Abstract
Human chromosome 16p11.2 microdeletion is the most common gene copy number variation in autism, but the synaptic pathophysiology caused by this mutation is largely unknown. Here we show using a mouse with the same genetic deficiency that metabotropic glutamate receptor 5-(mGluR5-) dependent synaptic plasticity and protein synthesis is altered in the hippocampus, and that hippocampus-dependent memory is impaired. Remarkably, chronic treatment with a negative allosteric modulator of mGluR5 reverses the cognitive deficit.
Introduction
Autism spectrum disorder (ASD) is characterized by behavioral, cognitive and language impairment. Over the past decade, studies on monogenetic syndromes with high prevalence of ASD, such as fragile X (FX) and tuberous sclerosis (TS), have provided insights into the pathophysiology of diseases that can cause autism1. For example, it has been shown that altered signaling downstream of metabotropic glutamate receptor 5 (mGluR5) plays a pivotal role in the pathogenesis of FX, and that genetic and pharmacological modulation of mGluR5 can ameliorate numerous impairments in FX animal models2.
Chromosomal copy number variations (CNVs) have been associated with 5–10% of patients with ASD3, 4. Variation at human chromosome 16p11.2 is the most common of these and accounts for approximately 0.5–1% of all ASD cases4. The affected region harbors ~27 annotated protein-coding genes, many of which are expressed in the brain5, 6. The common clinical presentations in individuals carrying chr16p11.2 microdeletion are language impairment, intellectual disability (ID), ASD, anxiety, attention deficit hyperactive disorder (ADHD), and epilepsy7. Recent studies on animal models of human chr16p11.2 microdeletion have demonstrated morphological, behavioral and electrophysiological deficits8–10, however the synaptic pathophysiology remains largely unexplored.
Using electrophysiology, biochemistry and behavioral tests we characterized hippocampal function of a mouse model for human chr16p11.2 microdeletion8. We uncovered alterations in mGluR5-mediated synaptic plasticity, Arc expression, basal protein synthesis, and hippocampus-dependent learning that are reminiscent of previous observations in mouse models of syndromic autism and ID1. Importantly, we were able to ameliorate the behavioral abnormalities using an mGluR5 negative allosteric modulator (NAM), 2-chloro-4-((2,5-dimethyl-1-(4-(trifluoromethoxy)phenyl)-1H-imidazol-4-yl)ethynyl)pyridine (CTEP), suggesting a pathophysiology shared with FX.
Results and Discussion
Mutant mice (termed 16p11.2 df/+), engineered to be heterozygous null at the region of chromosome 7qF3 that is syntenic to human chromosome 16p11.28, were back-crossed for 5–10 generations to C57BL/6N mice (Charles River) to allow a comparison of synaptic physiology with previous studies1, 2. As noted previously8, loss of the genes in this region can compromise survival, and we found that this effect was amplified in successive generations on the C57BL/6N background (Supplementary Fig. 1).
We first characterized basal synaptic transmission at the Schaffer collateral-CA1 synapse using hippocampal slices from 4–5 week old mice and found no difference from wild type (WT) in input-output or paired-pulse facilitation (PPF) (Supplementary Fig. 2). To investigate NMDA receptor-dependent synaptic plasticity, we induced long-term potentiation (LTP) with theta-burst stimulation (TBS), and long-term depression (LTD) with low-frequency (1 Hz) stimulation (LFS). Again, there was no difference from WT (Fig. 1a–b), suggesting basic excitatory synaptic transmission and plasticity mechanisms are intact in the mutant mice.
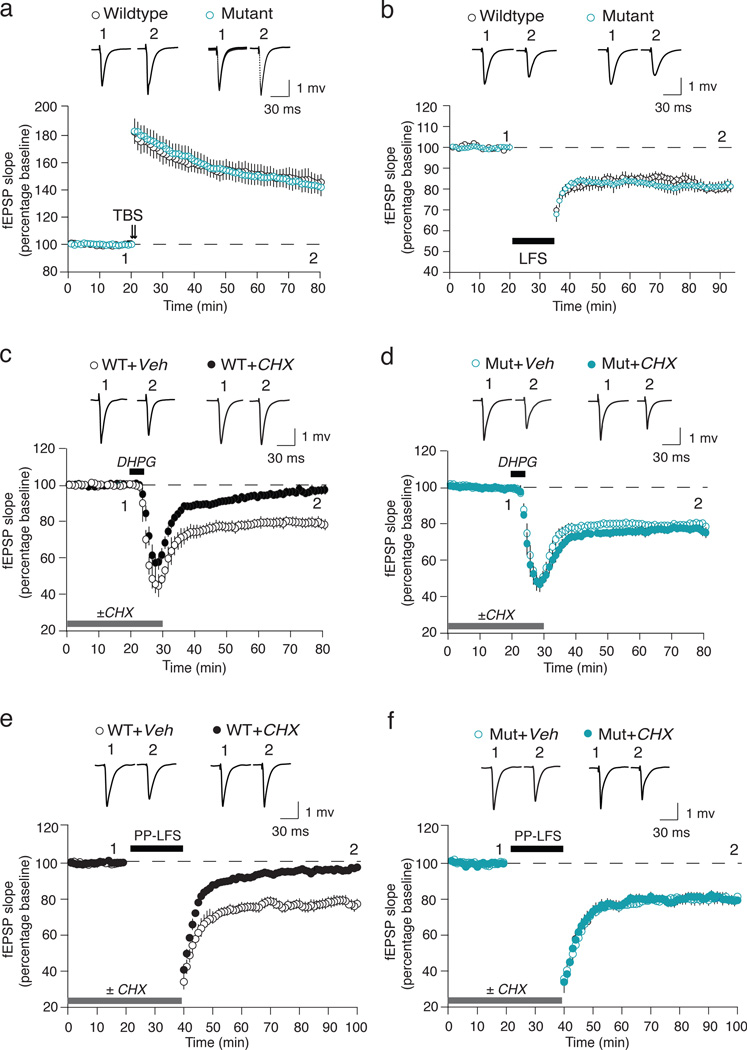
Figure 1. mGluR-LTD is protein synthesis independent in 16p11.2 df/+ mice.
(a) TBS-LTP is unchanged in mutant (n = 9 animals, 16 slices) compared with WT (n = 7 animals, 15 slices) mice. (b) LFS-LTD is unchanged in mutant (n = 6 animals, 10 slices) compared with WT (n = 6 animals, 10 slices) mice. (c, d) The magnitude of DHPG-LTD is comparable in hippocampal slices from the WT (n = 17 animals, 18 slices) and mutant (Mut, n = 16 animals, 20 slices) mice in the absence of CHX. However, CHX blocks DHPG-LTD in WT (n = 17 animals, 21 slices) but not mutant slices (n = 16 animals, 20 slices) (Two-way ANOVA, genotype × CHX, p = 0.0074). (e, f) The magnitude of PP-LFS-LTD is comparable in hippocampal slices from WT (n = 12 animals, 14 slices) and mutant (n = 8 animals, 10 slices) mice in the absence of CHX. CHX significantly attenuates PP-LFS-LTD in the WT (n = 12 animals, 14 slices) but not mutant slices (n = 8 animals, 9 slices) (Two-way ANOVA, genotype × CHX, p = 0.013). Representative fEPSP traces (average of 10 sweeps) were taken at the times indicated by numerals. All data are plotted as mean ± SEM.
We next assayed mGluR5 mediated long-term depression (mGluR-LTD). mGluR-LTD was induced either by brief application of the mGluR1/5 agonist S-3,5-dihydroxyphenylglycine (DHPG) or by applying a series of paired pulses at 50 ms interval (PP-LFS)1. We again found no difference between WT and the 16p11.2 df/+ mutant with either induction protocol (Fig. 1c–f).
A distinctive property of mGluR-LTD in WT animals is a requirement for mRNA translation at the time of induction11. In slices from Fmr1−/y mice, however, mGluR-LTD is unaffected by translation inhibitors12 because basal synaptic synthesis of LTD-regulatory proteins such as Arc13–15 is elevated downstream of constitutive mGluR5 activity due to loss of the translational repressor FMRP16. We were therefore compelled to investigate the protein synthesis-dependence of mGluR LTD in the 16p11.2 df/+ mice, and discovered a striking difference from WT. Like FX model mice, mGluR-LTD in the 16p11.2 df/+ mice was unaffected by cycloheximide (CHX) (Fig. 1d, f).
As reported previously in WT animals, DHPG induces LTD via two mechanisms: a postsynaptic reduction in AMPA receptors and a presynaptic reduction in glutamate release probability. Only the postsynaptic mechanism is CHX sensitive1. To test whether the different sensitivity of LTD to CHX in the mutant was due to a qualitatively different expression mechanism, we analyzed PPF at the beginning and end of each DHPG-LTD experiment. No difference was observed between the WT and mutant slices (Supplementary Fig. 3). These findings point to a deficiency in postsynaptic regulation of protein synthesis in the 16p11.2 df/+ mice.
We next tested the mutant mice in two hippocampus-dependent behavioral assays, contextual fear conditioning (CFC) and inhibitory avoidance (IA), which have been shown in previous studies to reveal cognitive impairments in Fmr1+/y mice1, 2. CFC requires intact mGluR5 signaling and new protein synthesis at the time of conditioning17. In this assay, mice are exposed to a distinctive environmental context in which a foot-shock is delivered, and 24 h later the mice are returned to either the same (familiar) or a different (novel) context (Fig. 2a). WT mice expressed fear memory by freezing in the familiar context, and demonstrated an ability to discriminate different contexts by freezing less in the novel context. In contrast, the mutant mice showed significantly less freezing in the familiar context, and were unable to distinguish between the familiar and novel context (Fig. 2b). Mutant and WT mice exhibited comparable sensitivity to foot-shock (Fig. 2c), suggesting the difference in freezing at 24 h was due to an impairment in memory formation in the mutant mice.
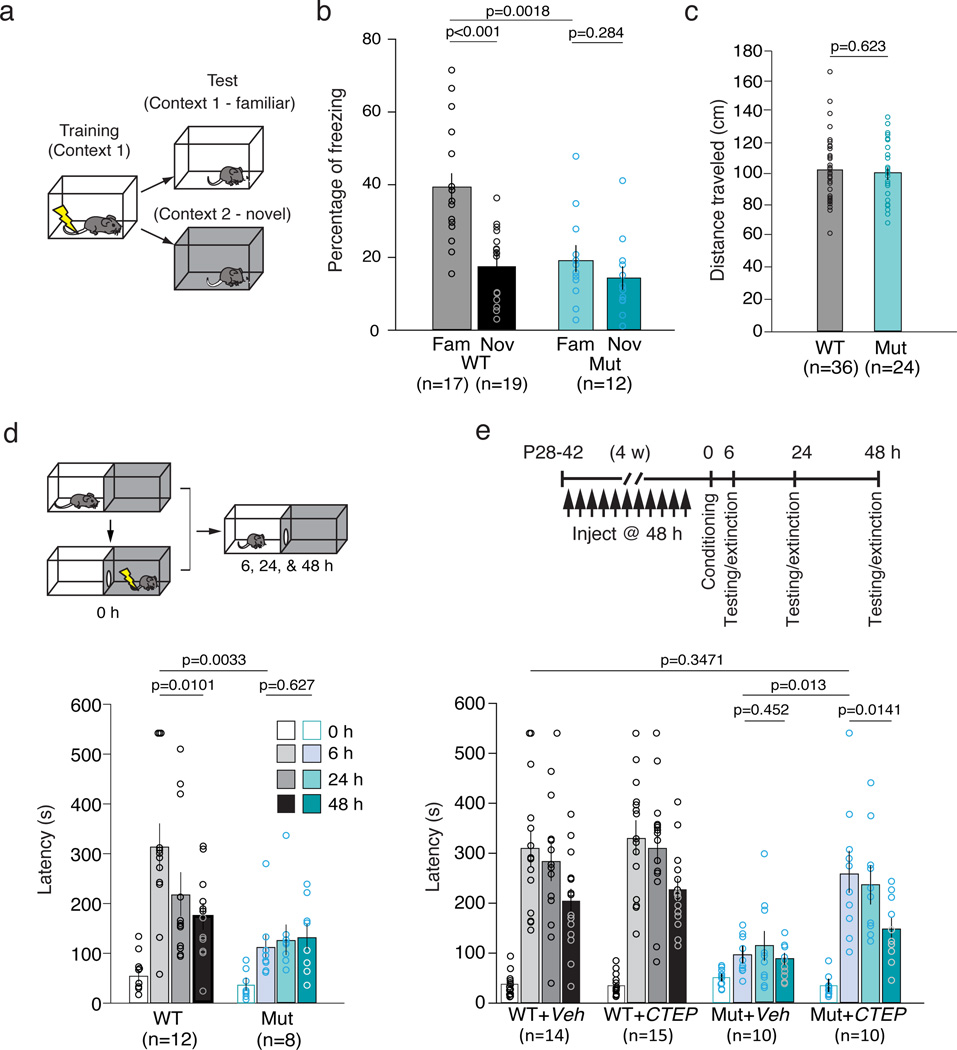
Figure 2. 16p11.2 df/+ mice exhibit deficits in hippocampal-associated contextual fear conditioning (CFC) and inhibitory avoidance (IA).
(a) CFC experimental design. (b) Mutant mice show significantly less freezing in the familiar context compared with WT (unpaired t-test, p = 0.0018). While WT mice are able to distinguish a novel from familiar context (unpaired t-test, p < 0.0001), the mutant mice are impaired (unpaired t-test, p = 0.2840). Two-way ANOVA, genotype × context, p = 0.0166. (c) Mutant and WT mice have the same running response to foot-shock during the training session (unpaired t-test, p = 0.6234). (d) Mutant mice are impaired in IA acquisition (WT vs Mut, 0 hr vs 6 hr, repeated measures two-way ANOVA, p = 0.0108; WT vs Mut at 6 hr, post-hoc unpaired t-test, p = 0.0033). Unlike WT (6 hr vs 48 hr, post-hoc paired t-test, p = 0.0101), mutant mice show no extinction of fear memory (WT vs Mut, 6 hr vs 48 hr, repeated measures two-way ANOVA, p = 0.0197; Mut, 6 hr vs 48 hr, post-hoc paired t-test, p = 0.6278). (e) CTEP treatment ameliorates behavioral deficits in mutant mice in IA. In mutant mice, CTEP treatment enhances acquisition (Mut+Veh vs Mut+CTEP, 0 hr vs 6 hr, repeated measures two-way ANOVA, p = 0.0016; Mut+Veh vs Mut+CTEP at 6 hr, post-hoc unpaired t-test, p = 0.0013) and extinction of fear memory (Mut+Veh vs Mut+CTEP, 6 hr vs 48 hr, repeated measures two-way ANOVA, p = 0.0039; Mut+Veh, 6 hr vs 48 hr, post-hoc paired t-test, p = 0.4281; Mut+CTEP, 6 hr vs 48 hr, post-hoc paired t-test, p = 0.0140). There is no statistically significant difference between WT+Veh and Mut+CTEP at 6 hr (unpaired t-test, p = 0.3471). In WT mice, CTEP has no effect on either acquisition (WT+Veh vs WT+CTEP, 0 hr vs 6 hr, repeated measures two-way ANOVA, p = 0.6564) or extinction of fear memory (WT+Veh vs WT+CTEP, 6 hr vs 48 hr, repeated measures two-way ANOVA, p = 0.9882). All data are plotted as mean ± SEM with individual values superimposed.
In the IA assay, mice received a foot shock upon entry into the dark side of a two-chamber box (Fig. 2d). Memory strength and extinction were measured as the latency to enter the dark side when given the opportunity at 6, 24, and 48 h intervals2, 18. The 16p11.2 df/+ mice showed impaired acquisition and extinction of IA memory. Similar IA deficits in Fmr1−/y mice have been ameliorated by chronic post-adolescent treatment with the mGluR5 NAM CTEP2. Therefore we repeated the IA assay on WT and mutant mice that were treated every second day with CTEP (2 mg/kg p.o.) or vehicle for 4 weeks2. Although treatment had no effect in the WT mice, it corrected the deficits in the mutants both in terms of acquisition and extinction (Fig. 2e).
We were motivated to investigate the possibility that FX and 16p11.2 microdeletion have shared pathophysiology for several reasons, including the fact that four deleted genes are targets of FMRP (MAZ, SEZ62L, TAOK2, ALDOA)19 and disruption of several genes in this region are predicted to affect mGluR5 signaling (MVP, CDIPT, MAPK3) or protein turnover (KCTD13)9. The most straightforward prediction from our results is that synaptic protein synthesis downstream of mGluR5 is exaggerated by the 16p11.2 microdeletion, and this gives rise to cognitive impairment. In the Fmr1−/y mouse, bulk protein synthesis in hippocampal slices is elevated and corrected by manipulations of mGluR516, 18. However, we found that basal protein synthesis in hippocampal slices in the 16p11.2 df/+ mice was reduced, possibly explained by the decrease in ERK pathway activity (Fig. 3). Nevertheless, immunoblots for Arc protein showed a significant increase. The mGluR5-dependent synthesis of Arc protein normally gates LTD and synapse elimination13–15. Therefore, constitutive elevation of Arc could render mGluR-LTD insensitive to acute inhibition of protein synthesis and contribute to cognitive impairment.
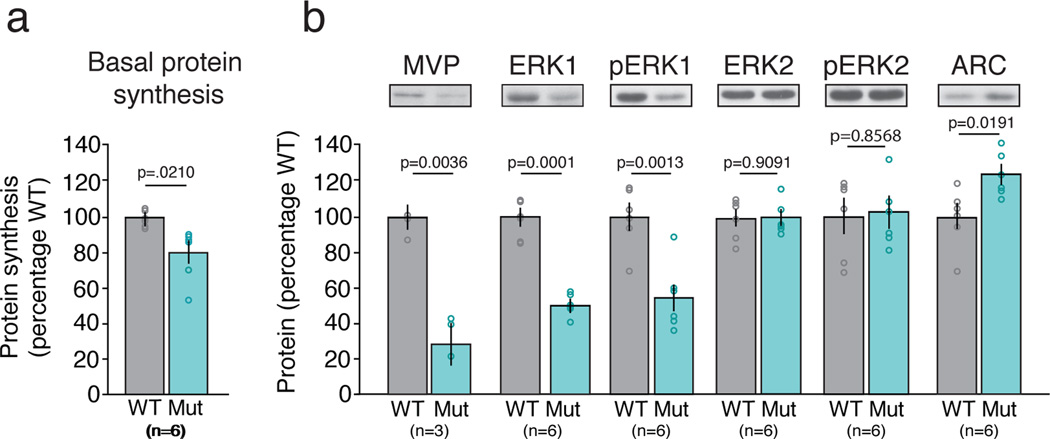
Figure 3. 16p11.2 df/+ mice exhibit a decrease in basal protein synthesis which is accompanied by an increase in Arc protein levels.
(a) Metabolic labeling of hippocampal slices reveals a significant reduction of basal protein synthesis in 16p11.2 df/+ compared to WT mice (unpaired t-test, p = 0.0210). (b) MVP, ERK1 and pERK1 are decreased in 16p11.2 df/+ mice relative to WT mice (unpaired t-test, p = 0.0036; p = 0.0001; p = 0.0013 respectively), whereas ERK2 and pERK2 levels are comparable between 16p11.2 df/+ and WT mice (unpaired t-test, p = 0.9091; p = 0.8568 respectively). Arc protein levels are significantly increased in 16p11.2 df/+ mice as compared to WT mice (unpaired t-test, p = 0.0191). All data are plotted as mean ± SEM with individual values superimposed; n indicates number of animals.
These findings support two important conclusions. First, the data suggest that some cognitive and neuropsychiatric symptoms of 16p11.2 microdeletion disorder arise from altered synaptic signaling that is amenable to targeted drug therapy. Second, the data strengthen the hypothesis that multiple causes of ASD and ID converge on common pathophysiological processes, and one of these is the synaptic regulation of protein synthesis20.
Supplementary Material
Acknowledgments
Acknowledgements and author contributions: M.B. and D.T. conceived and designed the study. M.B. and A.H. supervised the study. D.T. performed hippocampal electrophysiology and contextual fear conditioning. D.T. and A.H. performed inhibitory avoidance test. L.S. performed hippocampal protein synthesis and immunoblot experiments. D.T., A.H., L.S., and M.B. wrote the manuscript. L.L. and G.J. provided CTEP. A.M. provided the 16p11.2 df/− mice prior to publication and edited the manuscript. This work was partly supported by grants and funding to M.B. from the NIMH (R21MH090452), NICHD (R01HD046943), Simons Foundation (SFARI #240559) and the Simons Center for the Social Brain at MIT, and a physician-scientist career development award from NICHD (5K08HD053824) to D.T. Laura Stoppel was supported by NIMH training grant (5T32MH074249). We thank Benjamin Auerbach, Erik Sklar, and Suzanne Meagher for technical and administrative assistance.
Footnotes
Competing interests: M.F.B. holds patents on the use of mGluR5 inhibitors for treatment of fragile X and autism; M.F.B. and D.T. have patents pending on use of mGluR5 inhibitors for treatment of 16p11.2 microdeletion; L.L., G.J. are employees of Roche Pharmaceuticals.
References
- 1.Auerbach BD, Osterweil EK, Bear MF. Mutations causing syndromic autism define an axis of synaptic pathophysiology. Nature. 2011;480:63–68. doi: 10.1038/nature10658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Michalon A, et al. Chronic pharmacological mGlu5 inhibition corrects fragile X in adult mice. Neuron. 2012;74:49–56. doi: 10.1016/j.neuron.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levy D, et al. Rare de novo and transmitted copy-number variation in autistic spectrum disorders. Neuron. 2011;70:886–897. doi: 10.1016/j.neuron.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 4.Malhotra D, Sebat J. CNVs: harbingers of a rare variant revolution in psychiatric genetics. Cell. 2012;148:1223–1241. doi: 10.1016/j.cell.2012.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar RA, et al. Recurrent 16p11.2 microdeletions in autism. Hum Mol Genet. 2008;17:628–638. doi: 10.1093/hmg/ddm376. [DOI] [PubMed] [Google Scholar]
- 6.Weiss LA, et al. Association between microdeletion and microduplication at 16p11.2 and autism. N Engl J Med. 2008;358:667–675. doi: 10.1056/NEJMoa075974. [DOI] [PubMed] [Google Scholar]
- 7.Zufferey F, et al. A 600 kb deletion syndrome at 16p11.2 leads to energy imbalance and neuropsychiatric disorders. Journal of medical genetics. 2012;49:660–668. doi: 10.1136/jmedgenet-2012-101203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horev G, et al. Dosage-dependent phenotypes in models of 16p11.2 lesions found in autism. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:17076–17081. doi: 10.1073/pnas.1114042108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Golzio C, et al. KCTD13 is a major driver of mirrored neuroanatomical phenotypes of the 16p11.2 copy number variant. Nature. 2012;485:363–367. doi: 10.1038/nature11091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Portmann T, et al. Behavioral abnormalities and circuit defects in the basal ganglia of a mouse model of 16p11.2 deletion syndrome. Cell reports. 2014;7:1077–1092. doi: 10.1016/j.celrep.2014.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:7746–7750. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nosyreva ED, Huber KM. Metabotropic receptor-dependent long-term depression persists in the absence of protein synthesis in the mouse model of fragile X syndrome. Journal of neurophysiology. 2006;95:3291–3295. doi: 10.1152/jn.01316.2005. [DOI] [PubMed] [Google Scholar]
- 13.Waung MW, Pfeiffer BE, Nosyreva ED, Ronesi JA, Huber KM. Rapid translation of Arc/Arg3.1 selectively mediates mGluR-dependent LTD through persistent increases in AMPAR endocytosis rate. Neuron. 2008;59:84–97. doi: 10.1016/j.neuron.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jakkamsetti V, et al. Experience-induced Arc/Arg3.1 primes CA1 pyramidal neurons for metabotropic glutamate receptor-dependent long-term synaptic depression. Neuron. 2013;80:72–79. doi: 10.1016/j.neuron.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilkerson JR, et al. A role for dendritic mGluR5-mediated local translation of Arc/Arg3.1 in MEF2-dependent synapse elimination. Cell reports. 2014;7:1589–1600. doi: 10.1016/j.celrep.2014.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Osterweil EK, Krueger DD, Reinhold K, Bear MF. Hypersensitivity to mGluR5 and ERK1/2 leads to excessive protein synthesis in the hippocampus of a mouse model of fragile X syndrome. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:15616–15627. doi: 10.1523/JNEUROSCI.3888-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stiedl O, Palve M, Radulovic J, Birkenfeld K, Spiess J. Differential impairment of auditory and contextual fear conditioning by protein synthesis inhibition in C57BL/6N mice. Behav Neurosci. 1999;113:496–506. doi: 10.1037//0735-7044.113.3.496. [DOI] [PubMed] [Google Scholar]
- 18.Dolen G, et al. Correction of fragile X syndrome in mice. Neuron. 2007;56:955–962. doi: 10.1016/j.neuron.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Darnell JC, et al. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell. 2011;146:247–261. doi: 10.1016/j.cell.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelleher RJ, 3rd, Bear MF. The autistic neuron: troubled translation? Cell. 2008;135:401–406. doi: 10.1016/j.cell.2008.10.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.