Abstract
We describe a titanium-mediated reaction for the convergent coupling of allenic alcohols with aromatic imines. Overall, the bond formation occurs at the central carbon of the allene, proceeds with net allylic transposition, and provides substituted 1,3-dienes bearing allylic amine functionality in a regio- and stereoselective fashion.
Keywords: Imines, allenes, cross-coupling, metallacycles, titanium
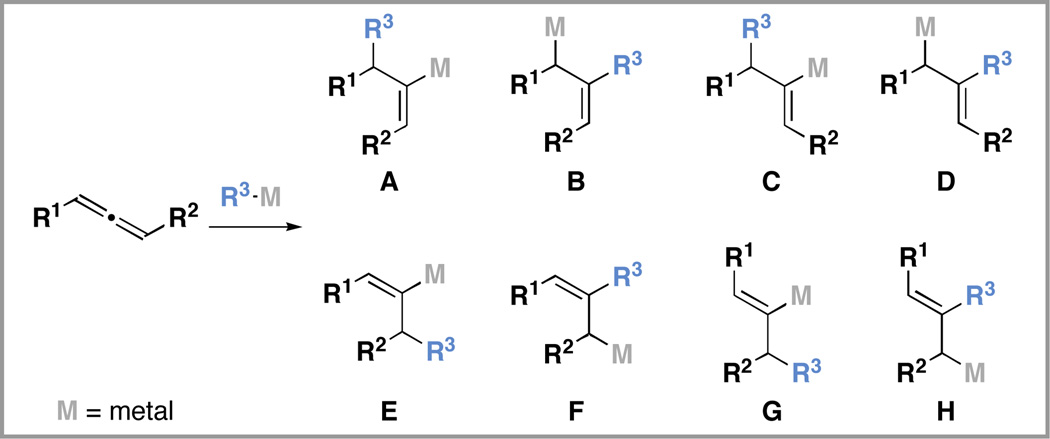
Site- and stereoselective functionalization of substituted allenes represents a broad class of reactions of great utility for the synthesis of stereodefined small molecules.1 The subset of these transformations that proceed in an intermolecular fashion, and provide a carbon– carbon bond in a regio- and stereoselective manner, are particularly powerful in complex molecule synthesis.2 Carbometalation as a pathway for such functionalization is rather complex owing to the numerous regioand stereoisomeric products possible in the bimolecular bond forming event (A–H; Figure 1). Here, we describe an allene–imine cross-coupling3 reaction that proceeds via site- and stereoselective union of a preformed azametallacyclopropane4 with an allenic alcohol, and provides a convenient pathway to the synthesis of substituted 1,3-dienes.
Figure 1.
Initial products possible in bimolecular carbometalation of substituted allenes.
Recently, we demonstrated that allenes bearing tethered hydroxyl functionality are viable substrates for bimolecular reaction with preformed metallacyclopropenes.5 In some cases, these cross-coupling reactions provide a means to access highly substituted cross-conjugated trienes, presumably by a pathway that involves directed carbometalation followed by syn elimination.5b
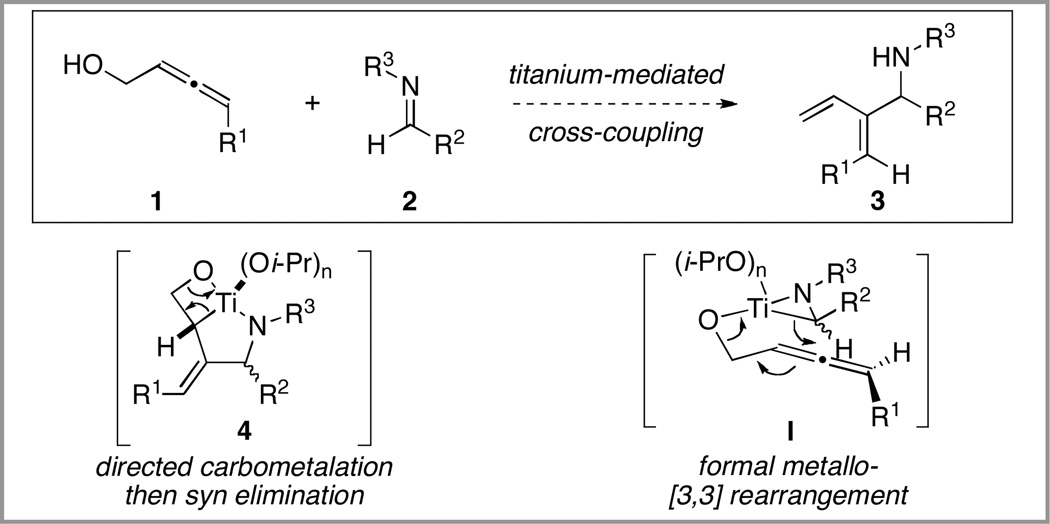
In an effort to explore the utility of this type of control in bimolecular functionalization of substituted allenes, efforts were directed to the study of an imine–allene cross-coupling reaction for the synthesis of substituted 1,3-dienes 3. As depicted in Figure 2, preformation of an azametallacyclopropane from imine 2, followed by addition of an allenic alkoxide was anticipated to provide a pathway to stereodefined 1,3-dienes 3 via formation of an intermediate metallacycle 4, followed by syn elimination. As described in our reports of allene–alkyne5b and allylic alcohol–alkyne6 cross-coupling, such bond construction may be conceptualized by formal metallo-[3,3] rearrangment by way of boat-like geometry I.
Figure 2.
Plan for allene–imine cross-coupling.
Formation of the azametallacyclopropane of aromatic imine 5 (ClTi(Oi-Pr)3, c-C5H9MgCl, Et2O; −78 to −40 °C), followed by addition of the lithium alkoxide of allene 6 (as a solution in Et2O) and warming to 0 °C provides, on aqueous work up, the substituted 1,3-diene 7 in 57% yield (entry 1; Table 1).7 As demonstrated in entry 2, aromatic chlorides are well tolerated in this cross-coupling reaction. Reaction of 8 with the simple allene 6 provides the functionalized diene 9 in 60% yield.
Table 1.
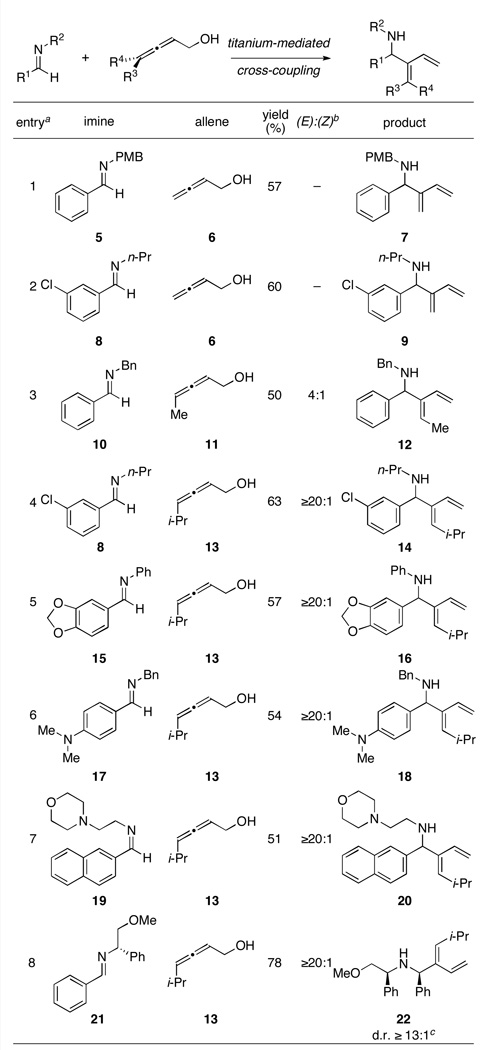 |
Typical reaction conditions: imine (1.0 eq), ClTi(OiPr)3 (1.25 eq), cC5H9MgCl (2.5 eq), Et2O (−78 to −40 °C), then add allenyl alkoxide (3–4 eq) (−40 to 0 °C).
Refers to the establishment of the stereodefined substituted olefin derived from the allene.
The relative stereochemistry was assigned based on analogy to alkyne–imine cross-coupling with imine 21 (see ref. 4f); this reaction was warmed from 0 to 18 °C over six hours (see Supporting Information for details).
When the allene is substituted at the site distal to the hydroxymethyl substituent, one can achieve stereoselectivity in the cross-coupling reaction. As depicted in entry 3, the methyl-substituted allenic alcohol 11 can be coupled with imine 10 to provide the stereodefined 1,3-diene 12 (E:Z = 4:1). When the size of the distal substituent is increased, the stereoselection of the cross-coupling reaction improves significantly. For example, reaction of the chlorinated aromatic imine 8 with the isopropyl-substituted allene 13 provides the stereodefined diene 14 in 63% yield, with ≥20:1 stereoselection. As illustrated in entries 6 and 7, electron rich aromatic imines 15 and 17 are equally effective in this cross-coupling reaction, providing the substituted 1,3-dienes 16 and 18 in 57 and 54% yield, respectively. Simple heterocyclic motifs are compatible with this stereoselective cross-coupling reaction. As demonstrated in entry 7, the morpholine-containing imine 19 can be coupled to allene 13, in this case providing the stereodefined diene 20 with ≥ 20:1 stereoselection. Finally, this reaction can be performed in a diastereoselective fashion. For example, coupling of the chiral imine 214e, 4g with racemic allene 13 provides allylic amine 22 in 78% yield (dr ≥ 13:1).
In summary, we have developed a new strategy for the cross-coupling of aromatic imines with substituted allenes. Selectivity is presumed to derive from a reaction pathway that utilizes an allenic alkoxide as a key organizing structural motif. We propose a pathway for bimolecular C–C bond formation by ligand exchange at titanium, followed by intramolecular carbometalation and syn elimination. Overall, the selectivity of the process is consistent with an empirical model based on formal metallo-[3,3] rearrangement. Studies aimed at exploring the generality of this new cross-coupling reaction, and application to the synthesis of complex heterocycles is underway.
Acknowledgment
We gratefully acknowledge financial support of this work by the American Cancer Society (RSG-06-117-01), the American Chemical Society (PRF-45334-G1), the Arnold and Mabel Beckman Foundation, Boehringer Ingelheim, Eli Lilly & Co., and the National Institutes of Health – NIGMS (GM80266). HLS acknowledges Bristol-Myers Squibb for a graduate fellowship. RN acknowledges support from the STARS program at Yale, and the ACS-PRF.
References and Notes
- 1.For a recent review, see: Krause N, Hashmi ASK. Modern Allene Chemistry. 1–2. Wiley-VCH; 2004.
- 2.For application of allenes in natural product synthesis, see: Brummond KM, Chen H. Allenes in Natural Product Synthesis. Modern Allene Chemistry Krause N, Hashmi ASK, editors. Vol. 2. Wiley-VCH; 2004. p. 1041. Alcaide B, Almendros P. Eur. J. Org. Chem. 2004:3377–3383. Marshall JA. J. Org. Chem. 2007;72:8153–8166. doi: 10.1021/jo070787c. Wei L, Xiong H, Hsung RP. Acc. Chem. Res. 2003. 36. pp. 773–782. Sydnes LK. Chem. Rev. 2003;103:1133–1150. doi: 10.1021/cr010025w. Krause N, Hoffmann-Roder A, Canisius J. Synthesis. 2002. pp. 1759–1774. Zimmer R. Synthesis. 1993. pp. 165–178.
- 3.For examples of palladium-catalyzed coupling of allenes with imines, see: Cooper IR, Grigg R, MacLachlan WS, Thornton-Pett M, Sridharan V. Chem. Comm. 2002:1372–1373. Hopkins CD, Malinakova HC. Org. Lett. 2006;8:5971–5974. doi: 10.1021/ol0624528. For examples of cross-coupling between allenylsulfides and imines, see: Hayashi Y, Shibata T, Narasaka K. Chem. Lett. 1990. pp. 1693–1696. Narasaka K, Shibata T, Hayashi Y. Bull. Chem. Soc. Jpn. 1992;65:1392–1396. For cross-coupling of vinyl allenes with imines, see: Regás D, Afonso MM, Rodríguez ML, Palenzuela JA. J. Org. Chem. 2003;68:7845–7852. doi: 10.1021/jo034480z. For cross-coupling of electron deficient allenes with imines, see: Gandhi RP, Ishar MPS, Wali A. Tetrahedron Lett. 1987;28:6679–6682. Xu Z, Lu X. Tetrahedron Lett. 1997;38:3461–3464. Xu Z, Lu X. J. Org. Chem. 1998;63:5031–5041. Ishar MPS, Kumar K, Kaur S, Kumar S, Girdhar NK, Sachar S, Marwaha A, Kapoor A. Org. Lett. 2001;3:2133–2136. doi: 10.1021/ol010026a. Zhu X-F, Lan J, Kwon O. J. Am. Chem. Soc. 2003;125:4716–4717. doi: 10.1021/ja0344009. Zhao G-L, Huang J-W, Shi M. Org. Lett. 2003;5:4737–4739. doi: 10.1021/ol0359416. Zhao G-L, Shi M. J. Org. Chem. 2005;70:9975–9984. doi: 10.1021/jo051763d. For a vanadium-catalyzed allenolimine cross-coupling, see: Trost BM, Jonasson C. Angew. Chem. Int. Ed. 2003;42:2063–2066. doi: 10.1002/anie.200350890.
- 4.For examples of cross-coupling with azametallacyclopropanes, see: Buchwald SL, Watson BT, Wannamaker MW, Dewan JC. J. Am. Chem. Soc. 1989;111:4486–4494. Jensen M, Livinghouse T. J. Am. Chem. Soc. 1989;111:4495–4496. Brossman RB, Davis WM, Buchwald SL. J. Am. Chem. Soc. 1991;113:2321–2322. Gao Y, Harada K, Sato F. Tetrahderon Lett. 1995;36:5913–5916. Fukuhara K, Okamoto S, Sato F. Org. Lett. 2003;5:2145–2148. doi: 10.1021/ol034599u. McLaughlin M, Takahashi M, Micalizio GC. Angew. Chem. Int. Ed. 2007;46:3912–3914. doi: 10.1002/anie.200605060. Takahashi M, Micalizio GC. J. Am. Chem. Soc. 2007;129:7514–7516. doi: 10.1021/ja071974v.
- 5.Shimp HL, Micalizio GC. Chem. Comm. 2007:4531–4533. doi: 10.1039/b708256h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolundzic F, Micalizio GC. J. Am. Chem. Soc. 2007;129:15112–15113. doi: 10.1021/ja075678u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Experimental procedure for the preparation of 7: A solution of imine 5 (0.075g, 0.333 mmol) in diethyl ether (2.2 mL) was cooled to −60 °C, and ClTi(Oi-Pr)3 (1.0 M solution in hexanes, 0.416 mmol) was added dropwise with a gas-tight syringe. The reaction was then cooled to −78 °C and cC5H9MgCl (2.02 M solution in diethyl ether, 0.832 mmol) was added dropwise with a gas-tight syringe. During the addition the solution turned light brown in color. The reaction was then slowly warmed to −45 °C over 30 minutes and stirred at −45 °C for an additional 90 minutes. Next, a solution of allenyl alkoxide 6, generated from the deprotenation of the corresponding alcohol (0.093g, 1.33 mmol) with n-BuLi (2.5 M in hexanes, 0.261 mmol), in diethyl ether (2 mL) at −78 °C warming to 0 °C over 30 minutes, was added via teflon cannula. The reaction was then warmed to 0 °C over 60 minutes and stirred at 0 °C 6 hours. The reaction was then quenched at 0 °C with five mL of saturated aqueous NH4Cl and the resulting biphasic mixture was rapidly stirred overnight at room temperature. The reaction mixture was further diluted with saturated NaHCO3, and extracted with ethyl acetate. The combined organic phases were washed with brine, dried over MgSO4, and concentrated in vacuo. The crude material was purified by column chromatography on silica gel (4%→8% ethyl acetate/hexanes) to yield amine 9 as a colorless oil (53 mg, 57%). 1H NMR (500 MHz, CDCl3) δ 7.42–7.39 (m, 2H), 7.34–7.30 (m, 2H), 7.27–7.23 (m, 3H), 6.88–6.85 (m, 2H), 6.32 (dd, J = 17.7, 11.3 Hz, 1H), 6.39 (s, 1H), 5.31 (s, 1H), 5.28 (d, J = 17.9 Hz, 1H), 5.01 (d, J = 11.35 Hz, 1H), 4.52 (s, 1H), 3.80 (s, 3H), 3.68 (dd, J = 21.8, 12.9 Hz, 2H), 1.61 (s, broad, 1H); 13C NMR (126 MHz, CDCl3) δ 158.9, 147.6, 142.5, 137.1, 132.9, 129.6, 128.5, 127.9, 127.3, 115.9, 114.7, 114.0, 63.2, 55.5, 51.4; IR (thin film, NaCl) 3004, 2935, 2833, 1512, 1246, 1611 cm−1; LRMS (EI, H) calcd for: C19H22NO 280.2 m/z (M + H); found (M + H)+ 280.1 m/z.