Abstract
AIM: To elucidate the clinicopathological characteristics and prognostic factors of gastric stump cancer (GSC).
METHODS: The clinical data for 92 patients with GSC were collected at Fudan University Shanghai Cancer Center. The prognostic factors were analyzed with Cox proportional hazard models.
RESULTS: GSC tended to occur within 25 years following the primary surgery, when the initial disease is benign, whereas it primarily occurred within the first 15 years post-operation for gastric cancer. Patients with regular follow-up after primary surgery had a better survival rate. The multivariate Cox regression analysis revealed that Borrmann type I/II (HR = 3.165, 95%CI: 1.055-9.500, P = 0.040) and radical resection (HR = 1.780, 95%CI: 1.061-2.987, P = 0.029) were independent prognostic factors for GSC. The overall 1-, 3-, and 5-year survival rates of the 92 patients were 78.3%, 45.6% and 27.6%, respectively. The 1-, 3-, and 5-year survival rates of those undergoing radical resection were 79.3%, 52.2%, and 37.8%, respectively. The 5-year survival rates for stages I, II, III, and IV were 85.7%, 47.4%, 16.0%, and 13.3%, respectively (P = 0.005).
CONCLUSION: The appearance of GSC occurs sooner in patients with primary malignant cancer than in patients with a primary benign disease. Therefore, close follow-up is necessary. The overall survival of patients with GSC is poor, and curative resection can improve their prognosis.
Keywords: Gastric stump cancer, Clinicopathological characteristics, Prognosis
Core tip: We retrospectively analyzed 92 patients. This study indicated that gastric stump cancer (GSC) has unique clinicopathologic characteristics, early detection of GSC is indeed possible, close follow-up is necessary and the radical resection may significantly improve the survival.
INTRODUCTION
The concept of gastric stump cancer (GSC) was originally described in the 1920s[1] , and was defined as a carcinoma occurring in the gastric remnant at least 5 years post-surgery for benign peptic ulcer disease. Currently, the concept of GSC has been expanded to include recurrence after gastric cancer resection[2,3].
Gastric stump as a precancerous condition remains a substantial clinical concern. The incidence of GSC accounts for 1%-7% of all gastric carcinomas following gastrectomy, and this frequency continues to increase[3-5]. Nevertheless, GSC is often described as a tumor with poor prognosis, and poor curative resection rates (38%-40%)[6,7]. The 5-year survival rate is only 7%-20% because GSC has unique biological features compared with conventional stomach cancer, and GSC is commonly diagnosed at an advanced stage[4,8,9].
The present study aimed to clarify the clinicopathological characteristics and operative methods for patients with GSC in order to improve their long-term outcomes.
MATERIALS AND METHODS
Patients
We retrospectively analyzed 92 patients who had undergone stomach resection between January 2003 and December 2012 at the Gastric Surgery Department of Fudan University Shanghai Cancer Center, China. All the patients were diagnosed through barium meal, endoscopic, and pathological examinations. Patient information was obtained from the medical records of Fudan University Shanghai Cancer Center. The clinical symptoms included abdominal pain, emesis, dysphagia, weight loss, anemia, and weakness. The clinical variables included age, gender, initial gastric disease, interval from initial surgery, reconstruction type of the first operation, type of gastric resection, tumor location, and tumor stage.
Follow-up
All patients were regularly contacted by telephone, and all patients received a follow-up. The duration of the follow-up period was defined as the interval from treatment date to the date of death or the last follow-up. The last follow-up occurred on March 31, 2014. Sixty-seven patients died at the last follow-up.
Evaluation
Tumor-node-metastasis (TNM) classification of gastric carcinoma was based on the seventh edition of the American Joint Committee on Cancer staging system. The surgical and pathological findings were recorded according to the Japanese Classification of Gastric Carcinoma, and the histological types were classified as differentiated or undifferentiated. The differentiated type included papillary adenocarcinoma and well/moderately differentiated adenocarcinoma, while the undifferentiated type included poorly or undifferentiated adenocarcinoma, signet ring cell carcinoma, and mucinous carcinoma. To calculate the survival curves, only patients who underwent tumor resection were included because these were the only patients with complete histopathological data and staging data.
Statistical analysis
Statistical analyses were performed using the SPSS version 19.0 statistical software package (SPSS IBM, United States). All continuous variables are presented as the median (range). The cumulative cause-specific overall survival rates were calculated using the Kaplan-Meier method. The log-rank test was used to assess differences between clinical factors. Cox proportional hazard models were used to determine which clinicopathological variables were predictive of GSC. P < 0.05 was regarded as significant.
RESULTS
The median age was 60.8 (range: 37-91) years, and the male:female ratio was 4.4:1. The median interval time from the initial operation to the development of GSC was 16.5 years (range: 1-40 years). The latency periods were different between benign disease and gastric cancer. GSC tended to occur within 25 years post-operation with a benign initial disease and within the first 15 years post-operation for gastric cancer (Table 1, Figure 1). Seventy-six (82.6%) patients had clinical symptoms, including abdominal pain, emesis, dysphagia, weight loss, anemia and weakness. Other patients were diagnosed via routine endoscopy.
Table 1.
Clinicopathologic features of the 92 patients with gastric stump cancer n (%)
| Characteristic | Value |
| Age (yr)1 | 60.8 ± 10.0 |
| Sex | |
| Male | 75 (81) |
| Female | 17 (19) |
| Initial gastric disease | |
| Benign | 38 (41) |
| Malignant | 54 (59) |
| Interval time from initial surgery (yr)1 | 16.5 ± 13.0 |
| Regular follow-up after primary surgery | |
| Yes | 76 (83) |
| No | 16 (17) |
| Reconstruction of primary surgery | |
| Billroth-I | 26 (28) |
| Billroth-II | 65 (71) |
| Roux-en-Y | 1 (1) |
| Location | |
| Anastomotic | 59 (64) |
| Non-anastomotic | 33 (36) |
| Tumor size (mm)1 | 4.0 ± 2.0 |
| Borrmann type | |
| I | 3 (3) |
| II | 13 (14) |
| III | 69 (75) |
| IV | 7 (8) |
| Depth of invasion | |
| T1 | 0 (0) |
| T2 | 6 (7) |
| T3 | 5 (5) |
| T4 | 81(88) |
| Lymph node involvement | |
| N0 | 35 (38) |
| ≥ N1 | 57 (62) |
| Presence of distant metastasis | |
| M0 | 63 (68) |
| ≥ M1 | 29 (32) |
| Stage | |
| I | 8 (9) |
| II | 25 (26) |
| III | 30 (33) |
| IV | 29 (32) |
| Histology | |
| Differentiated | 18 (20) |
| Undifferentiated | 74 (80) |
| Type of treatment | |
| Curative resection | 58 (63) |
| Palliative resection | 12 (13) |
| Chemotherapy/radiotherapy | 16 (17) |
| No treatment | 6 (7) |
Age, tumor size and disease-free time are mean ± SD.
Figure 1.
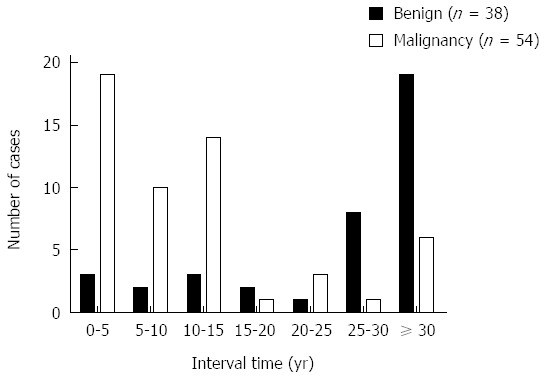
Time trend of stump carcinoma every 5 years for the primary gastric disease.
The detailed clinicopathological characteristics of all the patients are listed in Table 1. Concerning the initial gastric disease, 38 (41%) patients had benign disease, and 54 (59%) patients had gastric cancer. In total, 26 (28%) patients underwent Billroth-I reconstruction, and 65 (71%) patients received Billroth-II reconstruction. Most patients (59, 64%) had malignant lesions at the anastomotic site, and the median tumor size was 4.0 cm. Additionally, Borrmann III (69, 75%) was the most common type based on the gross appearance. Histology revealed that the most common type of cancer was undifferentiated (80%). The TNM classification was as follows: stage I in 8 patients (9%), stage II in 25 (26%), stage III in 30 (33%), and stage IV in 29 (32%). The lymph node metastasis rate in patients with GSC was 38% (35/92).
Seventy patients received surgical treatments, and 58 patients were qualified for radical surgery. The resectability rate was 59.8%, and 12 patients underwent palliative resection. Twenty-two patients declined surgery. Among them, 6 patients declined treatment, and 16 patients chose to receive chemotherapy/radiotherapy.
All 92 patients provided complete follow-up data. At the time of the last follow-up, 67 (72.8%) patients had died, and 25 (27.2%) patients were alive. The median survival duration was 29 mo, and the cumulative 1-, 3-, and 5-year overall survival rates were 78.3%, 45.6%, and 27.6%, respectively (Figure 2A). The survival curves also suggested that patients with regular follow-up after primary surgery had a better survival rate (Figure 2B and C). Their 1-, 3-, and 5-year survival rates were 93.8%, 75.0%, and 66.7%. However, the 1-, 3-, and 5-year survival rates were 87.5%, 47.7%, and 25.6%, respectively, for patients without regular follow-up after the primary surgery. Figure 2D demonstrates that patients with Borrmann type II cancer had the best survival rate (P = 0.0083). The 1-, 3-, and 5-year survival rates of the 58 patients who underwent radical resection were 79.3%, 52.2%, and 37.8%, respectively. Among them, 3 (3.3%) patients died during the peri-operative period. The peri-operative mortality was similar to that of patients with conventional gastric cancer (2%-3%)[10]. The 1-, 3-, and 5-year survival rates of the 12 patients who received palliative resection were 66.7%, 25.0%, and 0%, respectively, and there were significant differences between the three groups (P = 0.0007) (Figure 2E). Table 2 shows the group characteristics according to the tumor stage. The 5-year survival rates for stages I, II, III, and IV were 85.7%, 47.4%, 16.0%, and 13.3%, respectively (P = 0.005).
Figure 2.
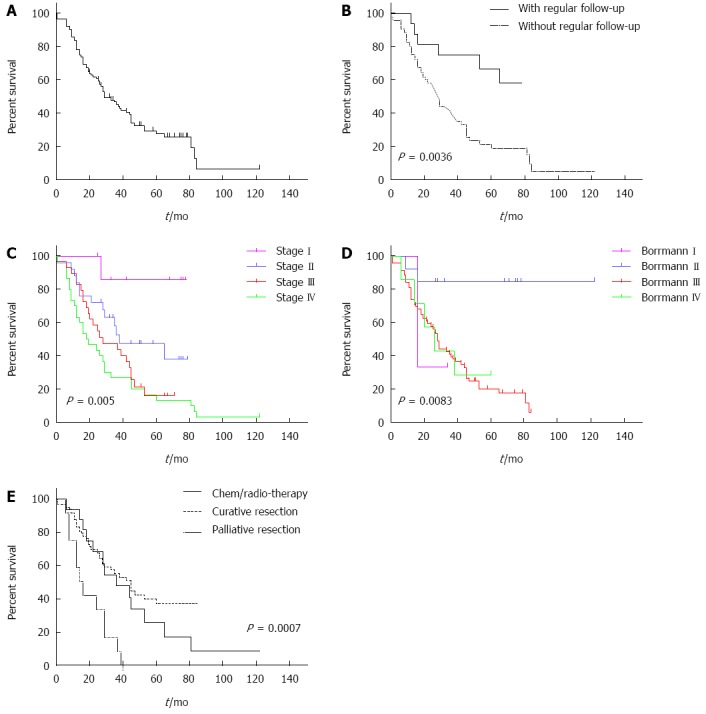
Overall survival proportions. A: Overall survival proportions of 92 patients with gastric stump cancer; B: Overall survival proportions between patients with and without regular follow-up after primary surgery are significantly different. The patients without regular follow-up after primary surgery had significantly poorer overall survival; C: Overall survival proportions for patients with different stages; D: Overall survival proportions for patients with different Borrmann type; E: Overall survival proportions in gastric stump cancer according to type of treatments.
Table 2.
Comparison of baseline characteristics according to tumor stage n (%)
| Variable | I (n = 8) | II (n = 25) | III (n = 30) | IV (n = 29) | |
| Histology | Differentiated | 4 (4.3) | 6 (6.5) | 6 (6.5) | 2 (2.2) |
| Undifferentiated | 4 (4.3) | 19 (20.6) | 24 (26.1) | 27 (29.3) | |
| Tumor Location | Anastomotic | 4 (4.3) | 17 (18.5) | 21 (22.8) | 17 (18.5) |
| Non-anastomotic | 4 (4.3) | 8 (8.7) | 9 (9.8) | 12 (13.0) | |
| Initial gastric disease | Benign disease | 4 (4.3) | 8 (8.7) | 13 (14.1) | 13 (14.1) |
| Gastric cancer | 9 (9.8) | 17 (18.5) | 17 (18.5) | 16 (17.4) | |
Values in parentheses are percentages.
DISCUSSION
Despite a decline in the overall incidence of gastric cancer[11], the incidence rate of GSC has increased during recent decades[3-5]. The incidence of GSC accounts for 1%-2% of all gastric cancers in Japan[2]. This increase indicates that we will face increasing challenges in the future.
To the best of our knowledge, the type of initial gastric disease has significant impact on the latency to GSC. The median interval for patients with benign disease is 30 years, and that for patients with gastric cancer is 12 years[12]. The incidence of GSC after gastric resection increases over time[13]. In the present study, the interval for benign disease was significantly longer than that for gastric cancer. Additionally, we observed different interval periods between benign disease and gastric cancer (Figure 1). The median interval time from initial operation to the development of GSC was 16.5 years (range: 1-40 years), which is consistent with other studies[9]. Figure 1 indicates that GSC tends to occur within 25 years following the initial benign disease, and during the 15 years post-operation for patients who have gastric cancer.
The clinical symptoms of GSC have no obvious specificity, and GSC usually manifests as upper abdominal pain, loss of appetite, swallowing difficulty, vomiting, weight loss, and anemia. GSC is often diagnosed at an advanced stage due to the lack of early symptoms. It is easily misdiagnosed as an ulcer recurrence or anastomotic inflammation, which can lead to a delayed diagnosis and the loss of the best treatment opportunity. The remnant stomach is viewed as a precancerous lesion. The specific etiology of GSC is currently unclear. According to our data, 16 (17%) patients had no clinical symptoms, and the malignant lesions of the remnant stomach were identified through periodic endoscopies. Most of these patients had early-stage GSC, and their 5-year survival rate was 66.7%. The 5-year survival rate of patients who had clinical symptoms was 19.0%. The survival curves also suggested that patients who were followed had better survival rates (Figure 2B). Regular follow-up can facilitate early detection and early therapy, which improve the survival rate.
Previous studies report the unique clinicopathological characteristics for GSC[2,8,14]. Our analysis demonstrated that the histological type was associated with prognosis (Table 3). The prognosis of patients with GSC was ultimately determined by the Borrmann lesion type and radical resection. Tumor location was also an important factor for predicting surgical outcomes[15-17]. The tumors commonly developed at the site of the gastrojejunal anastomosis. Some scholars thought that the type of reconstruction was associated with GSC[18-20]. In our study the Billroth-II reconstruction and anastomotic groups were more likely to develop GSC, although there was no significant difference due to the small number of patients.
Table 3.
Univariate and multivariate survival analyses in gastric stump cancer patients
| Variable | Univariate1 |
Multivariate2 |
||
| P value | HR | 95%CI | P value | |
| Age (yr) (≥ 65 vs < 65) | 0.896 | |||
| Sex ratio (male vs female) | 0.302 | |||
| Initial gastric disease (benign vs cancer) | 0.443 | |||
| Interval time from initial surgery (yr) (≥ 15 vs < 15) | 0.428 | |||
| Reconstruction of first operation (B-I vs B-II) | 0.357 | |||
| Regular follow-up (yes vs no) | 0.004 | 1.332 | 0.539-3.287 | 0.535 |
| Tumor size (mm) (≥ 40 vs < 40) | 0.040 | 0.707 | 0.415-1.206 | 0.203 |
| Location (anastomotic vs non-anastomotic) | 0.857 | |||
| Histology(differentiated vs undifferentiated) | 0.005 | 0.470 | 0.212-1.038 | 0.062 |
| Borrmann type (I-II vs III-IV) | 0.001 | 3.165 | 1.055-9.500 | 0.040 |
| Depth of invasion (T1-T2-T3 vs T4) | 0.305 | |||
| Lymph node involvement (NO vs ≥ N1) | 0.254 | |||
| Presence of distant metastasis (M0 vs M1) | 0.004 | |||
| Stage (I-II vs III-IV) | 0.002 | 0.603 | 0.323-1.124 | 0.111 |
| Type of treatment (curative vs other treatments) | 0.001 | 1.780 | 1.061-2.987 | 0.029 |
The Kaplan-Meier method, and significance was determined by the log-rank test;
Multivariate survival analysis was performed using Cox proportional hazard models.
Some drugs have been used to treat GSC in Japan[21]. However, in our study, surgery was still the most effective treatment. Although there were no significant differences in the histopathologic categories and tumor location of GSC compared with primary proximal gastric cancer[22-24], GSC is the most frequently occurring tumor after the initial surgery and occurs in the remnant stomach due to an abnormal anatomy. Additionally, because surgery for GSC is the second surgery, there is an increased number of adhesions around the residual stomach. Therefore, radical surgery is more difficult than ordinary surgery. There were no severe complications during and after our operations, therefore, radical surgery is feasible. The survival rate in the radical resection group was significantly higher than those in the palliative resection group and the chemotherapy/radiotherapy group. Our results demonstrated that chemotherapy/radiotherapy has a good short-term curative effect, but the long-term curative effect is still poor. Previous studies have reported higher incidences of postoperative complications in patients with GSC than in patients with primary gastric cancer[25]. Radical resection is still the best treatment option and may improve the survival outcomes of patients with GSC.
Admittedly, our study had a relatively small sample and was based on a retrospective analysis. However, even after acknowledging these limitations, we can draw some meaningful conclusions regarding GSC.
In conclusion, the findings in the present study led us to draw the following conclusions. Borrmann type I/II and radical resection are independent prognostic factors for patients with GSC. Early detection of GSC is possible. Regular endoscopies and gastric biopsies for subtotal gastrectomy patients have significant impact on postoperative survival. Therefore, it is necessary and feasible to perform repeated endoscopic follow-ups. If GSC is diagnosed, surgery should be performed. Additionally, radical resection may significantly improve the long-term survival of patients.
COMMENTS
Background
Gastric stump as a precancerous condition remains a substantial clinical concern. However, few studies explored the clinicopathological characteristics and prognostic factors for gastric stump cancer (GSC).
Research frontiers
GSC is often described as a tumor with a poor prognosis because of the unique biological features and it is commonly found at an advantage stage. Therefore, it is necessary to explore the clinicopathological characteristics in a great number of clinical cases.
Innovations and breakthroughs
This is the first comprehensive clinical trial about GSC in China. We observed the unique clinicopathological characteristics and prognostic factors for GSC, and the radical resection may significantly improve the long-term survival.
Applications
The results of the present study suggest that regular follow-up by endoscopy and gastric biopsy is necessary for subtotal gastrectomy patients, and the radical resection may significantly improve the long-term survival.
Terminology
The incidence of GSC accounts for 1%-7% of all gastric carcinomas following gastrectomy, and this frequency continues to increase. Gastric stump as a precancerous condition remains a substantial clinical concern. GSC is often described as a tumor with poor prognosis.
Peer-review
The manuscript is a remarkable article regarding GSC, which remains a substantial clinical concern and has its unique clinicopathological characteristics, as the appearance of GSC is earlier in the primary malignant cases than in the primary benign cases. So it is necessary to follow patients closely.
Footnotes
Supported by National Natural Science Foundation of China, No. 81272726; Specialized Research Fund for the Doctoral Program of Higher Education, China, No. 20110071120097; and Shanghai Municipal Health Bureau Research Project, No. 20114174.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: August 13, 2014
First decision: August 27, 2014
Article in press: October 15, 2014
P- Reviewer: Alshehabi Z, Higgins PJ, Shen LZ S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Liu XM
References
- 1.Balfour DC. Factors influencing the life expectancy of patients operated on for gastric ulcer. Ann Surg. 1922;76:405–408. doi: 10.1097/00000658-192209000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ohashi M, Katai H, Fukagawa T, Gotoda T, Sano T, Sasako M. Cancer of the gastric stump following distal gastrectomy for cancer. Br J Surg. 2007;94:92–95. doi: 10.1002/bjs.5538. [DOI] [PubMed] [Google Scholar]
- 3.Ahn HS, Kim JW, Yoo MW, Park do J, Lee HJ, Lee KU, Yang HK. Clinicopathological features and surgical outcomes of patients with remnant gastric cancer after a distal gastrectomy. Ann Surg Oncol. 2008;15:1632–1639. doi: 10.1245/s10434-008-9871-8. [DOI] [PubMed] [Google Scholar]
- 4.Sinning C, Schaefer N, Standop J, Hirner A, Wolff M. Gastric stump carcinoma - epidemiology and current concepts in pathogenesis and treatment. Eur J Surg Oncol. 2007;33:133–139. doi: 10.1016/j.ejso.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Nozaki I, Nasu J, Kubo Y, Tanada M, Nishimura R, Kurita A. Risk factors for metachronous gastric cancer in the remnant stomach after early cancer surgery. World J Surg. 2010;34:1548–1554. doi: 10.1007/s00268-010-0518-0. [DOI] [PubMed] [Google Scholar]
- 6.Sasako M, Maruyama K, Kinoshita T, Okabayashi K. Surgical treatment of carcinoma of the gastric stump. Br J Surg. 1991;78:822–824. doi: 10.1002/bjs.1800780718. [DOI] [PubMed] [Google Scholar]
- 7.Newman E, Brennan MF, Hochwald SN, Harrison LE, Karpeh MS. Gastric remnant carcinoma: just another proximal gastric cancer or a unique entity? Am J Surg. 1997;173:292–297. doi: 10.1016/S0002-9610(96)00403-5. [DOI] [PubMed] [Google Scholar]
- 8.Tanigawa N, Nomura E, Lee SW, Kaminishi M, Sugiyama M, Aikou T, Kitajima M. Current state of gastric stump carcinoma in Japan: based on the results of a nationwide survey. World J Surg. 2010;34:1540–1547. doi: 10.1007/s00268-010-0505-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park JH, Lee JH, Rhee PL, Kim JJ, Rhee JC, Kim S, Park CK. Endoscopic Screening for Remnant Gastric Cancer: Points to be Considered. Gut Liver. 2007;1:22–26. doi: 10.5009/gnl.2007.1.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ikeguchi M, Oka S, Gomyo Y, Tsujitani S, Maeta M, Kaibara N. Postoperative morbidity and mortality after gastrectomy for gastric carcinoma. Hepatogastroenterology. 2001;48:1517–1520. [PubMed] [Google Scholar]
- 11.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 12.Komatsu S, Ichikawa D, Okamoto K, Ikoma D, Tsujiura M, Nishimura Y, Murayama Y, Shiozaki A, Ikoma H, Kuriu Y, et al. Progression of remnant gastric cancer is associated with duration of follow-up following distal gastrectomy. World J Gastroenterol. 2012;18:2832–2836. doi: 10.3748/wjg.v18.i22.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greene FL. Management of gastric remnant carcinoma based on the results of a 15-year endoscopic screening program. Ann Surg. 1996;223:701–706; discussion 706-708. doi: 10.1097/00000658-199606000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu X, Tian DY, Cao L, Yu Y. Progression and prognosis of gastric stump cancer. J Surg Oncol. 2009;100:472–476. doi: 10.1002/jso.21370. [DOI] [PubMed] [Google Scholar]
- 15.An JY, Youn HG, Ha TK, Choi MG, Kim KM, Noh JH, Sohn TS, Kim S. Clinical significance of tumor location in remnant gastric cancers developed after partial gastrectomy for primary gastric cancer. J Gastrointest Surg. 2008;12:689–694. doi: 10.1007/s11605-007-0437-z. [DOI] [PubMed] [Google Scholar]
- 16.Papachristou DN, Karas M, Fortner JG. Anastomotic recurrence in the oesophagus complicating gastrectomy for adenocarcinoma of the stomach. Br J Surg. 1979;66:609–612. doi: 10.1002/bjs.1800660904. [DOI] [PubMed] [Google Scholar]
- 17.Namikawa T, Kitagawa H, Iwabu J, Okabayashi T, Kobayashi M, Hanazaki K. Tumors arising at previous anastomotic site may have poor prognosis in patients with gastric stump cancer following gastrectomy. J Gastrointest Surg. 2010;14:1923–1930. doi: 10.1007/s11605-010-1298-4. [DOI] [PubMed] [Google Scholar]
- 18.Sano T, Aiko T. New Japanese classifications and treatment guidelines for gastric cancer: revision concepts and major revised points. Gastric Cancer. 2011;14:97–100. doi: 10.1007/s10120-011-0040-6. [DOI] [PubMed] [Google Scholar]
- 19.Nomura E, Lee SW, Tokuhara T, Nitta T, Kawai M, Uchiyama K. Functional outcomes according to the size of the gastric remnant and the type of reconstruction following distal gastrectomy for gastric cancer: an investigation including total gastrectomy. Jpn J Clin Oncol. 2013;43:1195–1202. doi: 10.1093/jjco/hyt141. [DOI] [PubMed] [Google Scholar]
- 20.Fukuhara K, Osugi H, Takada N, Takemura M, Higashino M, Kinoshita H. Reconstructive procedure after distal gastrectomy for gastric cancer that best prevents duodenogastroesophageal reflux. World J Surg. 2002;26:1452–1457. doi: 10.1007/s00268-002-6363-z. [DOI] [PubMed] [Google Scholar]
- 21.Kashima Y, Adachi T, Hara N, Sohtome I, Shirasaka T. [A case of successful S-1 alternate-day administration for far-advanced remnant gastric cancer] Gan To Kagaku Ryoho. 2012;39:469–472. [PubMed] [Google Scholar]
- 22.Schaefer N, Sinning C, Standop J, Overhaus M, Hirner A, Wolff M. Treatment and prognosis of gastric stump carcinoma in comparison with primary proximal gastric cancer. Am J Surg. 2007;194:63–67. doi: 10.1016/j.amjsurg.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 23.An JY, Choi MG, Noh JH, Sohn TS, Kim S. The outcome of patients with remnant primary gastric cancer compared with those having upper one-third gastric cancer. Am J Surg. 2007;194:143–147. doi: 10.1016/j.amjsurg.2006.10.034. [DOI] [PubMed] [Google Scholar]
- 24.Tokunaga M, Sano T, Ohyama S, Hiki N, Fukunaga T, Yamada K, Yamaguchi T. Clinicopathological characteristics and survival difference between gastric stump carcinoma and primary upper third gastric cancer. J Gastrointest Surg. 2013;17:313–318. doi: 10.1007/s11605-012-2114-0. [DOI] [PubMed] [Google Scholar]
- 25.Sano T, Sasako M, Yamamoto S, Nashimoto A, Kurita A, Hiratsuka M, Tsujinaka T, Kinoshita T, Arai K, Yamamura Y, et al. Gastric cancer surgery: morbidity and mortality results from a prospective randomized controlled trial comparing D2 and extended para-aortic lymphadenectomy--Japan Clinical Oncology Group study 9501. J Clin Oncol. 2004;22:2767–2773. doi: 10.1200/JCO.2004.10.184. [DOI] [PubMed] [Google Scholar]


