Abstract
Background:
Despite increased cases published on breast implant–associated anaplastic large cell lymphoma (BIA-ALCL), important clinical issues remain unanswered. We conducted a second structured expert consultation process to rate statements related to the diagnosis, management, and surveillance of this disease, based on their interpretation of published evidence.
Methods:
A multidisciplinary panel of 12 experts was selected based on nominations from national specialty societies, academic department heads, and recognized researchers in the United States.
Results:
Panelists agreed that (1) this disease should be called “BIA-ALCL”; (2) late seromas occurring >1 year after breast implantation should be evaluated via ultrasound, and if a seroma is present, the fluid should be aspirated and sent for culture, cytology, flow cytometry, and cell block to an experienced hematopathologist; (3) surgical removal of the affected implant and capsule (as completely as possible) should occur, which is sufficient to eradicate capsule-confined BIA-ALCL; (4) surveillance should consist of clinical follow-up at least every 6 months for at least 5 years and breast ultrasound yearly for at least 2 years; and (5) BIA-ALCL is generally a biologically indolent disease with a good prognosis, unless it extends beyond the capsule and/or presents as a mass. They firmly disagreed with statements that chemotherapy and radiation therapy should be given to all patients with BIA-ALCL.
Conclusions:
Our assessment yielded consistent results on a number of key, incompletely addressed issues regarding BIA-ALCL, but additional research is needed to support these statement ratings and enhance our understanding of the biology, treatment, and outcomes associated with this disease.
Breast implant–associated anaplastic large cell lymphoma (BIA-ALCL) is an uncommon entity. We previously published a systematic literature review of 29 cases of BIA-ALCL1 (recapitulated in a 2011 Food and Drug Administration alert)2 and a subsequent report from a structured, expert consultation panel, which agreed that there is a positive association between breast implants and anaplastic large cell lymphoma (ALCL) development; anaplastic lymphoma kinase (ALK)-negative ALCL that develops around breast implants is a clinically indolent disease with a favorable prognosis that is distinct from systemic ALK-negative ALCL; management should consist of removal of the involved implant and capsule, which is likely to prevent recurrence, and evaluation for other sites of disease; and adjuvant radiation or chemotherapy should not be offered to women with capsule-confined disease.3 Since then, additional BIA-ALCL case reports and series have been published,4–30 which we have summarized in an updated systematic review.31 Although BIA-ALCL has become increasingly recognized in the plastic surgery community, there has been little additional guidance made available to hematology/oncology providers as to how the diagnosis, management, and surveillance of this disease should be undertaken. Because much of the information in the literature is still incomplete and does not address important clinical topics related to BIA-ALCL, we conducted a follow-up structured expert consultation process that combined published evidence with expert assessment to garner additional insight on these important issues.
METHODS
The expert consultation process is based on the RAND/UCLA Appropriateness Method, which provides a structured and quantifiable way to combine findings from a review of the evidence with input from a multidisciplinary expert panel.32 Guidelines developed using this method are reproducible,33 consistent clinically,34 and correlated with clinical outcomes.35 This approach has been used to address clinical issues in a wide variety of malignancies,36–54 including lymphoma.55
Literature Review and Item Development
We conducted a literature search focused on breast implants and ALCL, which has been previously described.31 After the data from the literature were abstracted by 2 trained, clinician reviewers (B.K. and C.A.G.), the authors identified recurring themes and potential evidence gaps. Systematic literature review findings, combined with a number of identical or similar items from our prior BIA-ALCL expert consultation process, were then utilized to compose an initial set of 65 evaluable statements addressing the nomenclature, evaluation, treatment, surveillance, and prognosis related to BIA-ALCL.
Expert Panel Recruitment and Rating Process
We identified a pool of potential panelists with either content or methodology expertise using a process that is typical for expert panel recruitment. We sought nominations from national specialty societies with a stake in this field (Table 1). We also used the results of our literature search to identify recognized researchers and heads of academic departments who had contributed to seminal articles on BIA-ALCL and/or had expertise in this area. The curricula vitae of all nominees were reviewed (by B.K. and C.A.G.) before inviting the experts for participation. Overall, 12 panel members who represented a range of relevant academic and clinical specialties (3 medical oncologists, 4 hematopathologists, 2 plastic surgeons, 1 surgical oncologist, and 2 radiation oncologists) from leading universities across the United States agreed to participate (Table 2). Each panelist received a draft of the literature review tables and a background document on BIA-ALCL. Panelists were instructed to rate each of the 65 statements on a scale from 1 to 9 according to their level of agreement. Low scores (1–3) represented disagreement, middle scores (4–6) uncertainty, and high scores (7–9) agreement with the statement. If the item was outside the panelist’s area of expertise, the panelist was allowed to indicate this and not provide a numeric rating.
Table 1.
National Specialty Societies Providing Panel Nominations

Table 2.
Affiliations of Structured Expert Panel Members
The first round of ratings was completed before the panel meeting. The initial ratings were tabulated, summarized, and presented to the entire expert panel at a subsequent 2-day, face-to-face meeting in March 2014. At this meeting, panel members were able to review aggregated ratings, discuss their interpretation of the evidence, and share reasons for their level of agreement or disagreement with each statement. Representatives from plastic surgery specialty societies and an implant manufacturer were also present to observe the proceedings. Based on the discussion during the meeting, some statements were deleted or revised to improve clarity and incorporate important clinical nuances before the panelists were asked to conduct a second and final round of ratings. Of note, this modified Delphi method does not strive to achieve consensus but typically leads to a convergence in panelists’ ratings after the discussion.
Data Analysis
RAND investigators compiled the final ratings and analyzed panelists’ disagreement, uncertainty, or agreement with each item. Results were then summarized and aggregated in tabular form, with reporting of, for each item, the median and dispersion—a statistical measure of the ratings’ spread, defined as the average absolute distance from the median. Median ratings ≤3.0 were interpreted as indicating disagreement, 4.0–6.0 uncertainty, and ≥7.0 agreement. The concordance of each median rating was defined as high if the dispersion was ≤1.00, moderate if 1.01–1.99, and low if ≥2.00.
The study was reviewed and considered exempt by the Human Subjects Protection Committee/Institutional Review Board at RAND.
RESULTS
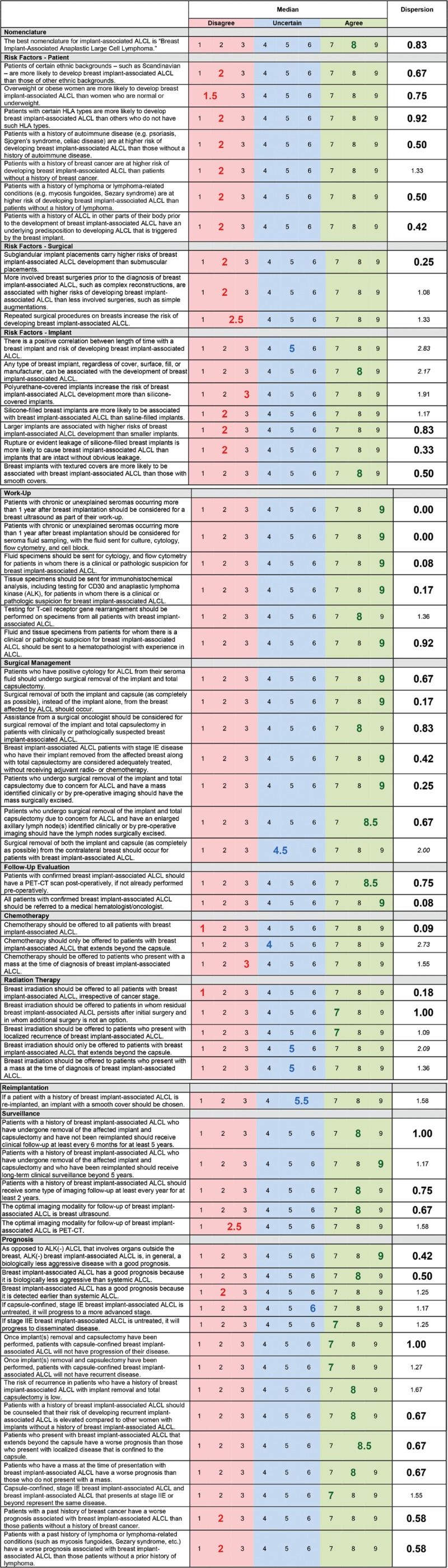
The 61 final rating results are reported in Figure 1. Overall, panelists disagreed with 21 of the 61 statements (large, bold, red numbers; 34.4%), were uncertain with 7 statements (large, bold, blue numbers; 11.5%), and agreed with 33 statements (large, bold, green numbers; 54.1%). In terms of the dispersion of panelists’ ratings, it ranged from 0.00 (perfect concordance) to 2.83. There was high concordance for 38 of the 61 items (large, bold, black numbers; 62.3%), moderate concordance for 18 items (small, black numbers; 29.5%), and low concordance for 5 items (small, italicized, black numbers; 8.2%).
Fig. 1.
Final ratings of BIA-ALCL statements.
Nomenclature
Panelists believed that the best nomenclature for ALCL occurring in the setting of a breast implant is “Breast Implant–Associated Anaplastic Large Cell Lymphoma” (rating, 8; dispersion, 0.83).
Risk Factors
Across all patient risk factors queried, panelists firmly disagreed that certain ethnic backgrounds (2, 0.67), obesity (1.5, 0.75), certain human leukocyte antigen (HLA) types (2, 0.92), and history of autoimmune disease (2, 0.50), lymphoma (2, 0.50), or ALCL in other locations (2, 0.42) are associated with an increased risk of developing BIA-ALCL. They also disagreed that women with a prior breast cancer have an increased risk of BIA-ALCL, although with moderate discordance (2, 1.33). In terms of surgical risk factors, there was consistent disagreement with the statement that subglandular versus submuscular implantations increase a woman’s BIA-ALCL risk (2, 0.25), although panelists’ disagreement with the statements that more involved (2, 1.08) or repeated (2.5, 1.33) breast surgeries increase the risk of developing BIA-ALCL was more varied. Regarding implant-associated risk factors, there was uncertainty and low concordance whether the length of time a woman has an implant positively correlates with their risk of developing BIA-ALCL (5, 2.83). Although the median rating of the statement agreed that any type of breast implant, regardless of cover, surface, fill, or manufacturer, can be associated with BIA-ALCL development, there was substantial variance in the panelists’ assessments (8, 2.17). Panel members disagreed with moderate concordance that polyurethane- versus silicone-covered (3, 1.91) or silicone- versus saline-filled (2, 1.17) implants are associated with an increased risk of BIA-ALCL and with strong concordance that larger implants (2, 0.83) and rupture or leakage of silicone-filled breast implants (2, 0.33) increase the risk for BIA-ALCL development. However, there was firm agreement that breast implants with textured shells are more likely to be associated with BIA-ALCL than those with smooth shells (8, 0.50).
Workup
Panelists universally agreed that chronic or unexplained seromas occurring more than 1 year after breast implantation should be considered for a breast ultrasound (9, 0.00) and seroma fluid sampling, with the fluid sent for culture, cytology, flow cytometry, and cell block (9, 0.00). There was also consistent agreement that seroma fluid specimens should be sent for cytology and flow cytometry (9, 0.08), tissue specimens should be sent for immunohistochemical analysis (including for CD30 and ALK; 9, 0.17), and specimens should be sent to a hematopathologist with experience in diagnosing ALCL (9, 0.92), with less consistent agreement that T-cell receptor gene rearrangement should also be performed (8, 1.36).
Surgical Management
There was firm agreement with all statements related to the surgical management of the breast affected by BIA-ALCL. Panelists agreed that patients with positive cytology from seroma fluid should undergo affected implant removal and total capsulectomy (9, 0.67). They also supported the statements that surgical removal of both the affected implant and capsule (as completely as possible) should occur (9, 0.17) and one should consider operative assistance from a surgical oncologist if BIA-ALCL is clinically or pathologically suspected (8, 0.83). Should the affected implant be excised and total capsulectomy be performed, panel members agreed that such patients are considered adequately treated without the need for adjuvant radiotherapy or chemotherapy (9, 0.42). For those patients with BIA-ALCL who present with a mass or enlarged axillary lymph node(s), there was agreement that both of these should also be removed (9, 0.25 and 8.5, 0.67; respectively). On the other hand, panelists were uncertain whether contralateral implant removal and total capsulectomy in the unaffected breast need to occur (4.5, 2.00).
Follow-up Evaluation and Treatment
There was highly concordant agreement that, postoperatively, patients with BIA-ALCL should undergo positron emission tomography-computed tomography (PET-CT) for staging purposes (if not performed preoperatively; 8, 0.75) and be referred to a medical hematologist/oncology for treatment recommendations (9, 0.08).
Chemotherapy
Panel members firmly disagreed that chemotherapy should be offered to all patients with BIA-ALCL (1, 0.09). They were uncertain whether chemotherapy should be offered to those with BIA-ALCL that extends beyond the capsule (4, 2.73). Although panelists disagreed that chemotherapy should be offered to patients with BIA-ALCL who present with a mass, there was moderate discordance with this assertion (3, 1.55).
Radiation Therapy
As with chemotherapy, there was firm disagreement that breast irradiation should be offered to all patients with BIA-ALCL (1, 0.18). However, panelists agreed that radiation therapy should be offered to patients with persistent disease after surgery and for whom additional surgery is not an option, with high concordance (7, 1.00); for patients with localized recurrence, the panelists expressed this opinion with slightly less concordance (7, 1.09). They were uncertain whether breast irradiation should be offered to those with BIA-ALCL that extends beyond the capsule (5, 2.09) or presents with a mass (5, 1.36).
Reimplantation
If reimplantation occurs, panelists were uncertain if an implant with a smooth cover (as opposed to textured) should be chosen (5.5, 1.58).
Surveillance
After affected implant removal and total capsulectomy, there was consistent agreement with having BIA-ALCL patients receive clinical follow-up at least every 6 months for at least 5 years (8, 1.00) and agreement, but with moderate concordance, that those who have undergone reimplantation should undergo surveillance beyond 5 years (9, 1.17). Panelists firmly agreed that imaging (8, 0.75)—optimally, breast ultrasound (8, 0.67) and not PET-CT (2.5, 1.58)—should be performed every year for at least 2 years for surveillance.
Prognosis
With 2 separate statements, panel members firmly agreed that BIA-ALCL is generally a biologically less aggressive disease with a good prognosis, compared with ALK-negative ALCL involving organs outside the breast, because it is biologically less aggressive (9, 0.42 and 8, 0.50); they disagreed with moderate variance that BIA-ALCL’s good prognosis is due to its earlier detection than systemic ALCL (2, 1.25). There was uncertainty as to whether capsule-confined BIA-ALCL will progress to a more advanced stage if left untreated (6, 1.17), although moderately concordant agreement that it will if the disease has already spread to regional lymph nodes (7, 1.25). Once the affected implant and all of the associated capsule have been removed, panelists agreed that patients with capsule-confined BIA-ALCL will not have progression (7, 1.00) or recurrence (7, 1.27) of their disease with little and some variance, respectively. They agree with moderate concordance that the overall recurrence risk is low for patients with BIA-ALCL who have undergone affected implant removal and total capsulectomy (8, 1.67); however, they agree with high concordance that their recurrence risk is higher than for women who have never had BIA-ALCL in the past (8, 0.67).
On the other hand, If BIA-ALCL extends beyond the capsule or has an associated mass, panelists consistently agree that the prognosis is worse compared with capsule-confined (8.5, 0.67) or nonmass BIA-ALCL (8, 0.67), respectively. Biologically, panelists agree that stage IE and IIE or beyond BIA-ALCL represent the same disease with moderate concordance (7, 1.55).
Finally, panel members firmly disagree that the prognosis associated with BIA-ALCL is adversely affected by a history of breast cancer (2, 0.58) or lymphoma (2, 0.58).
DISCUSSION
In the past several years, the number of published BIA-ALCL cases has increased; however, important clinical questions related to the diagnosis and treatment of women with breast implants who develop ALK-negative ALCL remain incompletely addressed. BIA-ALCL is a rare condition, with 2 recent systematic literature reviews identifying a total of only 83 cases.1,31 Because the infrequency of this disease makes conducting pivotal clinical trials challenging at best, we undertook a second structured expert consultation process to revisit and fill in important evidentiary gaps, as well as provide guidance to physicians who seek information in making informed clinical decisions for patients with this disease.
With summary tables from our updated systematic review and after the panel meeting, the current panelists judged only 11.5% of the final statements with a median rating in the uncertain range (35.4% in our previous panel).3 Although prior publications have termed this condition “breast implant-related anaplastic large cell lymphoma,”6 “implant-related primary anaplastic large cell lymphoma of the breast,”8 “lymphoma of the breast capsule,”11 and “effusion-associated anaplastic large cell lymphoma of the breast”28 and the panelists discussed using the term “lymphoproliferative disorder” instead of “lymphoma,” they ultimately consistently agreed that the nomenclature of this disease should be “breast implant–associated anaplastic large cell lymphoma,” which will hopefully be considered by the World Health Organization’s advisory committee responsible for the classification of lymphomas. There was firm disagreement with potential patient, surgical, and implant risk factors for increasing the risk of developing BIA-ALCL, although there was firm agreement regarding the workup and surgical management of patients suspected of having BIA-ALCL, which is graphically illustrated in algorithm form (Fig. 2).
Fig. 2.
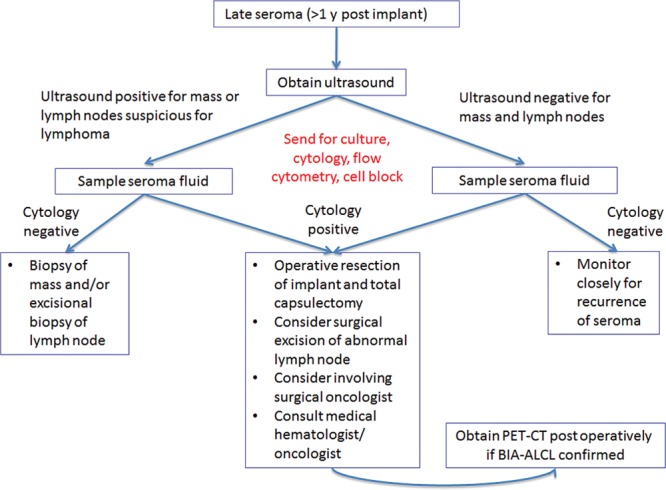
Algorithm for workup and surgical management of a late seroma following breast implantation. BIA-ALCL indicates breast implant–associated anaplastic large cell lymphoma; PET-CT, positron emission tomography-computed tomography.
In terms of adjuvant treatment, a recent case series of 60 patients with BIA-ALCL corroborated the recommendations agreed upon by the previous panel that proper management for women with capsule-confined BIA-ALCL should be limited to capsulectomy and implant removal.3 The authors’ extended, postdiagnosis follow-up (median, 2 years) of these patients revealed that women who present with a mass have a more aggressive clinical course that may be fatal, which, in their opinion, justifies the use of adjuvant chemotherapy.56 On the other hand, although BIA-ALCL is mentioned in the National Comprehensive Cancer Network’s Non-Hodgkin’s Lymphoma guidelines, they state that currently, the optimal management strategy for BIA-ALCL localized to the capsule or seroma is unclear.57 Our updated systematic literature review revealed that among BIA-ALCL patients with stage IE disease, over 50% received chemotherapy, suggesting a lack of familiarity with BIA-ALCL, treatment guidance received, or agreement with expert opinion on the recommended conservative management by medical oncologists for most cases of BIA-ALCL localized to the affected breast. Along these lines, our panelists firmly disagreed that adjuvant chemotherapy and radiation therapy should be offered to all patients with BIA-ALCL.
With regard to surveillance, breast imaging with ultrasound every 6 months for at least 5 years was agreed upon with high concordance (and longer if reimplantation is performed); panelists disagreed with moderate concordance that PET/CT should be the optimal imaging modality, which goes against how oncologists usually monitor lymphoma patients for recurrence. Finally, the current panel reaffirmed that BIA-ALCL is generally a biologically less aggressive disease than systemic ALK-negative ALCL involving lymph nodes and organs outside the breasts and that removing the affected implant and as much of the surrounding fibrous capsule as possible should prevent progression and recurrence of the lymphoma. Some panelists highlighted that, to perform a complete capsulectomy, a plastic surgeon may need to call upon a surgical oncology colleague to assist with this procedure, which the plastic surgeon may not be accustomed to performing. However, if BIA-ALCL extends beyond the capsule and/or presents as a mass, they agreed that the prognosis is worse and may be fatal. In such cases, they were uncertain if chemotherapy or radiation therapy should only be offered to patients with BIA-ALCL who have disease beyond the capsule. Panelists were also uncertain if radiation therapy should be offered only to those who present with a mass, but they disagreed with moderate discordance that chemotherapy should only be offered to these patients. They agreed, though, that breast irradiation should be offered if the patient has persistent disease and is ineligible for additional surgery or if they present with localized recurrence.
There are a few limitations of our study. First, because evidence directly addressing many of the statements did not exist, panelists might have largely relied on their own preconceptions or input from other experts to determine their ratings. It is unclear, however, how this could have influenced the direction and magnitude of the aggregate results. Second, we only asked panelists to rate their level of agreement with each statement and not the level of evidence supporting each item’s validity. Rating the level of evidence for validity was not an aim of our process, because we were not attempting to develop clinical guidelines, but this would be an important question to ask in future studies as the evidence base strengthens. Third, we selected the panelists purposively to ensure a balance among disciplines and perspectives. Although we used the results of our literature search to guide our selection, the subjective nature of this recruitment process could bias our results. Finally, although we asked all nonpanelists to provide objective data to the panel only when called upon, clinical experience and information presented by the observers present at the meeting may have potentially influenced the panelists’ final ratings.
In conclusion, our study integrates the available evidence and the assessment of a multidisciplinary expert panel to provide clinical guidance to plastic surgeons, surgical oncologists, medical oncologists, and radiation oncologists on key, unresolved issues regarding BIA-ALCL. Our hope is that increased awareness of this disease will extend into the cancer community so that potentially unnecessary chemotherapy and radiation therapy will not be administered for most patients with capsule-confined BIA-ALCL and that the appropriate surveillance and prognostic information is conducted by oncologists and communicated to patients, respectively. Additional data collection of detailed clinical information in breast implant registries will be necessary to support these statement ratings and enhance our understanding of the biology, treatment, and outcomes associated with this disease.
ACKNOWLEDGMENTS
We thank the invaluable contributions from each structured expert consultation panel member, without whom this study could not have been performed. In addition, we thank Patrick Orr for his operational assistance organizing the panel meeting.
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. This study was supported, in part, by Allergan, LLC, which did not have a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Kim B, Roth C, Chung KC, et al. Anaplastic large cell lymphoma and breast implants: a systematic review. Plast Reconstr Surg. 2011;127:2141–2150. doi: 10.1097/PRS.0b013e3182172418. [DOI] [PubMed] [Google Scholar]
- 2.U.S. Food and Drug Administration Center for Devices and Radiological Health. Anaplastic large cell lymphoma (ALCL) in women with breast implants: preliminary FDA findings and analyses. Available at: http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/BreastImplants/ucm239996.htm. Accessed June 4, 2014.
- 3.Kim B, Roth C, Young VL, et al. Anaplastic large cell lymphoma and breast implants: results from a structured expert consultation process. Plast Reconstr Surg. 2011;128:629–639. doi: 10.1097/PRS.0b013e31821f9f23. [DOI] [PubMed] [Google Scholar]
- 4.Aladily TN, Medeiros LJ, Amin MB, et al. Anaplastic large cell lymphoma associated with breast implants: a report of 13 cases. Am J Surg Pathol. 2012;36:1000–1008. doi: 10.1097/PAS.0b013e31825749b1. [DOI] [PubMed] [Google Scholar]
- 5.Alexander W, Anthony S. Secondary cutaneous involvement by breast implant-associated, ALK-negative anaplastic large cell lymphoma (ALCL)—report of a case with fatal outcome. Am J Dermatopathol. 2011;33:423. [Google Scholar]
- 6.Arbelaez A, Catley L, Pool L. Breast implant related anaplastic large cell lymphoma ALK-(ALCL ALK-)—case report. Blood. 2013;122 [Google Scholar]
- 7.Bautista-Quach MA, Nademanee A, Weisenburger DD, et al. Implant-associated primary anaplastic large-cell lymphoma with simultaneous involvement of bilateral breast capsules. Clin Breast Cancer. 2013;13:492–495. doi: 10.1016/j.clbc.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 8.Carty MJ, Pribaz JJ, Antin JH, et al. A patient death attributable to implant-related primary anaplastic large cell lymphoma of the breast. Plast Reconstr Surg. 2011;128:112e–118e. doi: 10.1097/PRS.0b013e318221db96. [DOI] [PubMed] [Google Scholar]
- 9.De Silva IM, Teague JA, Blake WE. Breast implant associated anaplastic large cell lymphoma: a case report and reconstructive option. J Plast Reconstr Aesthet Surg. 2013;66:1773–1776. doi: 10.1016/j.bjps.2013.04.049. [DOI] [PubMed] [Google Scholar]
- 10.De Torres Olombrada M, Garcia T, Caballero P, et al. Anaplastic large cell lymphoma associated with breast implant: a case report and review of the literature. Rep Prac Oncol Radiother. 2013;18:S275–S276. [Google Scholar]
- 11.Do V, Shifrin DA, Oostendorp L. Lymphoma of the breast capsule in a silicone implant-reconstructed patient. Am Surg. 2010;76:1030–1031. [PubMed] [Google Scholar]
- 12.Farace F, Bulla A, Marongiu F, et al. Anaplastic large cell lymphoma of the breast arising around mammary implant capsule: an Italian report. Aesthetic Plast Surg. 2013;37:567–571. doi: 10.1007/s00266-013-0120-6. [DOI] [PubMed] [Google Scholar]
- 13.George EV, Pharm J, Houston C, et al. Breast implant-associated ALK-negative anaplastic large cell lymphoma: a case report and discussion of possible pathogenesis. Int J Clin Exp Pathol. 2013;6:1631–1642. [PMC free article] [PubMed] [Google Scholar]
- 14.Hanson SE, Gutowski KA. Primary T-cell lymphoma associated with breast implant capsule. Plast Reconstr Surg. 2010;126:39e–41e. doi: 10.1097/PRS.0b013e3181dab2e0. [DOI] [PubMed] [Google Scholar]
- 15.Hurst E, Wadehra V, Menon G, et al. Breast implant associated anaplastic large cell lymphoma [ALCL]. Hematol Oncol. 2013;31:243. [Google Scholar]
- 16.Lazzeri D, Agostini T, Giannotti G, et al. Null-type anaplastic lymphoma kinase-negative anaplastic large cell lymphoma arising in a silicone breast implant capsule. Plast Reconstr Surg. 2011;127:159e–162e. doi: 10.1097/PRS.0b013e318213a1bd. [DOI] [PubMed] [Google Scholar]
- 17.Lechner MG, Megiel C, Church CH, et al. Survival signals and targets for therapy in breast implant-associated ALK–anaplastic large cell lymphoma. Clin Cancer Res. 2012;18:4549–4559. doi: 10.1158/1078-0432.CCR-12-0101. [DOI] [PubMed] [Google Scholar]
- 18.Lee M, Cooper B, Becker D. Keeping abreast of axillary masses. Lancet. 2012;380:1530. doi: 10.1016/S0140-6736(12)61334-8. [DOI] [PubMed] [Google Scholar]
- 19.Mies C, Goyal A, Bagg A, et al. Breast implant capsule-associated anaplastic large cell lymphoma (BIC-ALCL). Lab Invest. 2012;92:54A. [Google Scholar]
- 20.Neppalli AK, Koshy NV. Breast implant associated ALK negative anaplastic large T cell lymphoma. J Investig Med. 2013;61 [Google Scholar]
- 21.Parthasarathy M, Orrell J, Mortimer C, et al. Chemotherapy-resistant breast implant-associated anaplastic large cell lymphoma. BMJ Case Rep. 2013 doi: 10.1136/bcr-2013-201950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Popplewell L, Thomas SH, Huang Q, et al. Primary anaplastic large-cell lymphoma associated with breast implants. Leuk Lymphoma. 2011;52:1481–1487. doi: 10.3109/10428194.2011.574755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh E, Frost E, Morris EJ, et al. Anaplastic lymphoma masquerading as breast abscess in a patient with silicone implants. Breast J. 2013;19:543–545. doi: 10.1111/tbj.12161. [DOI] [PubMed] [Google Scholar]
- 24.Smith TJ, Ramsaroop R. Breast implant related anaplastic large cell lymphoma presenting as late onset peri-implant effusion. Breast. 2012;21:102–104. doi: 10.1016/j.breast.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 25.Sørensen K, Murphy J, Lennard A, et al. Anaplastic large cell lymphoma in a reconstructed breast using a silicone implant: a UK case report. J Plast Reconstr Aesthet Surg. 2014;67:561–563. doi: 10.1016/j.bjps.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 26.Talagas M, Charles-Petillon F, Uguen A, et al. Fine needle aspiration cytology of ALK negative T-cell anaplastic large-cell lymphoma associated with breast implant. Acta Cytol. 2013;57:53. [Google Scholar]
- 27.Taylor KO, Webster HR, Prince HM. Anaplastic large cell lymphoma and breast implants: five Australian cases. Plast Reconstr Surg. 2012;129:610e–617e. doi: 10.1097/PRS.0b013e3182450aae. [DOI] [PubMed] [Google Scholar]
- 28.Thompson PA, Lade S, Webster H, et al. Effusion-associated anaplastic large cell lymphoma of the breast: time for it to be defined as a distinct clinico-pathological entity. Haematologica. 2010;95:1977–1979. doi: 10.3324/haematol.2010.026237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weathers WM, Wolfswinkel EM, Hatef DA, et al. Implant-associated anaplastic large cell lymphoma of the breast: insight into a poorly understood disease. Can J Plast Surg. 2013;21:95–98. doi: 10.1177/229255031302100209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zakhary JM, Hamidian Jahromi A, Chaudhery S, et al. Anaplastic large cell lymphoma in the setting of textured breast implant: a call for patients and physicians education. J La State Med Soc. 2013;165:26–29. [PubMed] [Google Scholar]
- 31.Gidengil CA, Predmore Z, Mattke S, et al. Breast implant-associated anaplastic large cell lymphoma: a systematic review. Plast Reconstr Surg. doi: 10.1097/PRS.0000000000001037. In Press. [DOI] [PubMed] [Google Scholar]
- 32.Fitch K, Bernstein SJ, Aguilar MS, et al. The RAND/UCLA Appropriateness Method User’s Manual. Vol. 126. Santa Monica, Calif.: RAND Corporation; 2001. [Google Scholar]
- 33.Shekelle PG, Kahan JP, Bernstein SJ, et al. The reproducibility of a method to identify the overuse and underuse of medical procedures. N Engl J Med. 1998;338:1888–1895. doi: 10.1056/NEJM199806253382607. [DOI] [PubMed] [Google Scholar]
- 34.Hodgson DC, Brierley JD, Cernat G, et al. The consistency of panelists’ appropriateness ratings: do experts produce clinically logical scores for rectal cancer treatment? Health Policy. 2005;71:57–65. doi: 10.1016/j.healthpol.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 35.Hemingway H, Crook AM, Feder G, et al. Underuse of coronary revascularization procedures in patients considered appropriate candidates for revascularization. N Engl J Med. 2001;344:645–654. doi: 10.1056/NEJM200103013440906. [DOI] [PubMed] [Google Scholar]
- 36.Gale RP, Park RE, Dubois R, et al. Delphi-panel analysis of appropriateness of high-dose chemotherapy and blood cell or bone marrow autotransplants in women with breast cancer. Clin Transplant. 2000;14:32–41. doi: 10.1034/j.1399-0012.2000.140107.x. [DOI] [PubMed] [Google Scholar]
- 37.Dubois RW, Swetter SM, Atkins M, et al. Developing indications for the use of sentinel lymph node biopsy and adjuvant high-dose interferon alfa-2b in melanoma. Arch Dermatol. 2001;137:1217–1224. doi: 10.1001/archderm.137.9.1217. [DOI] [PubMed] [Google Scholar]
- 38.Poston GJ, Adam R, Alberts S, et al. OncoSurge: a strategy for improving resectability with curative intent in metastatic colorectal cancer. J Clin Oncol. 2005;23:7125–7134. doi: 10.1200/JCO.2005.08.722. [DOI] [PubMed] [Google Scholar]
- 39.Beets-Tan RG, Lambregts DM, Maas M, et al. Magnetic resonance imaging for the clinical management of rectal cancer patients: recommendations from the 2012 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol. 2013;23:2522–2531. doi: 10.1007/s00330-013-2864-4. [DOI] [PubMed] [Google Scholar]
- 40.Ludt S, Urban E, Eckardt J, et al. Evaluating the quality of colorectal cancer care across the interface of healthcare sectors. PLoS One. 2013;8:e60947. doi: 10.1371/journal.pone.0060947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pinto C, Barone CA, Girolomoni G, et al. American Society of Clinical Oncology; European Society of Medical Oncology. Management of skin toxicity associated with cetuximab treatment in combination with chemotherapy or radiotherapy. Oncologist. 2011;16:228–238. doi: 10.1634/theoncologist.2010-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brar SS, Mahar AL, Helyer LK, et al. Processes of care in the multidisciplinary treatment of gastric cancer: results of a RAND/UCLA expert panel. JAMA Surg. 2014;149:18–25. doi: 10.1001/jamasurg.2013.3959. [DOI] [PubMed] [Google Scholar]
- 43.Dixon M, Cardoso R, Tinmouth J, et al. Gastric Cancer Processes of Care Expert Panel. What studies are appropriate and necessary for staging gastric adenocarcinoma? Results of an international RAND/UCLA expert panel. Gastric Cancer. 2014;17:377–382. doi: 10.1007/s10120-013-0262-x. [DOI] [PubMed] [Google Scholar]
- 44.Dixon M, Mahar A, Paszat L, et al. What provider volumes and characteristics are appropriate for gastric cancer resection? Results of an international RAND/UCLA expert panel. Surgery. 2013;154:1100–1109. doi: 10.1016/j.surg.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 45.Dixon M, Seevaratnam R, Wirtzfeld D, et al. A RAND/UCLA appropriateness study of the management of familial gastric cancer. Ann Surg Oncol. 2013;20:533–541. doi: 10.1245/s10434-012-2584-z. [DOI] [PubMed] [Google Scholar]
- 46.Brar S, Law C, McLeod R, et al. International Multidisciplinary Expert Panel. Defining surgical quality in gastric cancer: a RAND/UCLA appropriateness study. J Am Coll Surg. 2013;217:347–357.e1. doi: 10.1016/j.jamcollsurg.2013.01.067. [DOI] [PubMed] [Google Scholar]
- 47.Higashi T, Nakamura F, Shimada Y, et al. Quality of gastric cancer care in designated cancer care hospitals in Japan. Int J Qual Health Care. 2013;25:418–428. doi: 10.1093/intqhc/mzt041. [DOI] [PubMed] [Google Scholar]
- 48.Coburn N, Seevaratnam R, Paszat L, et al. Optimal management of gastric cancer: results from an international RAND/UCLA expert panel. Ann Surg. 2014;259:102–108. doi: 10.1097/SLA.0b013e318288dd2b. [DOI] [PubMed] [Google Scholar]
- 49.Gore ME, Bellmunt J, Eisen T, et al. Evaluation of treatment options for patients with advanced renal cell carcinoma: assessment of appropriateness, using the validated semi-quantitative RAND corporation/University of California, Los Angeles methodology. Eur J Cancer. 2012;48:1038–1047. doi: 10.1016/j.ejca.2012.02.058. [DOI] [PubMed] [Google Scholar]
- 50.Tombal B, Andriole GL, de la Taille A, et al. Clinical judgment versus biomarker prostate cancer gene 3: which is best when determining the need for repeat prostate biopsy? Urology. 2013;81:998–1004. doi: 10.1016/j.urology.2012.11.069. [DOI] [PubMed] [Google Scholar]
- 51.Tombal B, Ameye F, de la Taille A, et al. Biopsy and treatment decisions in the initial management of prostate cancer and the role of PCA3; a systematic analysis of expert opinion. World J Urol. 2012;30:251–256. doi: 10.1007/s00345-011-0721-0. [DOI] [PubMed] [Google Scholar]
- 52.Gale RP, Park RE, Dubois RW, et al. Delphi-panel analysis of appropriateness of high-dose therapy and bone marrow autotransplants in newly diagnosed multiple myeloma. Leuk Lymphoma. 1999;33:511–519. doi: 10.3109/10428199909058455. [DOI] [PubMed] [Google Scholar]
- 53.Gale RP, Park RE, Dubois RW, et al. Delphi-panel analysis of appropriateness of high-dose therapy and bone marrow transplants in chronic myelogenous leukemia in chronic phase. Leuk Res. 1999;23:817–826. doi: 10.1016/s0145-2126(99)00097-1. [DOI] [PubMed] [Google Scholar]
- 54.Gale RP, Park RE, Dubois RW, et al. Delphi-panel analysis of appropriateness of high-dose therapy and bone marrow transplants in adults with acute myelogenous leukemia in 1st remission. Leuk Res. 1999;23:709–718. doi: 10.1016/s0145-2126(99)00044-2. [DOI] [PubMed] [Google Scholar]
- 55.Gale RP, Park RE, Dubois R, et al. Delphi-panel analysis of appropriateness of high-dose chemotherapy and blood cell or bone marrow autotransplants in diffuse large-cell lymphoma. Leuk Lymphoma. 1998;32:139–149. doi: 10.3109/10428199809059254. [DOI] [PubMed] [Google Scholar]
- 56.Miranda RN, Aladily TN, Prince HM, et al. Breast implant-associated anaplastic large-cell lymphoma: long-term follow-up of 60 patients. J Clin Oncol. 2014;32:114–120. doi: 10.1200/JCO.2013.52.7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology, non-Hodgkin’s lymphomas. Available at: http://www.nccn.org/professionals/physician_gls/pdf/nhl.pdf. Accessed June 4, 2014. [DOI] [PubMed]


