Abstract
Since it was first described nearly three decades ago, endoscopic papillectomy (EP) has been utilized as a less invasive, alternative therapy for adenoma of the major duodenal papilla. In this article, we review the recent advances in EP, especially those pertaining to endoscopic ultrasonography (EUS), intraductal ultrasonography (IDUS), and pancreatic stent placement for the prevention of postpapillectomy pancreatitis. Because EUS and IDUS have similar diagnostic accuracies, either modality can be used for the preprocedural evaluation of ampullary tumors. Nevertheless, further technical refinements are required for a more precise evaluation. Given the paucity of data on the usefulness of EUS and/or IDUS during follow-up after EP, a well-designed study is warranted. Furthermore, pancreatic stent placement appears to have a protective effect against postpapillectomy pancreatitis; however, a prospective, randomized, controlled study with a larger number of patients is needed to assess this finding. Moreover, since pancreatic stent placement after EP is not always successful, various novel techniques have been developed to ensure reliable stent placement. Despite the recent advances in EP, further technical refinements and studies are needed to confirm their efficacy.
Keywords: Ampulla of Vater, Ampullary tumors, Endoscopic resection, Endoscopic papillectomy
INTRODUCTION
Since the first report of an endoscopic papillectomy (EP) in 1983,1 EP has been established as a less invasive, alternative therapy for adenoma of the major duodenal papilla. Both prospective and retrospective studies have reported the safety and efficacy of this procedure.2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21 The complete resection rate of EP ranges from 54% to 92% and the recurrence rate ranges from 0% to 33%.2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21 The rate of complications after EP has varied from study to study, and is as high as 29%.2,3,4,5,6,7,8,9,11 The overall mortality rate after EP is 0.4%.11 The indications, techniques, and outcomes of EP are described comprehensively in previously published reviews and guidelines.22,23,24,25,26,27,28,29
This review will focus on the recent advances in EP, especially those pertaining to endoscopic ultrasonography (EUS) and the placement of pancreatic duct stents for the prevention of postpapillectomy pancreatitis.
ENDOSCOPIC ULTRASONOGRAPHY AND INTRADUCTAL ULTRASONOGRAPHY
Role in preprocedural evaluation
After an endoscopic exam and biopsy of the ampullary tumor, an assessment of tumor extent is vital before commencing EP. Intraductal extension of the tumor may preclude complete endoscopic resection and may be a criterion for surgical resection.5,7 Endoscopic retrograde cholangiopancreatography (ERCP) can demonstrate intraductal extension of the tumor, but may be complicated by ERCP-related complications such as pancreatitis. On the other hand, EUS is a less invasive alternative to ERCP for the assessment of tumor extent and periampullary lymph node involvement (Fig. 1). The techniques of EUS examination have been meticulously described in a previous review.30 To obtain high quality EUS images of the ampulla, the transducer is placed perpendicular to the ampulla of Vater, duodenal peristalsis is paralyzed with anticholinergics, and water is infused to achieve submersion without bubbles. Since the ampulla can be easily compressed by the transducer and the lesion on the ampulla may be friable and bleed easily, the transducer should be maintained at some distance from the ampulla.
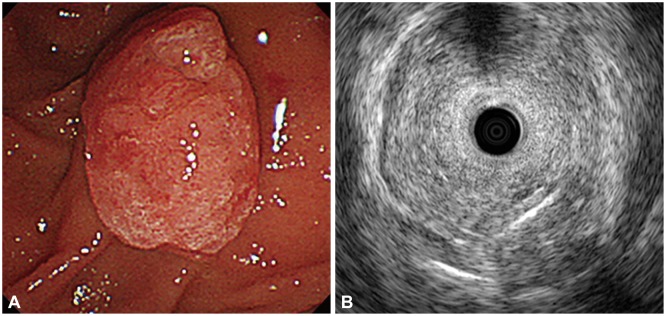
Fig. 1.

Pre-procedural evaluation of ampullary adenoma. (A) Endoscopic exam demonstrates a pale, granular lesion on the major duodenal papilla. (B) Endoscopic ultrasonography with radial echoendoscope shows that the adenoma is limited to mucosa.
Previous studies repeatedly demonstrated the superiority of EUS to transabdominal ultrasonography, computed tomography, and magnetic resonance imaging in terms of the assessment of the extent of ampullary tumors.31,32,33,34,35,36,37,38 In a meta-analysis of 14 studies involving 422 patients, the pooled sensitivity and specificity of EUS in the diagnosis of T1-stage tumors were 77% and 78%, respectively.33 In a recent retrospective cohort study of 119 patients with ampullary tumors, the sensitivities and specificities of EUS (80% and 93%) and ERCP (83% and 93%) were comparable.19 The overall accuracy of EUS for the assessment of tumor extent was 90%. The authors concluded that ERCP and attempts at endoscopic resection of the ampullary tumor should be avoided in selected cases of local tumor invasion or intraductal extension detected by EUS. However, the findings of that study are limited by its retrospective design, and a prospective study with predefined criteria for proceeding from EUS to ampullary tumor resection is needed. While EP is a potentially curative treatment for ampullary adenoma, it can only serve as a diagnostic method and/or palliative treatment for ampullary carcinoma, even in capable hands. EUS would be useful in minimizing incomplete endoscopic resection of ampullary tumors.
There has been some disagreement regarding the need for EUS before the resection of ampullary adenomas. The American Society of Gastrointestinal Endoscopy guidelines recommended an EUS examination before endoscopic or surgical resection when available.28 There has been no consensus with respect to the type of echoendoscope. Many endoscopists use a radial echoendoscope alone, some use a linear echoendoscope alone, and some use both echoendoscope types.6,19,20,31,32,33,39
In intraductal ultrasonography (IDUS), an ultrasound probe is inserted through the working channel of the duodenoscope and into the bile and pancreatic ducts.40,41 IDUS may be better than EUS for detailed imaging of the anatomy of the ampulla of Vater because it has a higher ultrasound frequency and obtains images in a perpendicular direction to the duct (Fig. 2).40,42 In a recently published study of 48 patients with ampullary tumors, EUS and IDUS showed the same (85%) overall diagnostic accuracy.41 There were no complications related to EUS or IDUS. The authors proposed that either EUS or IDUS can be used for the preprocedural evaluation of ampullary tumors. However, EUS could not visualize the muscularis propria of the duodenum in two patients and had difficulty visualizing the pancreatic duct in one patient. In addition, IDUS could not visualize the sphincter of Oddi in one patient. The diagnosis of foci of adenocarcinoma or focal invasion of the duodenal wall layer presented difficulties with both modalities. As an editorial on this study aptly pointed out, further technical refinements of EUS and IDUS are required for a more precise assessment of ampullary tumors.43
Fig. 2.
Another case of preprocedural evaluation of ampullary adenoma. (A) Endoscopic exam shows a protuberant, hyperemic lesion at the major duodenal papilla. (B) Intraductal ultrasonography reveals that the adenoma is limited to mucosa and muscularis propria is intact (Kindly provided by Drs. Jong Ho Moon and Hyun Jong Choi from SoonchunHyang University Bucheon Hospital).
Role in follow-up after endoscopic papillectomy
There is still no consensus regarding the interval and method of follow-up after EP. Usually, a duodenoscopic exam and biopsy are used. In contrast to the robust data on the usefulness of EUS and/or IDUS in preprocedural evaluation, there is a paucity of data concerning the usefulness of EUS and/or IDUS during follow-up after EP. A multicenter prospective study involving 93 patients used a duodenoscopic exam, biopsy, and EUS with or without ERCP during post-EP follow-up.20 Another prospective study used EUS during follow-up, but only "if required" in addition to a clinical exam, transabdominal ultrasonography, and endoscopy with biopsy.18 However, neither study was designed to evaluate the usefulness of EUS during post-EP follow-up. An assessment of the usefulness of EUS and/or IDUS during follow-up after EP would require a multicenter study conducted over an extended period of time.
PANCREATIC STENT PLACEMENT TO PREVENT POSTPAPILLECTOMY PANCREATITIS
Rationale for pancreatic stent placement
Along with the tumor, EP removes tissue around the bile duct and pancreatic duct orifices located at the major duodenal papilla.27 Therefore, it is associated with an increased risk of postprodecural pancreatitis. A meta-analysis of five studies involving 481 patients showed that patients in the no stent group had a 3-fold increased risk of post-ERCP pancreatitis.44 Prophylactic pancreatic duct stent placement has been widely used to prevent post-ERCP pancreatitis (Fig. 3).
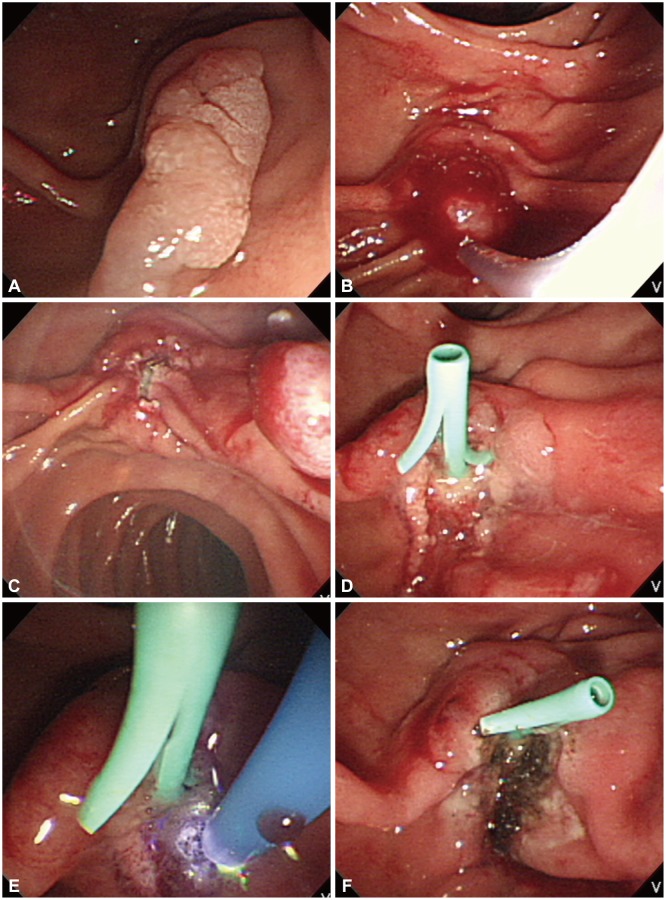
Fig. 3.
Endoscopic papillectomy of ampullary adenoma. (A) A pale, elongated lesion is seen on the major duodenal papilla. (B) The adenoma is grasped with a standard polypectomy snare. (C) The tumor is removed en bloc after application of electrosurgical current. (D) A 5 Fr, 3 cm pancreatic stent is placed. (E) Hemostasis is achieved with argon plasma coagulation. (F) Postpapillectomy ulcer is clear without bleeding.
After three decades of EP, the ability of pancreatic duct stent placement to prevent postpapillectomy pancreatitis has not yet been established. Many endoscopists recommend prophylactic pancreatic stent placement in every patient who undergoes EP.7,10,13,15,25,45 Some recommend selective pancreatic stent placement-i.e., only when the pancreatic duct is not patulous and cannot be identified easily.2,5,6,7,11,12,15,20,46
One of the most cited studies in favor of pancreatic stent placement is a prospective, randomized, controlled trial conducted by Harewood et al.10 This study enrolled 19 patients who underwent en bloc EP, 10 of whom were randomized to pancreatic stent placement. Postpapillectomy pancreatitis occurred in three patients who were all from the unstented group (33% vs. 0%, p=0.03). Prophylactic pancreatic stent placement has been recommended and practiced on the basis of this finding. However, this study is limited by the small number of enrolled patients. Hence, further large-scale studies are needed to confirm the protective effect of prophylactic pancreatic stent placement.
Methods of facilitating pancreatic stent placement
Although some endoscopists claim that it is easy to identify and cannulate the pancreatic duct orifice after EP, this is not always the case. The orifice may be buried under the coagulum in the base of a postpapillectomy ulcer. Therefore, quite a few studies examined methods of facilitating pancreatic stent placement (Table 1).4,43,47,48,49,50,51
Table 1.
Techniques to Facilitate Pancreatic Stent Placement during Endoscopic Papillect

Routine preresection biliary and pancreatic sphincterotomy was used with pancreatic stent placement in a retrospective analysis of 41 patients with ampullary tumors.4 This method inevitably resulted in piecemeal resection. There was a single episode of postpapillectomy pancreatitis and a single episode of moderately severe postsphincterotomy bleeding requiring the transfusion of seven units of packed red blood cells. Although 92% of the ampullary adenoma patients remained recurrence-free over a mean follow-up of 19 months, multiple sessions (range, 1 to 5) of ERCP were needed for eradication. However, long-term results are not available for this method.
Another interesting method is pancreatic duct wire-guided EP.43,47 This method secures a route for pancreatic stent placement prior to EP. Before the ampullary tumor is snared, a 0.035-inch guidewire is placed in the pancreatic duct. Immediately after EP, a 5 Fr pancreatic stent with a flap is passed over the indwelling guidewire and placed across the pancreatic duct orifice. In all six patients enrolled in the pilot study, wire-guided EP resulted in en bloc resection without acute pancreatitis.47 After 9 years, the authors published their results with pancreatic duct wire-guided EP in 72 patients.43 Pancreatic stent placement after EP was successful in all patients. En bloc resection of the ampullary adenoma was achieved in 83% of patients. Postpapillectomy pancreatitis occurred in 8%, but it was mild in all cases and resolved with conservative management.
A case study of three patients with ampullary adenoma reported that the EUS-guided intraductal injection of methylene blue resulted in successful pancreatic stent placement after EP in all patients.52 Using a 25-gauge needle primed with diluted methylene blue, the main pancreatic duct was punctured under EUS guidance. Next, methylene blue was slowly injected into the duct until its flow was visible from the orifice. There were no episodes of postpapillectomy pancreatitis. This case study did not clarify whether the injection was performed after failed attempts at pancreatic cannulation.
One case report described the use of EUS-guided rendezvous pancreatic duct access for pancreatic stent placement during EP.48 After successful excision of the ampullary adenoma, the pancreatic orifice could not be located despite multiple protracted and futile attempts. Because the authors feared that the pancreatic duct was "thermally sealed shut," they undertook an EUS-guided transduodenal puncture of the main pancreatic duct. A 0.025-inch guidewire was advanced through the 19-gauge needle across the papilla and into the duodenum. Pancreatic duct cannulation was achieved alongside the guidewire and a 5 Fr pancreatic stent was placed, which traversed the puncture site. No postpapillectomy pancreatitis occurred. In the presence of an experienced endosonographer, EUS-guided pancreatic duct access seems feasible. There have been no further case reports or studies to verify this assumption.
One pilot study investigated the preresection placement of a newly developed insulated pancreatic stent.49 The ampullary tumor was snared and resected with the stent in place, and retrieved after perpendicular incision with a needle knife. This 5 Fr stent was composed of polytetrafluoroethylene, which was originally used in the inner tube of the delivery catheter of an esophageal metal stent. In 11 consecutive patients, there was no case of postpapillectomy pancreatitis. However, this method is limited by the difficulty of retrieving the resected specimen with the stent left in place. Hence, further studies with larger numbers of patients and long-term follow-up are needed to confirm these results.
To ensure reliable post-EP pancreatic stent placement, a method for placement above the pancreatic duct orifice (inside pancreatic stenting papillectomy) was devised and used in a prospective 10-patient pilot study.50 A 5 Fr, 5 cm straight plastic stent with flaps at the proximal and distal ends was used. The flap at the proximal end was cutoff and 4-cm-long braided medical silk was tied to the distal end. Prior to the snaring of the ampullary tumor, this pancreatic stent was inserted deeply and deliberately left inside the main pancreatic duct. Only 2 cm of the silk suture was left outside and endoscopically visible. After EP and specimen retrieval, the suture was grasped and pulled with biopsy forceps and the pancreatic stent was placed across the orifice. This novel method was successful in nine out of 10 patients. En bloc resection was performed in eight patients. The suture remained intact in eight patients after the application of an electrosurgical current. There were no episodes of postpapillectomy pancreatitis. Inside pancreatic stenting papillectomy seems practical, but its efficacy requires confirmation in further studies with larger patient numbers.
Another study of 56 consecutive patients with ampullary adenoma evaluated whether intraductal methylene blue injection prior to EP facilitated pancreatic stent placement.51 Before snaring of the ampullary tumor, the pancreatic duct was cannulated with a hydrophilic guidewire and 2 mL of diluted methylene blue was injected slowly and distributed along the main pancreatic duct. After resection, a 5 or 7 Fr straight pancreatic stent was placed. Pancreatic cannulation was successful in 89% of the patients. Postpapillectomy pancreatitis occurred in six patients: three who underwent pancreatic stent placement and three who did not (p=0.013). One case of pancreatitis occurred in a patient with successful pancreatic cannulation and methylene blue injection. One case of pancreatitis occurred in a patient with failed pancreatic cannulation. All pancreatitis cases were mild or moderate in severity and resolved with conservative management. Even though intraductal methylene blue injection prior to EP is a simple and seemingly safe method of facilitating pancreatic stent placement, the findings of this study are limited by the small sample size. Further controlled studies are necessary.
CONCLUSIONS
Over the last three decades, there have been many studies of EP as well as improvements in the techniques and modalities used for EP. As a result, EP has been established as a first-line effective therapy for adenoma of the major duodenal papilla. Despite recent advances pertaining to the roles of EUS and IDUS in preprocedural evaluation and the placement of pancreatic duct stents for the prevention of postpapillectomy pancreatitis, further technical refinements and studies to confirm their efficacy are needed.
Acknowledgments
The authors acknowledge and thank Drs. Jong Ho Moon and Hyun Jong Choi who provided high quality images of intraductal ultrasonography.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Suzuki K, Kantou U, Murakami Y. Two cases with ampullary cancer who underwent endosopic excision. Prog Dig Endosc. 1983;23:236–239. [Google Scholar]
- 2.Binmoeller KF, Boaventura S, Ramsperger K, Soehendra N. Endoscopic snare excision of benign adenomas of the papilla of Vater. Gastrointest Endosc. 1993;39:127–131. doi: 10.1016/s0016-5107(93)70051-6. [DOI] [PubMed] [Google Scholar]
- 3.Zádorová Z, Dvofák M, Hajer J. Endoscopic therapy of benign tumors of the papilla of Vater. Endoscopy. 2001;33:345–347. doi: 10.1055/s-2001-13693. [DOI] [PubMed] [Google Scholar]
- 4.Desilets DJ, Dy RM, Ku PM, et al. Endoscopic management of tumors of the major duodenal papilla: refined techniques to improve outcome and avoid complications. Gastrointest Endosc. 2001;54:202–208. doi: 10.1067/mge.2001.116564. [DOI] [PubMed] [Google Scholar]
- 5.Norton ID, Gostout CJ, Baron TH, Geller A, Petersen BT, Wiersema MJ. Safety and outcome of endoscopic snare excision of the major duodenal papilla. Gastrointest Endosc. 2002;56:239–243. doi: 10.1016/s0016-5107(02)70184-3. [DOI] [PubMed] [Google Scholar]
- 6.Kahaleh M, Shami VM, Brock A, et al. Factors predictive of malignancy and endoscopic resectability in ampullary neoplasia. Am J Gastroenterol. 2004;99:2335–2339. doi: 10.1111/j.1572-0241.2004.40391.x. [DOI] [PubMed] [Google Scholar]
- 7.Catalano MF, Linder JD, Chak A, et al. Endoscopic management of adenoma of the major duodenal papilla. Gastrointest Endosc. 2004;59:225–232. doi: 10.1016/s0016-5107(03)02366-6. [DOI] [PubMed] [Google Scholar]
- 8.Cheng CL, Sherman S, Fogel EL, et al. Endoscopic snare papillectomy for tumors of the duodenal papillae. Gastrointest Endosc. 2004;60:757–764. doi: 10.1016/s0016-5107(04)02029-2. [DOI] [PubMed] [Google Scholar]
- 9.Bohnacker S, Seitz U, Nguyen D, et al. Endoscopic resection of benign tumors of the duodenal papilla without and with intraductal growth. Gastrointest Endosc. 2005;62:551–560. doi: 10.1016/j.gie.2005.04.053. [DOI] [PubMed] [Google Scholar]
- 10.Harewood GC, Pochron NL, Gostout CJ. Prospective, randomized, controlled trial of prophylactic pancreatic stent placement for endoscopic snare excision of the duodenal ampulla. Gastrointest Endosc. 2005;62:367–370. doi: 10.1016/j.gie.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 11.Han J, Lee SK, Park DH, et al. Treatment outcome after endoscopic papillectomy of tumors of the major duodenal papilla. Korean J Gastroenterol. 2005;46:110–119. [PubMed] [Google Scholar]
- 12.Jung MK, Cho CM, Park SY, et al. Endoscopic resection of ampullary neoplasms: a single-center experience. Surg Endosc. 2009;23:2568–2574. doi: 10.1007/s00464-009-0464-9. [DOI] [PubMed] [Google Scholar]
- 13.Irani S, Arai A, Ayub K, et al. Papillectomy for ampullary neoplasm: results of a single referral center over a 10-year period. Gastrointest Endosc. 2009;70:923–932. doi: 10.1016/j.gie.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 14.Boix J, Lorenzo-Zúñiga V, Moreno de Vega V, Domènech E, Gassull MA. Endoscopic resection of ampullary tumors: 12-year review of 21 cases. Surg Endosc. 2009;23:45–49. doi: 10.1007/s00464-008-9866-3. [DOI] [PubMed] [Google Scholar]
- 15.Yamao T, Isomoto H, Kohno S, et al. Endoscopic snare papillectomy with biliary and pancreatic stent placement for tumors of the major duodenal papilla. Surg Endosc. 2010;24:119–124. doi: 10.1007/s00464-009-0538-8. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen N, Shah JN, Binmoeller KF. Outcomes of endoscopic papillectomy in elderly patients with ampullary adenoma or early carcinoma. Endoscopy. 2010;42:975–977. doi: 10.1055/s-0030-1255875. [DOI] [PubMed] [Google Scholar]
- 17.Heinzow HS, Lenz P, Lenze F, Domagk D, Domschke W, Meister T. Feasibility of snare papillectomy in ampulla of Vater tumors: meta-analysis and study results from a tertiary referral center. Hepatogastroenterology. 2012;59:332–335. doi: 10.5754/hge11414. [DOI] [PubMed] [Google Scholar]
- 18.Will U, Muller AK, Fueldner F, Wanzar I, Meyer F. Endoscopic papillectomy: data of a prospective observational study. World J Gastroenterol. 2013;19:4316–4324. doi: 10.3748/wjg.v19.i27.4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ridtitid W, Tan D, Schmidt SE, et al. Endoscopic papillectomy: risk factors for incomplete resection and recurrence during long-term follow-up. Gastrointest Endosc. 2014;79:289–296. doi: 10.1016/j.gie.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Napoleon B, Gincul R, Ponchon T, et al. Endoscopic papillectomy for early ampullary tumors: long-term results from a large multicenter prospective study. Endoscopy. 2014;46:127–134. doi: 10.1055/s-0034-1364875. [DOI] [PubMed] [Google Scholar]
- 21.Ismail S, Marianne U, Heikki J, Jorma H, Leena K. Endoscopic papillectomy, single-centre experience. Surg Endosc. 2014;28:3234–3239. doi: 10.1007/s00464-014-3596-5. [DOI] [PubMed] [Google Scholar]
- 22.Moon JH, Choi HJ, Lee YN. Current status of endoscopic papillectomy for ampullary tumors. Gut Liver. 2014;8:598–604. doi: 10.5009/gnl14099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Palma GD. Endoscopic papillectomy: indications, techniques, and results. World J Gastroenterol. 2014;20:1537–1543. doi: 10.3748/wjg.v20.i6.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El Hajj II, Coté GA. Endoscopic diagnosis and management of ampullary lesions. Gastrointest Endosc Clin N Am. 2013;23:95–109. doi: 10.1016/j.giec.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 25.Patel R, Varadarajulu S, Wilcox CM. Endoscopic ampullectomy: techniques and outcomes. J Clin Gastroenterol. 2012;46:8–15. doi: 10.1097/MCG.0b013e318233a844. [DOI] [PubMed] [Google Scholar]
- 26.Hernandez LV, Catalano MF. Endoscopic papillectomy. Curr Opin Gastroenterol. 2008;24:617–622. doi: 10.1097/MOG.0b013e3283088e12. [DOI] [PubMed] [Google Scholar]
- 27.Han J, Kim MH. Endoscopic papillectomy for adenomas of the major duodenal papilla (with video) Gastrointest Endosc. 2006;63:292–301. doi: 10.1016/j.gie.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 28.Standards of Practice Committee. Adler DG, Qureshi W, et al. The role of endoscopy in ampullary and duodenal adenomas. Gastrointest Endosc. 2006;64:849–854. doi: 10.1016/j.gie.2006.08.044. [DOI] [PubMed] [Google Scholar]
- 29.Bohnacker S, Soehendra N, Maguchi H, Chung JB, Howell DA. Endoscopic resection of benign tumors of the papilla of vater. Endoscopy. 2006;38:521–525. doi: 10.1055/s-2006-925263. [DOI] [PubMed] [Google Scholar]
- 30.Castillo C. Endoscopic ultrasound in the papilla and the periampullary region. World J Gastrointest Endosc. 2010;2:278–287. doi: 10.4253/wjge.v2.i8.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cannon ME, Carpenter SL, Elta GH, et al. EUS compared with CT, magnetic resonance imaging, and angiography and the influence of biliary stenting on staging accuracy of ampullary neoplasms. Gastrointest Endosc. 1999;50:27–33. doi: 10.1016/s0016-5107(99)70340-8. [DOI] [PubMed] [Google Scholar]
- 32.Skordilis P, Mouzas IA, Dimoulios PD, Alexandrakis G, Moschandrea J, Kouroumalis E. Is endosonography an effective method for detection and local staging of the ampullary carcinoma? A prospective study. BMC Surg. 2002;2:1. doi: 10.1186/1471-2482-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trikudanathan G, Njei B, Attam R, Arain M, Shaukat A. Staging accuracy of ampullary tumors by endoscopic ultrasound: meta-analysis and systematic review. Dig Endosc. 2014;26:617–626. doi: 10.1111/den.12234. [DOI] [PubMed] [Google Scholar]
- 34.Chen CH, Tseng LJ, Yang CC, Yeh YH, Mo LR. The accuracy of endoscopic ultrasound, endoscopic retrograde cholangiopancreatography, computed tomography, and transabdominal ultrasound in the detection and staging of primary ampullary tumors. Hepatogastroenterology. 2001;48:1750–1753. [PubMed] [Google Scholar]
- 35.Artifon EL, Couto D, Jr, Sakai P, da Silveira EB. Prospective evaluation of EUS versus CT scan for staging of ampullary cancer. Gastrointest Endosc. 2009;70:290–296. doi: 10.1016/j.gie.2008.11.045. [DOI] [PubMed] [Google Scholar]
- 36.Chen CH, Tseng LJ, Yang CC, Yeh YH. Preoperative evaluation of periampullary tumors by endoscopic sonography, transabdominal sonography, and computed tomography. J Clin Ultrasound. 2001;29:313–321. doi: 10.1002/jcu.1041. [DOI] [PubMed] [Google Scholar]
- 37.Manta R, Conigliaro R, Castellani D, et al. Linear endoscopic ultrasonography vs magnetic resonance imaging in ampullary tumors. World J Gastroenterol. 2010;16:5592–5597. doi: 10.3748/wjg.v16.i44.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen CH, Yang CC, Yeh YH, Chou DA, Nien CK. Reappraisal of endosonography of ampullary tumors: correlation with transabdominal sonography, CT, and MRI. J Clin Ultrasound. 2009;37:18–25. doi: 10.1002/jcu.20523. [DOI] [PubMed] [Google Scholar]
- 39.Wee E, Lakhtakia S, Gupta R, et al. The diagnostic accuracy and strength of agreement between endoscopic ultrasound and histopathology in the staging of ampullary tumors. Indian J Gastroenterol. 2012;31:324–332. doi: 10.1007/s12664-012-0248-3. [DOI] [PubMed] [Google Scholar]
- 40.Itoh A, Goto H, Naitoh Y, Hirooka Y, Furukawa T, Hayakawa T. Intraductal ultrasonography in diagnosing tumor extension of cancer of the papilla of Vater. Gastrointest Endosc. 1997;45:251–260. doi: 10.1016/s0016-5107(97)70267-0. [DOI] [PubMed] [Google Scholar]
- 41.Okano N, Igarashi Y, Hara S, et al. Endosonographic preoperative evaluation for tumors of the ampulla of vater using endoscopic ultrasonography and intraductal ultrasonography. Clin Endosc. 2014;47:174–177. doi: 10.5946/ce.2014.47.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Menzel J, Hoepffner N, Sulkowski U, et al. Polypoid tumors of the major duodenal papilla: preoperative staging with intraductal US, EUS, and CT: a prospective, histopathologically controlled study. Gastrointest Endosc. 1999;49(3 Pt 1):349–357. doi: 10.1016/s0016-5107(99)70012-x. [DOI] [PubMed] [Google Scholar]
- 43.Moon JH. Endoscopic diagnosis of ampullary tumors using conventional endoscopic ultrasonography and intraductal ultrasonography in the era of endoscopic papillectomy: advantages and limitations. Clin Endosc. 2014;47:127–128. doi: 10.5946/ce.2014.47.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singh P, Das A, Isenberg G, et al. Does prophylactic pancreatic stent placement reduce the risk of post-ERCP acute pancreatitis? A meta-analysis of controlled trials. Gastrointest Endosc. 2004;60:544–550. doi: 10.1016/s0016-5107(04)02013-9. [DOI] [PubMed] [Google Scholar]
- 45.Chini P, Draganov PV. Diagnosis and management of ampullary adenoma: the expanding role of endoscopy. World J Gastrointest Endosc. 2011;3:241–247. doi: 10.4253/wjge.v3.i12.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chang WI, Min YW, Yun HS, et al. Prophylactic pancreatic stent placement for endoscopic duodenal ampullectomy: a single-center retrospective study. Gut Liver. 2014;8:306–312. doi: 10.5009/gnl.2014.8.3.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moon JH, Cha SW, Cho YD, et al. Wire-guided endoscopic snare papillectomy for tumors of the major duodenal papilla. Gastrointest Endosc. 2005;61:461–466. doi: 10.1016/s0016-5107(04)02649-5. [DOI] [PubMed] [Google Scholar]
- 48.Keenan J, Mallery S, Freeman ML. EUS rendezvous for pancreatic stent placement during endoscopic snare ampullectomy. Gastrointest Endosc. 2007;66:850–853. doi: 10.1016/j.gie.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 49.Hwang JC, Kim JH, Lim SG, Yoo BM, Cho SW. Endoscopic resection of ampullary adenoma after a new insulated plastic pancreatic stent placement: a pilot study. J Gastroenterol Hepatol. 2010;25:1381–1385. doi: 10.1111/j.1440-1746.2010.06273.x. [DOI] [PubMed] [Google Scholar]
- 50.Nakahara K, Okuse C, Suetani K, et al. A novel endoscopic papillectomy after a pancreatic stent placement above the pancreatic duct orifice: inside pancreatic stenting papillectomy. J Clin Gastroenterol. 2014;48:796–800. doi: 10.1097/MCG.0000000000000019. [DOI] [PubMed] [Google Scholar]
- 51.Poincloux L, Scanzi J, Goutte M, et al. Pancreatic intubation facilitated by methylene blue injection decreases the risk for postpapillectomy acute pancreatitis. Eur J Gastroenterol Hepatol. 2014;26:990–995. doi: 10.1097/MEG.0000000000000146. [DOI] [PubMed] [Google Scholar]
- 52.Carrara S, Arcidiacono PG, Diellou AM, et al. EUS-guided methylene blue injection into the pancreatic duct as a guide for pancreatic stenting after ampullectomy. Endoscopy. 2007;39(Suppl 1):E151–E152. doi: 10.1055/s-2007-966242. [DOI] [PubMed] [Google Scholar]