945/jn.113.188367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ulrich CM, Steindorf K, Berger NA, et al. Exercise, Energy Balance, and Cancer. Springer; New York: 2012. Biological Pathways Impacting Cancer Survival: Exercise as a Countermeasure for the Development and Progression of Cachexia; pp. 59–81. [Google Scholar]
- 49.Guo Y, Xu F, Lu T, et al. Interleukin-6 signaling pathway in targeted therapy for cancer. Cancer Treat Rev. 2012 Nov;38(7):904–10. doi: 10.1016/j.ctrv.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 50.Tuca A, Jimenez-Fonseca P, Gascon P. Clinical evaluation and optimal management of cancer cachexia. Crit Rev Oncol Hematol. 2013 Dec;88(3):625–36. doi: 10.1016/j.critrevonc.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 51.Wen HS, Li X, Cao YZ, et al. Clinical studies on the treatment of cancer cachexia with megestrol acetate plus thalidomide. Chemotherapy. 2012;58(6):461–7. doi: 10.1159/000346446. [DOI] [PubMed] [Google Scholar]
- 52.Amitani M, Asakawa A, Amitani H, et al. Control of food intake and muscle wasting in cachexia. Int J Biochem Cell Biol. 2013 Oct;45(10):2179–85. doi: 10.1016/j.biocel.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 53.Morley JE, von Haehling S, Anker SD. Are we closer to having drugs to treat muscle wasting disease? J Cachexia Sarcopenia Muscle. 2014 Jun;5(2):83–7. doi: 10.1007/s13539-014-0149-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bayliss TJ, Smith JT, Schuster M, et al. A humanized anti-IL-6 antibody (ALD518) in non-small cell lung cancer. Expert Opin Biol Ther. 2011 Dec;11(12):1663–8. doi: 10.1517/14712598.2011.627850. [DOI] [PubMed] [Google Scholar]
- 55.Hirata H, Tetsumoto S, Kijima T, et al. Favorable responses to tocilizumab in two patients with cancer-related cachexia. J Pain Symptom Manage. 2013 Aug;46(2):e9–e13. doi: 10.1016/j.jpainsymman.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 56.Ando K, Takahashi F, Kato M, et al. Tocilizumab, a proposed therapy for the cachexia of interleukin6-expressing lung cancer. PloS one. 2014;9(7):e102436. doi: 10.1371/journal.pone.0102436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berti A, Boccalatte F, Sabbadini MG, et al. Assessment of tocilizumab in the treatment of cancer cachexia. J Clin Oncol. 2013 Aug 10;31(23):2970. doi: 10.1200/JCO.2012.48.4147. [DOI] [PubMed] [Google Scholar]


