Abstract
AIM: To elucidate the molecular mechanisms underlying hepatitis B virus (HBV) occult infection of genotype C.
METHODS: A total of 10 types of hepatitis B surface antigen (HBsAg) variants from a Korean occult cohort were used. After a complete HBV genome plasmid mutated such that it does not express HBsAg and plasmid encoding, each HBsAg variant was transiently co-transfected into HuH-7 cells. The secretion capacity and intracellular expression of the HBV virions and HBsAgs in their respective variants were analyzed using real-time quantitative polymerase chain reaction assays and commercial HBsAg enzyme-linked immunosorbent assays, respectively.
RESULTS: All variants exhibited lower levels of HBsAg secretion into the medium compared with the wild type. In particular, in eight of the ten variants, very low levels of HBsAg secretion that were similar to the negative control were detected. In contrast, most variants (9/10) exhibited normal virion secretion capacities comparable with, or even higher than, the wild type. This provided new insight into the intrinsic nature of occult HBV infection, which leads to HBsAg sero-negativeness but has horizontal infectivity. Furthermore, most variants generated higher reactive oxidative species production than the wild type. This finding provides potential links between occult HBV infection and liver disease progression.
CONCLUSION: The presently obtained data indicate that deficiency in the secretion capacity of HBsAg variants may have a pivotal function in the occult infections of HBV genotype C.
Keywords: Occult infection, Hepatitis B virus, Hepatitis B surface antigen, Variants, Genotype C, Reactive oxidative species
Core tip: The presently obtained data indicate that deficiency in the secretion capacity of hepatitis B surface antigen (HBsAg), but not virion, may have a pivotal function in occult infections of hepatitis B virus (HBV) genotype C, at least in occult infections in South Korea. This provided new insight into the intrinsic nature of HBV occult infections, which lead to HBsAg sero-negativeness but horizontal infectivity. In addition, reactive oxidative species production via possible induction of endoplasmic reticulum stress in hepatocytes provide a probable explanation for the links between occult infection and liver disease progression.
INTRODUCTION
Hepatitis B virus (HBV) infection is a global health problem, and more than 350 million people are chronic carriers of the virus[1]. The infection is associated with a large spectrum of clinical manifestations ranging from acute or fulminant hepatitis to various forms of chronic infection, including asymptomatic carriers, chronic hepatitis, cirrhosis, and hepatocellular carcinoma (HCC)[2,3]. South Korea is recognized as an endemic area of HBV infection[4]. Furthermore, extraordinary prevalence of genotype C2 has been reported in this area[5]; this genotype is known to be more virulent and more prone to mutation than genotype B[6], and it might contribute to the distribution of characteristic HBV mutation patterns related to the progression of liver diseases[7-16].
Occult HBV infection is defined as the infection state negative for hepatitis B surface antigen (HBsAg) serology, but it has shown viral genome persistence in infected individuals[17-19]. In general, HBV infection is diagnosed when the circulating HBsAg is serologically detected. However, recent progress in molecular-based technology, such as polymerase chain reaction (PCR)-based methods, has enabled HBV infection to be proven from HBsAg negative individuals with or without circulating antibodies to HBsAg and/or hepatitis B core antigen[20-22]. A large body of evidence has demonstrated that HBV occult infection is highly prevalent, particularly in HBV endemic areas, and it has distinct clinical entities. In particular, it is significantly related to severe forms of liver disease, such as cirrhosis and HCC[23,24]. Furthermore, in hepatitis C virus (HCV) infected patients, liver disease could worsen HCV infection[25-27].
Recently, there has been significant progress in obtaining an understanding of the molecular mechanisms that underlie occult HBV infection, together with increased clinical concerns throughout the world[26]. Among earlier findings, mutation in the HBsAg region, particularly in the “a” determinant, is regarded as a significant mechanism in relation to occult HBV infection. Structural alterations in HBsAg induced through mutations in or even outside the “a” determinant led to reduced affinity against the antibody in HBV diagnostic assays[26]. Furthermore, HBsAg mutations are known to contribute to HBV occult infections via reduction of virion secretion[27]. Because HBsAg variants are a serious health concern not only because they are not detectable by some commercial HBsAg assays, but also because they can infect vaccinated individuals, the emergence of HBsAg variants with novel mutations must be monitored among populations within HBV endemic areas, such as South Korea.
Recently, we reported various types of novel HBsAg variants of genotype C2 from South Korean occult subjects[14]. The present study elucidates the mechanisms related to the occult infection of ten types of genotype C2 HBsAg variants that were discovered in our previous study, primarily focusing on the extracellular secretion capacity of HBV virion and HBsAg.
MATERIALS AND METHODS
HBV DNA extraction and PCR amplification
For purification of the HBV DNA from serum, a QIAamp DNA Blood Mini Kit (QIAGEN, Hilden, Germany) was used according to the manufacturer’s instructions. Briefly, a 200 μL aliquot of obtained serum was incubated with QIAGEN protease and buffer AL at 65 °C for 10 min. The lysate was applied to a QIAamp spin column, spun, and washed three times with buffer AW, and finally eluted with 50 μL of BQW. In order to investigate the mutation patterns of the occult HBV infection, we conducted a nested PCR targeting the small surface open reading frame in Korean HBsAg-negative subjects. The first round of PCR was performed using the sense primer PreS2-Del-F2 (positions 2814-2832, 5′ - GGG TCA CCA TAT TCT TGG G - 3′) and the antisense primer HB2R (positions 970-989, 5′ - CAT ACT TTC CAA TCA ATA GG - 3′), which target a large surface region, while the second round of amplification was performed using the sense primer Cystein-S-F1 (positions 155-179, 5′ - ATG GAG AGC ACA ACA TCA GGA TTC C - 3′) and the antisense primer Cystein-S-R1 (positions 811-835, 5′ - TCA AAT GTA TAC CCA AAG ACA AAA G - 3′). The PCR was initiated using the hot-start technique in a 50 μL PCR mixture containing 2.5 mmol/L MgCl2, 400 mmol/L dNTP, and 2.5 U of LA Taq polymerase (Takara Bio Inc., Shiga, Japan). The reaction mixture was subjected to 30 cycles of amplification (60 s at 95 °C, 45 s at 52 °C, and 90 s at 72 °C) followed by a 5 min extension at 72 °C. A 96-well thermocycler (Model 9600, Perkin-Elmer Cetus, Norwalk, United States) was also used. The obtained PCR products were analyzed via electrophoresis on 1% agarose gels, stained with ethidium bromide, and visualized on a ultraviolet trans-illuminator.
Cloning and sequencing analysis of small surface ORFs
In order to analyze the mutation patterns of the small surface regions of 41 occult subjects, the 681-bp of the PCR products was cloned using a TOPO TA cloning kit (Invitrogen, Carlsbad, United States). The sequencing was performed using an Applied Biosystems model 377 DNA automatic sequencer (Perkin-Elmer Applied Biosystems, Warrington, United Kingdom). The mutations were classified through comparisons with seven reference strains obtained from GenBank [accession numbers (Genotypes) M57663 (A), AB100695 (B), AB074755 (C1), AY641558 (C2), X02496 (D), AB106564 (E), and X75663 (F)]. The nucleotides were aligned and their similarities were calculated using the multiple-alignment algorithm in Megalign (DNASTAR, Windows Version 3.12e).
Cloning of HBV small surface for expression
The small surface regions of ten subjects with novel mutation patterns related to the occult infection including the wild type (Nor) and pIRES2-EGFP only (mock) were cloned for the expression of HBsAg in the HuH-7 cell line. The TOPO TA cloned small surface region was re-cloned into the pIRES2-EGFP expression vector (Clontech, Mountain View, United States) including the cytomegalovirus (CMV) promoter as per the manufacturer’s recommendation.
Cell culture and transient co-transfection
Human hepatoma cells (HuH-7) were cultured in Dulbecco’s modified Eagle’s medium (HyClone, Thermo Scientific, United States) containing 10% fetal bovine serum (GibcoBRL, Grand Island, United States) and 100 μg/mL of penicillin-streptomycin (GibcoBRL, Grand Island, United States). The cells were seeded on cell culture dishes (Falcon, San Jose, United States) and sub-cultured using a trypsin-EDTA (GibcoBRL, Grand Island, United States) treatment. All cells were maintained at 37 °C in a humidified 5% CO2 atmosphere. In order to detect the HBsAg, viral DNA, and ROS level in the transient co-transfected HuH-7 using the novel mutants in this study, the cells (2 × 105) were seeded in 6-well plates. After incubation for 24 h, the cells were transiently co-transfected with pHY92-1.1x-HBV-deleted small surface plasmids (pHY92-delS) and pIRES2-EGFP-HBV small surface plasmids presenting occult HBV variants using Lipofectamine 2000 (Invitrogen, Carlsbad, United States) and incubated at 37 °C with 5% CO2 for 48 h. After 48 h, the cell supernatant was collected and the pellets were washed with phosphate buffered saline (PBS) (GibcoBRL, Grand Island, United States). The washed pellets were lysed using mild Reporter Lysis Buffer (RLB) (Promega, WI, United States); these pellets were then used in the subsequent experiments.
Construction of HBV full genomic DNA with deleted HBsAg via site-directed mutagenesis
In order to verify the effects of the mutations related to the occult infections in HBV full genomic constructs, we created HBV full genome constructs that do not express HBsAg through site-directed mutagenesis of start codon of HBsAg (ATG to ATC). For this purpose, we used a plasmid and pHY92 vector containing a copy of the 1.1x-unit length HBV genome under the control of a CMV promoter (genotype A, serotype adw, HBV strain identical to GenBank AF305422), which was provided by Yang et al[28]. For each reaction, 5 ng of plasmid was incubated with 125 ng of mutagenic primer [the sense primer MUTA-Small-SF (position 134-175, 5′ - ACT GGG GAC CCT GCA CCG AAC ATC GAG AGC ACA ACA TCA GGA - 3′) and the anti-sense primer MUTA-Small-SR (same position, 5′ - TCC TGA TGT TGT GCT CTC GAT GTT CGG TGC AGG GTC CCC AGT - 3′)], 2.5 mmol/L of dNTP, and 2.5 U of Pyrococcus furiosus DNA polymerase (Takara Bio Inc., Shiga, Japan) with a final volume of 50 μL containing 10 mmol/L KCl, 6 mmol/L (NH4)2SO4, 20 mmol/L Tris-HCl, 2 mmol/L MgCl2, 1% Triton X-100, and 10 μg/mL bovine serum albumin. A cycling reaction was performed in a PCR machine (BIO-RAD, CA, United States). The reaction was initiated via heating for 5 min at 94 °C. The reaction conditions for the denaturation, annealing, and elongation were 45 s at 94 °C, 45 s at 52 °C, and 10 min at 72 °C, respectively, for a total of 15 cycles. The mixture was then subjected to digestion using 10 U of DpnI restriction enzyme for 20 min at 37 °C and transformation into TOP10 (Invitrogen, Carlsbad, United States). Each bacterial colony was confirmed via DNA sequencing.
HBsAg ELISA
In order to compare the secretion capacity between the occult infection-related HBsAg variants, an ELISA was conducted for HBsAg, according to the given experiment method, using a commercial Bioelisa HBsAg color ELISA Kit (BIOKIT, Barcelona, Spain), MONOLISA HBs Ag ULTRA (BIO-RAD, CA, United States), and an ETI-MAK-4 HBsAg Enzyme Immunoassay Kit (DiaSorin, Saluggia, Italy) from the supernatant and lysed pellet. Furthermore, for normalization of the HBsAg ELISA in the cloned pIRES2-EGFP of the target HBsAg, we also measured the β-galactosidase expression level of the respective pIRES2-EGFP plasmid using a β-galactosidase enzyme assay system kit (Promega, WI, United States). β-galactosidase is a commonly used reporter molecule. The β-galactosidase enzyme assay system with RLB is a convenient method for assaying β-galactosidase activity in lysates prepared from cells transfected with β-galactosidase reporter vectors, such as the pSV-β-galactosidase control vector. For normalization of pHY92-based full genomic constructs without the EGFP gene, the pSV-β-gal vector containing β-galactosidase was co-transfected; the β-galactosidase activities were analyzed according to the manufacturer’s recommendation.
HBV viral DNA purification and Q-PCR analyses
The HBV DNA replication from the full genome HBV construct with pIRES2-EGFP clones was evaluated via quantitative real-time PCR targeting the secreted HBV viral DNA in the supernatant or intracellular viral DNA in a pellet of the cell culture. PCR amplification was performed with a set of real-time PCR primers targeting the small S gene designed to amplify a 101 base pair product and primer sequences as follows: the sense primer Real-SF (position 218-240, 5′ - TTG ACA AGA ATC CTC ACA ATA CC - 3′) and the antisense primer Real-SR (position 309-328, 5′ - GGA GGT TGG GGA CTG CGA AT - 3′). The culture medium was centrifuged in order to remove the cellular debris. Afterwards, the supernatant underwent ultracentrifugation at 20000 rpm in a SW28 rotor (Beckman Coulter, United States) for 2 h at 4 °C. The collected pellet was re-suspended using PBS. The cell pellet was harvested using a trypsin-EDTA treatment and rinsed twice with PBS. The viral DNA was extracted using a Viral Gene-Spin DNA extraction Kit (iNtRON, Daejeon, South Korea) according to the manufacturer’s instructions from the rinsed cell pellet and the collected viral particle of the supernatant. The quantitative PCR assay was conducted using commercial iQ SYBR Green supermix (Bio-Rad, CA, United States) and primers specific to the S gene. The HBV DNA levels were analyzed with an Exicycler™ 96 Real Time Quantitative Thermal Block system (Bioneer, Daejeon, South Korea). Each PCR was conducted in duplicate in a 25 μL volume using the iQ SYBR Green supermix for 5 min at 95 °C for the initial denaturing, followed by 40 cycles of 95 °C for 15 s and 56 °C for 15 s. Detection of the fluorescence was set at the last step of each cycle. In order to determine the specificity of the amplification, a melting curve analysis was applied to all final PCR products after the cycling protocol. The results are representative of three independent experiments.
ROS measurement
Dihydrorhodamine123 (DHR123; Calbiochem, San Diego, United States) is a cell-permeable fluorogenic probe that is useful for detecting ROS such as peroxide and peroxynitrite. It is not fluorescent until oxidized by ROS to the highly fluorescent product Rhodamine123. The formation of Rhodamine123 can be monitored using a fluorescence spectroscopy with excitation and emission wavelengths of 500 and 536 nm, respectively. For the ROS level detection in the transient transfected HuH-7 using the novel mutants from this study, the cells (2 × 105) were seeded in 6-well plates. The day after the transfection, the cells were treated with DHR123 at a final concentration of 10 μmol/L for 30 min and lysed with a ROS lysis buffer (5 mmol/L KH2PO4, 0.1 mmol/L EDTA, 0.1% Triton-X-100). Then, 100 μL of the cell lysate was transferred to Nunc immuno-microwell 96-well polystyrene plates (Nunclon, Carlsbad, United States) and analyzed using TECAN Infinite m200 pro (TECAN, Seestrasse, Switzerland) at an excitation of 500 nm and emission of 536 nm.
Ethics statement
This retrospective study was reviewed and approved by the Institutional Review Board of Seoul National University Hospital (IRB Grant No. C-0803-013-237), and the patients’ medical records were anonymized and de-identified prior to the analyses.
Statistical analysis
Statistical analyses were conducted using GraphPad Prism v5.01 (GraphPad software, SD, United States) and figures were generated using the same program. Tables were prepared using Excel 2010 (Microsoft, United States). For continuous variables, a one-way ANOVA was used when the data exhibited a normal distribution. The results are expressed as a comparative ratio, the mean ± SD, or as medians (range). A P value of < 0.05 (two-tailed) was considered to be statistically significant.
RESULTS
Mutation patterns of 10 HBsAg variants from Korean occult subjects
Previously, from 41 of 624 occult subjects (6.6%), we obtained the PCR amplicons using a nested PCR strategy targeting the large surface proteins (LHBs)[14]. Finally, we selected ten unique types of HBsAg variant of genotype C via sequence analysis of amplified LHBs, which have not been reported in other studies, and these were used to elucidate the occult HBV infection mechanism. The respective variants were denoted by abbreviations derived from the distinct mutations in this study. The mutation patterns of the ten HBsAg variants and patient information were determined through comparisons with the amino acids of a reference strain from genotypes A to F in the same region. Briefly, in five (KD, LL, 172R, 182L, and STOP) of the ten HBsAg variants, the mutations were only located outside the “a” determinant (aa 124 to 147). It is noteworthy that two variants (PAHS and STOP) had a premature mutation of the 182th codon (sW182*), which has been reported to be related to the clinical severity of genotype C infected chronic patients[12]. The PAHS and STOP variants had a total of eight and two mutations including sW182* in the HBsAg, respectively. In addition, 182L had a substitution in the 182th codon (W→L), which has only been reported to exist in occult cases in South Korea, but not in chronic patients (Tables 1 and 2)[14].
Table 1.
Mutation patterns of ten variants from Korean occult subjects found in the hepatitis B surface antigen region
| Number | 17 | 32 | 33 | 36 | 44 | 47 | 52 | 56 | 61 | 66 | 73 | 85 | 101 | 107 | 111 | 117 | 126 | 127 | 129 | 130 | 134 | 140 | 145 | 146 | 158 | 170 | 172 | 177 | 182 | 195 | 204 | 206 | 220 | 227 |
| Aminoacid | A | L | D | W | G | T | N | P | S | P | R | F | Q | C | P | S | I | P | Q | G | F | T | G | N | F | F | W | V | W | I | S | Y | F | ★ |
| Genotype A | · | · | · | · | · | V | · | · | · | · | · | · | · | · | · | · | T | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | ★ |
| Genotype B | · | · | · | · | E | V | · | Q | · | · | · | C | · | · | · | · | T | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | ★ |
| Genotype C1 | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | ★ |
| Genotype C2 | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | ★ |
| Genotype D | · | · | · | · | · | V | · | · | · | · | · | · | · | · | · | · | T | · | · | · | Y | · | · | · | · | · | · | · | · | · | · | · | · | ★ |
| Genotype E | · | · | · | · | · | V | · | · | · | · | · | · | · | · | · | · | T | L | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | ★ |
| Genotype F | · | · | · | · | · | R | · | · | · | · | · | · | · | · | · | · | A | L | · | · | · | · | · | · | · | · | · | · | · | · | N | · | · | ★ |
| Nor (WT) | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | ★ |
| TN | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | T | · | N | · | · | · | · | · | · | · | · | · | · | · | · | · | · | ★ |
| TALR | T | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | A | · | · | L | · | · | · | · | · | · | R | · | ★ |
| ALK | · | · | G | · | · | A | · | · | L | · | · | · | K | · | · | · | · | · | · | · | · | · | · | D | · | · | · | · | · | M | · | · | · | ★ |
| KD | · | · | · | L | · | K | D | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | ★ |
| CNR | · | · | · | · | · | · | · | · | · | · | C | · | · | · | · | N | · | · | · | R | · | · | · | · | · | · | · | · | · | · | · | · | · | ★ |
| LL | · | · | · | · | · | · | · | · | · | · | · | · | · | · | L | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | L | ★ |
| PAHS | · | P | · | · | · | A | · | · | · | H | · | S | · | W | · | · | · | · | · | · | · | · | R | · | · | P | · | · | ★ | |||||
| 172R | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | R | · | · | · | · | · | · | ★ |
| 182L | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | L | · | · | · | · | ★ |
| Stop | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | A | ★ |
Black asterisk (★): Stop codon; Nor (WT): Normal (wild-type); TN: I126T, Q129N; TALR: A17T, T140A, F158L, Y206R; ALK: D33G, T47A, S61L, Q101K, N146D, I195M; KD: W36L, T47K, N52D; CNR: R73C, S117N, G130R; LL: P111L F220L; PAHS: L32P, T47A, P66H, F85S, C107W, G145R, F170P; 172R: W172R; 182L: W182L; Stop: V177A, W182Stop.
Table 2.
Clinical, genotype, hepatitis B virus bDNA, and serologic data of the ten subjects with hepatitis B surface antigen variants used in the present study
| Samples | Age | Sex | Genotype | HBV bDNA (copies/mL) | HBsAg | Anti-HBs | Mutations | Abbreviation |
| 1 | 60 | F | C2 | < 2000 | Negative | Negative | I126T, Q129N | TN |
| 2 | 53 | M | C2 | < 2000 | Negative | Negative | A17T, T140A, F158L, Y206R | TALR |
| 3 | 60 | F | C2 | < 2000 | Negative | Positive | D33G, T47A, S61L, Q101K, N146D, I195M | ALK |
| 4 | 54 | M | C2 | < 2000 | Negative | Negative | W36L, T47K, N52D | KD |
| 5 | 44 | M | C2 | < 2000 | Negative | Positive | R73C, S117N, G130R | CNR |
| 6 | 41 | F | C2 | < 2000 | Negative | Negative | P111L, F220L | LL |
| 7 | 42 | M | C2 | < 2000 | Negative | Negative | L32P, T47A, P66H, F85S, C107W, G145R, F170P, W182Stop | PAHS |
| 8 | 73 | M | C2 | < 2000 | Negative | Negative | W172R | 172R |
| 9 | 30 | M | C2 | < 2000 | Negative | Positive | W182L | 182L |
| 10 | 3 | F | C2 | < 2000 | Negative | Negative | V177A, W182Stop | Stop |
HBsAg: Hepatitis B surface antigen; Anti-HBs: Anti-hepatitis B surface; F: Female; M: Male; HBV: Hepatitis B virus.
Comparison of HBsAg and virion secretion capacity according to occult infection-related HBsAg variants
In order to address the issue of whether the occult HBV infection may be attributed to a reduced secretion capacity of the occult infection-related HBsAg variants, we analyzed the HBsAg secretion capacity of ten different types of HBsAg variants using a commercial HBsAg ELISA kit in a transient co-transfection system with both the pHY92-1.1x-full HBV genome construct (Genotype A, GeneBank No.AF305422), which is not capable of HBsAg expression via abrogation of the start codon (pHY92-delS), and the pIRES2-EGFP-HBV small surface plasmid vector into the HuH-7 cell (Figure 1).
Figure 1.
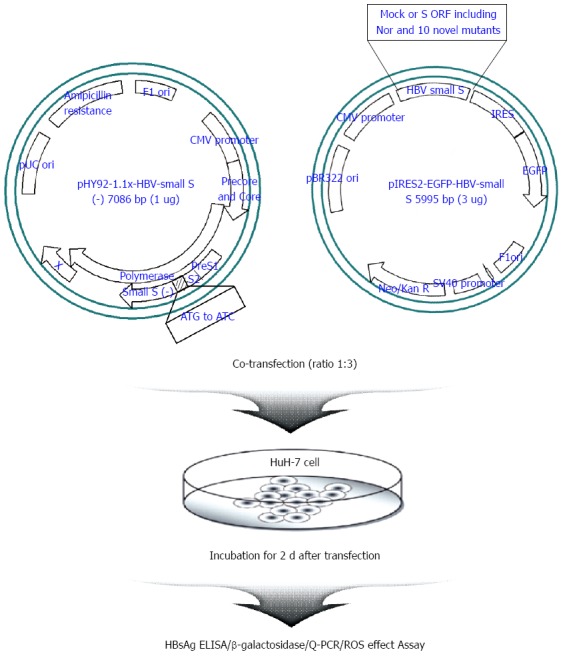
Schematic experimental strategy used in this study. Briefly, one microgram of pHY92-1.1x-HBV-small S (-) having a full-genome of hepatitis B virus (HBV) with a knock-out small surface open reading frame (ORF) was co-transfected with three micrograms of pIRES2-EGFP-HBV-small S expressing the sub-genome of small surface region, which has ten variants including mock and wild-type into HuH-7 cell line transiently. After co-transfection, HuH-7 cells were incubated for 2 d. Supernatant and lysed pellet were collected and used for various assays. The tests were performed in triplicate. HBsAg: Hepatitis B surface antigen; ELISA: Enzyme linked immunosorbent assay; Q-PCR: Quantitative polymerase chain reaction; ROS: Reactive oxygen species; Neo/Kan R: Neomycin and Kanamycin resistance.
All ten HBsAg variants exhibited a significantly reduced secretion capacity of HBsAg into the media compared with the wild type. In particular, eight variants, excluding the two variants TN and TALR, could not be secreted into the media, which is similar to the secretion level seen in the negative control (MOCK). This indicates that the HBV occult infections from these subjects may result from the lack of HBsAg secretion capacity. The eight variants exhibiting an HBsAg secretion capacity similar to the negative level were divided into two groups according to the detected intracellular HBsAg level. One group included two variants (ALK and KD) that exhibited higher levels of intracellular HBsAg than the wild type, which indicated that their reduced HBsAg secretion might result from increased intracellular accumulation of the HBsAg variants. The second group included six variants (CNR, LL, PAHS, 172R, 182L, and STOP) that exhibited very low levels of intracellular HBsAg, similar to the negative control (MOCK), and this indicated that their reduced secretion level might result from a problem of HBsAg protein stability or changed antigenicity through the mutations. Meanwhile, in order to investigate the effect of the occult-related variants on virion formation, we verified the amounts of viral DNAs from the medium and cell lysates of co-transfected HuH-7 cells using a real-time quantitative-DNA PCR. The results revealed that the level of extracellular viral DNA from nine occult-related variants (except “STOP”) was almost equal to (ALK, KD, 172R, and 182L) or higher (TN, TALR, CNR, LL, and PAHS) than that of the wild type. This indicates that most occult-related HBsAg variants do not affect the HBV virion formation. The levels of intracellular viral DNA of seven variants (TN, TALR, ALK, KD, CCNR, LL, and PAHS) were equal to or higher than that of the wild type. However, the remaining three variants (172R, 182L, and STOP) exhibited very low levels of intracellular and extracellular HBsAg, and they also exhibited significantly lower intracellular viral DNA levels than that of the wild type (Figure 2).
Figure 2.
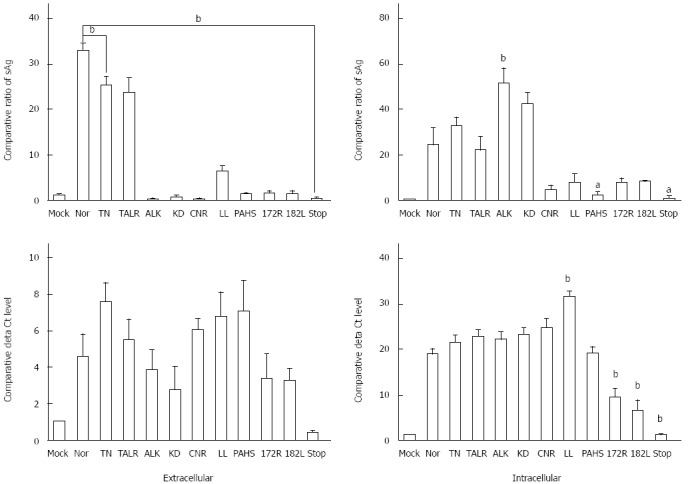
Secretion capacity and viral DNA formation of occult hepatitis B surface antigen variants. Extracellular secreted hepatitis B surface antigen (HBsAg) and intracellular expressed HBsAg from cell lysate were measured using a commercial HBsAg enzyme linked immunosorbent assay kit normalized via a β-galactosidase assay. After purification of the viral DNA from the supernatant and cell lysate using a total viral DNA preparation kit, detection of the viral DNA from both intracellular and extracellular was performed using real-time quantitative DNA-polymerase chain reaction assays. The hepatitis B virus DNA was normalized via a β-galactosidase assay. The tests were performed in triplicate (mean ± SD). sAg: Surface antigen. aP < 0.05, bP < 0.01 vs wild type (Nor) group.
Collectively considering the effects of the occult infection-related HBsAg variants on HBsAg and HBV virion formation, the ten different types of HBV variants analyzed in this study were categorized into five groups. Group I included two variants (TN and TALR) and they displayed intracellular and extracellular HBsAg secretion and viral DNA levels similar to that of the wild type. Group II also included two variants (ALK and KD) that exhibited very low levels of HBsAg secretion, but a higher level of accumulated intracellular HBsAg compared with the wild type. Group III included three variants (CNR, LL, and PAHS) that exhibited very low levels of both secreted and intracellular HBsAg secretion, but similar levels of viral DNA compared with the wild type. Group IV included two variants (172R and 182L) that exhibited very low levels of both secreted and intracellular HBsAg secretion, and also lower levels of viral DNA compared with the wild type. Group V included only the STOP variant, and it cannot produce HBsAg or virions in intracellular or secreted forms (Table 3).
Table 3.
Classification of the ten novel mutants according to the secretion level of hepatitis B surface antigen and viral DNA formation capacity
| Grouping | Mutants |
sAg level |
Viral DNA level |
||
| Extracellular | Intracellular | Extracellular | Intracellular | ||
| I | TN | Positive | Positive | Higher than WT | Similar to WT |
| TALR | Positive | Positive | Higher than WT | Similar to WT | |
| II | ALK | Negative | Highly positive | Similar to WT | Similar to WT |
| KD | Negative | Highly positive | Similar to WT | Similar to WT | |
| III | CNR | Negative | Weakly positive | Higher than WT | Similar to WT |
| LL | Weakly Positive | Weakly positive | Higher than WT | Higher than WT | |
| PAHS | Negative | Negative | Higher than WT | Similar to WT | |
| IV | 172R | Negative | Weakly positive | Lower than WT | Lower than WT |
| 182L | Negative | Weakly positive | Lower than WT | Lower than WT | |
| V | Stop | Negative | Negative | Negative | Negative |
sAg: Surface antigen; WT: Wild type.
Comparison with ROS production between the ten variants and the wild type
Alteration of the secretion capacity of the HBsAgs and HBV virions could lead to ROS production via the endoplasmic reticulum (ER) stress pathway[29,30]. In order to address the issue of whether the ten HBsAg variants used in this study could lead to ROS production compared with the wild type, we compared the ROS production of the 11 types of HBsAg (the ten variants and the wild type) using a DHR123 system, 24 h after co-transfection of both plasmids of pHY92-delS and pIRES2-EGFP-HBV small surface plasmid presenting occult HBV variants, respectively. Although generally all variants produced higher levels of ROS compared with the wild type, the ROS production differed substantially among the variants. While seven variants (TN, TALR, ALK, KD, CCNR, LL, and PAHS) belonging to Groups I, II, and III produced relatively high levels of ROS production, the remaining three variants (172R, 182L, and STOP) belonging to Groups IV and V produced ROS levels similar to that of the wild type. In particular, the ROS production of two variants (KD and PAHS) was statistically significantly higher than that of the wild type (Figure 3).
Figure 3.
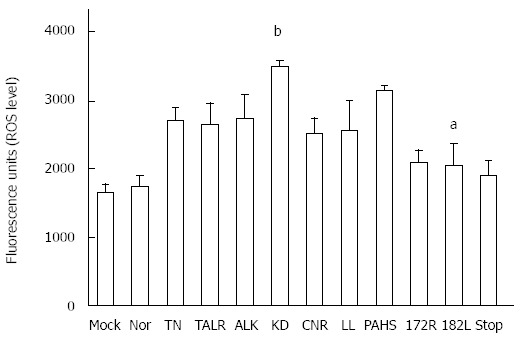
Effect of occult hepatitis B surface antigen variants on the reactive oxygen species system. The reactive oxygen species level according to the occult hepatitis B virus variants in transient transfected HuH-7 was measured using 20 μmol/L of DHR123 reagent, which can detect reactive oxygen species (ROS) such as peroxide and peroxynitrite. The tests were performed in triplicate (mean ± SD). aP < 0.05, bP < 0.01 vs wild type (Nor) group.
DISCUSSION
To date, studies of occult infection related HBsAg mutations have primarily focused on mutations in the “a” determinant to produce the occult infection or generation of vaccine escape variants through reduction of the binding affinity between the HBsAg and antibody to HBsAg[31-34]. Among these, the mutation from glycine to arginine in the 145th codon of HBsAg (i.e., G145R) has been most frequently encountered[33,34]. However, this is not the case in South Korea where the genotype C infection is prevalent. It has been reported that the mutation of G145R is not responsible (but if so, rarely) for the occult infection in Korean subjects. Our previous report demonstrated that, in South Korea, the HBsAg variants of genotype C related to the occult infection harbored multiple mutations within HBsAg, primarily located outside the “a” determinant[14]. In this study, we attempted to uncover the molecular mechanism underlying the occult infection of HBV endemic areas such as South Korea using ten novel types of HBsAg variants from Korean subjects.
The transient transfection study using a total of ten novel HBsAg variants offers several noteworthy findings. First, defects in the HBsAg secretions may be a significant mechanism underlying occult infections. Although all variants exhibited lower levels of HBsAg secretion capacity, substantial differences between types of HBsAg variants were found. The two variants of Group II (KD and ALK) exhibited high levels of intracellular accumulation, but not secretion, which could lead to ER accumulation of the mutated HBsAg without protein degradation via an impaired ER-associated degradation process[29]. The six variants of Groups III, IV, and V exhibited low levels of HBsAg in both intracellular and secretory forms. For this, mechanisms such as antigenicity modification or protein stability defects could be proposed. Considering most variants (four of the six) did not have “a” determinant mutations, defects in protein stability of the variants rather than antigenicity modification is more probable. Furthermore, despite the defects of the HBsAg secretion capacity, most variants had potential for virion secretion comparable with or even higher than that of the wild type. This indicates higher normal infectivity, but was not detectable using the commercial HBsAg ELISA Kit. However, it could provide a probable explanation regarding the relatively high levels of occult infection prevalence in South Korea, as reported previously[14].
Second, our previous report demonstrated that, including the W182L and sW182* mutations, the 182nd codon was the most frequently affected among the mutations of HBsAg in the occult subjects (15/41 subjects). sW182* was also reported to be related to liver disease progression in Korean chronic patients[12]. However, W182L was distinct in the occult infection, because it was not found in chronic Korean patients[14]. Three types of variants with mutations in the 182nd codon (PAHS and 182L of Group IV, and STOP of Group V) were included in this study. There were substantial differences in the virion formation between PAHS with eight mutations and STOP with two mutations, although both had sW182*. In STOP, HBsAg production and virion were not found, as demonstrated in the previous report using the full HBV pHY92 construct with sW182* generated using site-directed mutagenesis[12], which indicates abortive production of mutated HBsAg. However, PAHS exhibited recovered HBV virion formation despite having sW182* even with a higher secretory virion level than the wild type, which indicates a role of additional mutations for virion formation. Despite the single mutation, 182L also exhibited a secretion profile similar to that of PAHS, with secretory HBV virion, but without HBsAg secretion. Therefore, collectively, our data proved that the 182nd codon has a pivotal function in HBsAg secretion. Furthermore, our data also provided a probable explanation regarding why mutation in the 182th codon is so prevalent in the occult subjects.
Third, considering previous reports that implicated reduced HBsAg in the induction of the ER stress pathway[29], HBsAg secretion deficiency via accumulation of occult-related HBsAg mutations may also provide links between the occult infection and progression of liver disease. In order to address this issue, we compared the ROS production that was expected to be induced by the ER stress pathway between the ten HBsAg variants and the wild type. Our data strongly supported the hypothesis that occult-related HBsAg variants could induce ROS induction in hepatocytes via the ER stress pathway, which increases the potential of liver disease progression; however, our data also demonstrated disparities in potentials to elicit ROS production in the hepatocytes among the HBV variants. This indicates differences in the ER stress inducing potentials and kinetics according to the mutation patterns of the HBsAg variants. The exact role of the respective mutation in inducing the ER stress-ROS axis remains to be elucidated in a future study.
In conclusion, our data indicate that deficiency in the secretion capacity of HBsAg, but not virion, that is induced by HBsAg mutations (particularly outside the “a” determinant) may have a pivotal function in occult infections of HBV genotype C, at least in occult infections in South Korea. Furthermore, it also provided new insight into the intrinsic nature of HBV occult infections, which leads to HBsAg sero-negativeness but horizontal infectivity. In addition, proving ROS production via possible induction of ER stress by HBsAg variants in hepatocytes would provide a probable explanation for the links between occult infection and liver disease progression.
COMMENTS
Background
A large body of evidence has demonstrated that occult hepatitis B virus (HBV) infection is highly prevalent, particularly in HBV endemic areas. Recently, various types of novel hepatitis B surface antigen (HBsAg) variants of genotype C2 from Korean occult subjects have been introduced. This study elucidates the mechanisms related to occult infection of genotype C2 HBsAg variants introduced in a previous study, primarily focusing on the extracellular secretion capacity of HBV virion and HBsAg.
Research frontiers
These data indicate that deficiency in the secretion capacity of HBsAg, but not virion, that is induced by the HBsAg mutations (particularly outside the “a” determinant) may have a pivotal function in occult infections of HBV genotype C, at least in occult infections in South Korea.
Innovations and breakthroughs
Recent reports have highlighted the importance of “a” determinant mutations such as G145R as a major mechanism underlying occult infection. However, it is not the case in Korean occult subjects. This is the first study to report that deficiency in the secretion capacity of HBsAg variants (particularly outside the “a” determinant) may have a pivotal function in occult infections of HBV genotype C. Furthermore, this study proved reactive oxygen species production via possible induction of endoplasmic reticulum stress by HBsAg variants in hepatocytes, providing a probable explanation for the links between occult infection and liver disease progression.
Applications
The co-transient transfection system of both HBV full genomic DNA and occult infection related HBsAg variants introduced in this study could be effectively used for the development of a novel HBsAg detection method or for the screening of new vaccine escape or occult infection related mutants.
Terminology
Occult HBV infection is defined as the infection state negative for HBsAg serology, but it has shown viral genome persistence in infected individuals. In general, HBV infection is diagnosed when the circulating HBsAg is serologically detected. However, recent progress in molecular-based technology has enabled HBV infection to be proven from HBsAg negative individuals with or without circulating antibodies to HBsAg and/or hepatitis B core antigen.
Peer-review
The authors examined the underlying mechanism of HBV occult infection focusing on ten HBsAg variants from Korean occult subjects, recently introduced by the authors. It was revealed that all variants exhibited lower levels of HBsAg secretion into the medium, but similar level of virions, compared with the wild type. Furthermore, most variants generated higher reactive oxidative species production than the wild type. The results are interesting and may represent a molecular mechanism of HBV genotype C occult infection.
Footnotes
Supported by National Research Foundation of Korea grant funded by the Korean Government (Ministry of Education, Science, and Technology), Grant No. 2013-005810.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: June 12, 2014
First decision: July 21, 2014
Article in press: November 11, 2014
P- Reviewer: Kuhns MC, Peng CY S- Editor: Gou SX L- Editor: A E- Editor: Liu XM
References
- 1.Kao JH, Chen PJ, Lai MY, Chen DS. Genotypes and clinical phenotypes of hepatitis B virus in patients with chronic hepatitis B virus infection. J Clin Microbiol. 2002;40:1207–1209. doi: 10.1128/JCM.40.4.1207-1209.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lavanchy D. Worldwide epidemiology of HBV infection, disease burden, and vaccine prevention. J Clin Virol. 2005;34 Suppl 1:S1–S3. doi: 10.1016/s1386-6532(05)00384-7. [DOI] [PubMed] [Google Scholar]
- 3.Chen DS. From hepatitis to hepatoma: lessons from type B viral hepatitis. Science. 1993;262:369–370. doi: 10.1126/science.8211155. [DOI] [PubMed] [Google Scholar]
- 4.Korea Centers for Disease Control and Prevention. Korea Center for Disease Control and Prevention. 2007. National Health and Nutrition Survey. [Google Scholar]
- 5.Kim HJ, Park JH, Jee Y, Lee SA, Kim H, Song BC, Yang S, Lee M, Yoon JH, Kim YJ, et al. Hepatitis B virus X mutations occurring naturally associated with clinical severity of liver disease among Korean patients with chronic genotype C infection. J Med Virol. 2008;80:1337–1343. doi: 10.1002/jmv.21219. [DOI] [PubMed] [Google Scholar]
- 6.Orito E, Mizokami M, Sakugawa H, Michitaka K, Ishikawa K, Ichida T, Okanoue T, Yotsuyanagi H, Iino S. A case-control study for clinical and molecular biological differences between hepatitis B viruses of genotypes B and C. Japan HBV Genotype Research Group. Hepatology. 2001;33:218–223. doi: 10.1053/jhep.2001.20532. [DOI] [PubMed] [Google Scholar]
- 7.Song BC, Kim SH, Kim H, Ying YH, Kim HJ, Kim YJ, Yoon JH, Lee HS, Cha CY, Kook YH, et al. Prevalence of naturally occurring surface antigen variants of hepatitis B virus in Korean patients infected chronically. J Med Virol. 2005;76:194–202. doi: 10.1002/jmv.20354. [DOI] [PubMed] [Google Scholar]
- 8.Kim BJ. Hepatitis B virus mutations related to liver disease progression of Korean patients. World J Gastroenterol. 2014;20:460–467. doi: 10.3748/wjg.v20.i2.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mun HS, Lee SA, Jee Y, Kim H, Park JH, Song BC, Yoon JH, Kim YJ, Lee HS, Hyun JW, et al. The prevalence of hepatitis B virus preS deletions occurring naturally in Korean patients infected chronically with genotype C. J Med Virol. 2008;80:1189–1194. doi: 10.1002/jmv.21208. [DOI] [PubMed] [Google Scholar]
- 10.Lee SA, Mun HS, Kim H, Lee HK, Kim BJ, Hwang ES, Kook YH, Kim BJ. Naturally occurring hepatitis B virus X deletions and insertions among Korean chronic patients. J Med Virol. 2011;83:65–70. doi: 10.1002/jmv.21938. [DOI] [PubMed] [Google Scholar]
- 11.Mun HS, Lee SA, Kim H, Hwang ES, Kook YH, Kim BJ. Novel F141L pre-S2 mutation in hepatitis B virus increases the risk of hepatocellular carcinoma in patients with chronic genotype C infections. J Virol. 2011;85:123–132. doi: 10.1128/JVI.01524-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee SA, Kim K, Kim H, Kim BJ. Nucleotide change of codon 182 in the surface gene of hepatitis B virus genotype C leading to truncated surface protein is associated with progression of liver diseases. J Hepatol. 2012;56:63–69. doi: 10.1016/j.jhep.2011.06.028. [DOI] [PubMed] [Google Scholar]
- 13.Kim DW, Lee SA, Hwang ES, Kook YH, Kim BJ. Naturally occurring precore/core region mutations of hepatitis B virus genotype C related to hepatocellular carcinoma. PLoS One. 2012;7:e47372. doi: 10.1371/journal.pone.0047372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim H, Lee SA, Kim DW, Lee SH, Kim BJ. Naturally occurring mutations in large surface genes related to occult infection of hepatitis B virus genotype C. PLoS One. 2013;8:e54486. doi: 10.1371/journal.pone.0054486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim DW, Lee SA, Kim H, Won YS, Kim BJ. Naturally occurring mutations in the nonstructural region 5B of hepatitis C virus (HCV) from treatment-naïve Korean patients chronically infected with HCV genotype 1b. PLoS One. 2014;9:e87773. doi: 10.1371/journal.pone.0087773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee SA, Kim KJ, Kim DW, Kim BJ. Male-specific W4P/R mutation in the pre-S1 region of hepatitis B virus, increasing the risk of progression of liver diseases in chronic patients. J Clin Microbiol. 2013;51:3928–3936. doi: 10.1128/JCM.01505-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bowden S, Bartholomeusz A, Locarnini S. Lamivudine resistant occult HBV: implications for public health? J Hepatol. 2003;38:526–528. doi: 10.1016/s0168-8278(03)00079-5. [DOI] [PubMed] [Google Scholar]
- 18.Raimondo G, Pollicino T, Cacciola I, Squadrito G. Occult hepatitis B virus infection. J Hepatol. 2007;46:160–170. doi: 10.1016/j.jhep.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 19.Torbenson M, Thomas DL. Occult hepatitis B. Lancet Infect Dis. 2002;2:479–486. doi: 10.1016/s1473-3099(02)00345-6. [DOI] [PubMed] [Google Scholar]
- 20.Yu ML, Dai CY, Huang CF, Lee JJ, Yeh ML, Yeh SM, Kuo HT, Huang JF, Chang JM, Chen HC, et al. High hepatitis B virus surface antigen levels and favorable interleukin 28B genotype predict spontaneous hepatitis C virus clearance in uremic patients. J Hepatol. 2014;60:253–259. doi: 10.1016/j.jhep.2013.09.023. [DOI] [PubMed] [Google Scholar]
- 21.Morris CJ, Hill M, de Medina M, Herman C, Cloherty GA, Martin P. Comparison of detection and quantification of HBV DNA in chronic HBeAg negative and positive patients by Abbott RealTime HBV and Roche Cobas TaqMan HBV assays. J Virol Methods. 2013;193:391–393. doi: 10.1016/j.jviromet.2013.06.036. [DOI] [PubMed] [Google Scholar]
- 22.Chen CF, Lee WC, Yang HI, Chang HC, Jen CL, Iloeje UH, Su J, Hsiao CK, Wang LY, You SL, et al. Changes in serum levels of HBV DNA and alanine aminotransferase determine risk for hepatocellular carcinoma. Gastroenterology. 2011;141:1240–1248, 1248.e1-e2. doi: 10.1053/j.gastro.2011.06.036. [DOI] [PubMed] [Google Scholar]
- 23.Squadrito G, Cacciola I, Alibrandi A, Pollicino T, Raimondo G. Impact of occult hepatitis B virus infection on the outcome of chronic hepatitis C. J Hepatol. 2013;59:696–700. doi: 10.1016/j.jhep.2013.05.043. [DOI] [PubMed] [Google Scholar]
- 24.Huang X, Hollinger FB. Occult hepatitis B virus infection and hepatocellular carcinoma: a systematic review. J Viral Hepat. 2014;21:153–162. doi: 10.1111/jvh.12222. [DOI] [PubMed] [Google Scholar]
- 25.Sagnelli E, Coppola N, Scolastico C, Mogavero AR, Filippini P, Piccinino F. HCV genotype and “silent” HBV coinfection: two main risk factors for a more severe liver disease. J Med Virol. 2001;64:350–355. doi: 10.1002/jmv.1057. [DOI] [PubMed] [Google Scholar]
- 26.Samal J, Kandpal M, Vivekanandan P. Molecular mechanisms underlying occult hepatitis B virus infection. Clin Microbiol Rev. 2012;25:142–163. doi: 10.1128/CMR.00018-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwei K, Tang X, Lok AS, Sureau C, Garcia T, Li J, Wands J, Tong S. Impaired virion secretion by hepatitis B virus immune escape mutants and its rescue by wild-type envelope proteins or a second-site mutation. J Virol. 2013;87:2352–2357. doi: 10.1128/JVI.02701-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang H, Westland C, Xiong S, Delaney WE. In vitro antiviral susceptibility of full-length clinical hepatitis B virus isolates cloned with a novel expression vector. Antiviral Res. 2004;61:27–36. doi: 10.1016/j.antiviral.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 29.Chua PK, Wang RY, Lin MH, Masuda T, Suk FM, Shih C. Reduced secretion of virions and hepatitis B virus (HBV) surface antigen of a naturally occurring HBV variant correlates with the accumulation of the small S envelope protein in the endoplasmic reticulum and Golgi apparatus. J Virol. 2005;79:13483–13496. doi: 10.1128/JVI.79.21.13483-13496.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ji C, Kaplowitz N. ER stress: can the liver cope? J Hepatol. 2006;45:321–333. doi: 10.1016/j.jhep.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 31.Lada O, Benhamou Y, Poynard T, Thibault V. Coexistence of hepatitis B surface antigen (HBs Ag) and anti-HBs antibodies in chronic hepatitis B virus carriers: influence of “a” determinant variants. J Virol. 2006;80:2968–2975. doi: 10.1128/JVI.80.6.2968-2975.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Banerjee A, Chandra PK, Datta S, Biswas A, Bhattacharya P, Chakraborty S, Chakrabarti S, Bhattacharya SK, Chakravarty R. Frequency and significance of hepatitis B virus surface gene variant circulating among ‘antiHBc only’ individuals in Eastern India. J Clin Virol. 2007;40:312–317. doi: 10.1016/j.jcv.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 33.Mu SC, Lin YM, Jow GM, Chen BF. Occult hepatitis B virus infection in hepatitis B vaccinated children in Taiwan. J Hepatol. 2009;50:264–272. doi: 10.1016/j.jhep.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 34.Amini-Bavil-Olyaee S, Vucur M, Luedde T, Trautwein C, Tacke F. Differential impact of immune escape mutations G145R and P120T on the replication of lamivudine-resistant hepatitis B virus e antigen-positive and -negative strains. J Virol. 2010;84:1026–1033. doi: 10.1128/JVI.01796-09. [DOI] [PMC free article] [PubMed] [Google Scholar]


