Abstract
A 72-year-old woman with a sigmoid colon cancer and a synchronous colorectal liver metastasis (CRLM), which involved the right hepatic vein (RHV) and the inferior vena cava (IVC), was referred to our hospital. The metastatic lesion was diagnosed as initially unresectable because of its invasion into the confluence of the RHV and IVC. After she had undergone laparoscopic sigmoidectomy for the original tumor, she consequently had 3 courses of modified 5-fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) plus cetuximab. Computed tomography revealed a partial response, and the confluence of the RHV and IVC got free from cancer invasion. After 3 additional courses of mFOLFOX6 plus cetuximab, preoperative percutaneous transhepatic portal vein embolization (PTPE) was performed to secure the future remnant liver volume. Finally, a right hemihepatectomy was performed. The postoperative course was uneventful. The patient was discharged from the hospital on postoperative day 13. She had neither local recurrence nor distant metastasis 18 mo after the last surgical intervention. This multidisciplinary strategy, consisting of conversion chemotherapy using FOLFOX plus cetuximab and PTPE, could contribute in facilitating curative hepatic resection for initially unresectable CRLM.
Keywords: Initially unresectable, Colorectal liver metastasis, Conversion chemotherapy, Cetuximab, Percutaneous transhepatic portal vein embolization
Core tip: A 72-year-old woman with a sigmoid colon cancer and a synchronous colorectal liver metastasis (CRLM) was referred to our hospital. The metastatic lesion was diagnosed to be initially unresectable. After she had undergone laparoscopic sigmoidectomy for the original tumor, she consequently had 6 courses of modified 5-fluorouracil, leucovorin, and oxaliplatin plus cetuximab, resulting in conversion chemotherapy. Preoperative percutaneous transhepatic portal vein embolization was performed to secure the future remnant liver volume. Finally, a right hemihepatectomy was successfully performed. The postoperative course was uneventful. She had no recurrence for 18 mo. This multidisciplinary strategy could contribute in facilitating curative hepatic resection for initially unresectable CRLM.
INTRODUCTION
The incidence of colorectal cancer is increasing, and it is now the fourth leading cause of cancer deaths worldwide[1]. According to GLOBOCAN 2008 estimates, about 12.7 million cancer cases and 7.6 million cancer deaths were estimated to have occurred in 2008[2]. Over half of patients with colorectal cancer will develop metastatic disease, with a quarter having distant metastatic lesions at diagnosis, often in the liver[3]. Although hepatic resection remains the only potentially curative treatment in patients with colorectal liver metastasis (CRLM)[4-7], only 15% to 20% of patients with CRLM are suitable for surgical resection[8,9]. Here, we report a case of conversion chemotherapy using 5-fluorouracil, leucovorin, and oxaliplatin (FOLFOX) plus cetuximab, combined with portal vein embolization, which led to a successfully curative liver resection in a patient with initially unresectable synchronous CRLM.
CASE REPORT
A 72-year-old woman with a high level of carcinoembryonic antigen (CEA) was referred to our hospital for the further diagnosis from a previous physician. She had no previous history of serious diseases, operations, or hospitalizations. She had a family history of gastric cancer on her mother’s side. Laboratory workup showed that CEA level had increased to 27.0 ng/mL, and serum levels of transaminases were slightly elevated. Total colonoscopy revealed a tumor at the sigmoid colon. Computed tomography (CT) revealed a synchronous CRLM. It was diagnosed as unresectable due to its invasion of the right hepatic vein (RHV) and the inferior vena cava (IVC) (Figure 1A and B). Thus, she underwent just a sigmoidectomy with lymph node dissection. Histopathological analysis resulted in the diagnosis of a well-to-moderately differentiated adenocarcinoma. Since the cancer cells were found to have wild-type KRAS, a combination therapy of mFOLFOX6 with cetuximab [Day 1: 5-fluorouracil (5-FU) 400 mg/m2 bolus injection; leucovorin (LV) 200 mg/(m2.2 h) with oxaliplatin (L-OHP) 85 mg/(m2.2 h); cetuximab 250 mg/(m2.2 h); 5-FU 2400 mg/(m2.46 h) continuous infusion; Day 8: cetuximab 250 mg/(m2.1 h), every 2 wk] was chosen as the first-line chemotherapy, considering the possibility of conversion chemotherapy. Follow-up CT revealed that the CRLM had a partial response, and that the confluence of the RHV and IVC was free from cancer invasion after 3 courses of systemic chemotherapy (Figure 1C and D). In addition, the serum level of CEA decreased significantly (Figure 2). The residual liver volume was regarded as insufficient for right hemihepatectomy. Thus, percutaneous transhepatic portal embolization (PTPE) was carried out after an additional 3 courses of chemotherapy. Three weeks after PTPE, CT revealed an increase of the estimated future remnant liver ratio from 36.2% to 46.9%, with no detectable presence of any other metastatic lesion. This CRLM was finally regarded as resectable with a normal hepatic functional reserve (Table 1). Six weeks after the last course of chemotherapy, a right hemihepatectomy and cholecystectomy were performed because of the invasion to the right branch of the portal vein. After the mobilization of the right lobe and the completion of hepatic transection, the RHV and a part of the IVC was side-clamped with a Satinsky clamp and divided. The surgical margins, observed with a frozen section, had no malignancy. The side-clamped IVC was simply closed with a continuous suture without severe stenosis. The postoperative course was uneventful. The patient was discharged on postoperative day 13. The histopathological analysis revealed a well-differentiated adenocarcinoma, consistent with CRLM. The tumor was comprised of approximately 50% viable cancer cells, and the remainder was necrotic (Figure 3). The patient had an additional 6 courses of mFOLFOX6 after hepatectomy. She had neither local recurrence nor distant metastasis 18 mo after the last surgical intervention.
Figure 1.
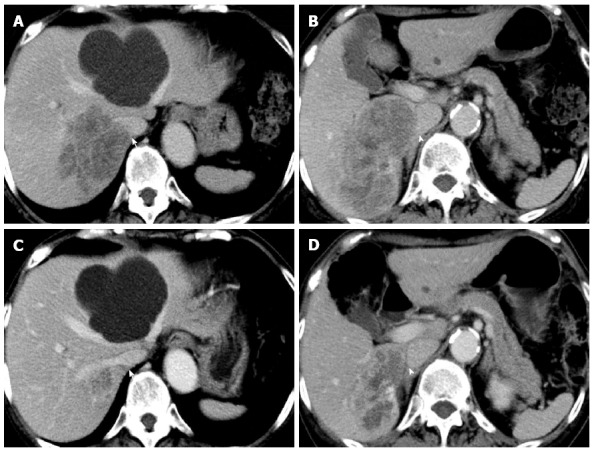
Enhanced computed tomography. A, B: Before chemotherapy. A huge synchronous colorectal liver metastasis was involving the right hepatic vein (RHV; arrow) and the inferior vena cava (IVC; arrowhead); C, D: After chemotherapy. The tumor was dramatically reduced, and the IVC was isolated from the tumor (arrowhead).
Figure 2.
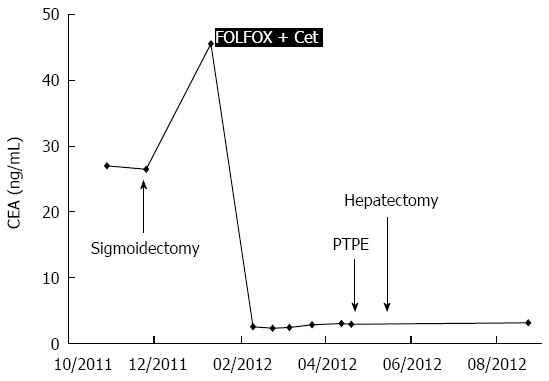
Serum levels of carcinoembryonic antigen. FOLFOX: 5-fluorouracil, leucovorin, and oxaliplatin; Cet: Cetuximab; PTPE: Percutaneous transhepatic portal vein embolization.
Table 1.
Laboratory data
| WBC | 6000/L |
| RBC | 343 × 104/L |
| Hb | 10.7 g/dL |
| Hct | 34.6% |
| Plt | 24.8 × 104/L |
| PT | 100.1% |
| HPT | 75.7% |
| AT3 | 107% |
| CEA | 2.9 ng/mL |
| CA19-9 | 14.1 U/mL |
| ICG-R15 | 6.7% |
| CRP | 0.077 mg/dL |
| T-Bil | 0.5 mg/dL |
| AST | 29 IU/L |
| ALT | 24 IU/L |
| LDH | 220 IU/L |
| γ-GTP | 72 IU/L |
| ALP | 385 IU/L |
| CHE | 112 IU/L |
| T-cho | 164 mg/dL |
| TP | 6.1 g/dL |
| Alb | 3.4 g/dL |
| BUN | 15 mg/dL |
| Cre | 0.62 mg/dL |
| Na | 141 mEq/L |
| K | 4.2 mEq/L |
| Cl | 108 mEq/L |
| Ca | 8.6 mEq/L |
Hb: Hemoglobin; Hct: Hematocrit; Plt: Platelet; PT: Prothrombin time; HPT: Hepaplastin test; AT3: Antithrombin III; CEA: Carcinoembryonic antigen; CA19-9: Carbohydrate antigen 19-9; ICG-R15: Indocyanine green retention rate at 15 min; CRP: C-reactive protein; T-Bil: Total bilirubin; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; LDH: Lactate dehydrogenase; γ-GTP: Gamma-glutamyl transpeptidase; ALP: Alanine phosphatase; CHE: Cholinesterase; T-cho: Total cholesterol; TP: Total protein; Alb: Albumin; BUN: Blood urea nitrogen; Cre: Creatinine.
Figure 3.
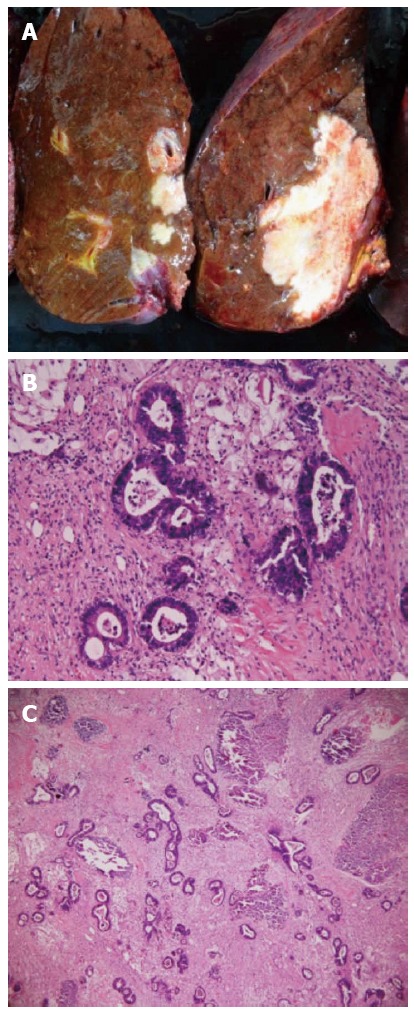
Resected specimen. A: Cut surface. The tumor was 70 mm × 40 mm in size. The tumor was grayish-white and stony hard; B, C: Hematoxylin and eosin (HE) staining of the resected specimen; B: HE staining, × 400. Adenocarcinoma; C: HE, × 100. Approximately 50% of the adenocarcinoma was necrotic.
DISCUSSION
Liver resection is the only potentially curative treatment with an expectation of long-term survival in patients with CRLM[4-7]. However, approximately 80% of patients with CRLM have unresectable disease, and long-term survival is poor in this setting[10]. In selected patients with unresectable metastases, CRLM may be down-staged by systemic chemotherapy with or without molecular targeted therapy, so that liver resection may be completed[11-15]. Once a complete curative resection is achieved, long-term survival would be expected, even in patients with initially unresectable CRLM[10,16-19]. Moreover, there is a positive correlation between the response rate to chemotherapy and the resection rate of liver metastases[20]. Therefore, response rates are very important when selecting patients for resection.
Currently, L-OHP and irinotecan have been widely used for patients with CRLM. The resection rate is significantly higher in patients receiving FOLFOX (5-FU + LV + L-OHP) than in those receiving FOLFIRI (5-FU + LV + irinotecan), according to a randomized GERCOR study[21]. To make matters worse, preoperative treatment with irinotecan has been reported to be associated with an increased risk of steatohepatitis. Steatohepatitis is associated with an increase in 90-day mortality after hepatic surgery[22]. While, L-OHP-based chemotherapy is associated with a significantly higher incidence of sinusoidal obstruction syndrome (SOS)[23,24]. SOS resulted in a poorer hepatic functional reserve and in a higher complication rate after major hepatectomy[24], but with no increase in mortality[22]. Therefore, we selected mFOLFOX6 as first-line chemotherapy, considering the future possibility of liver surgery.
The development of efficient molecular-targeted drugs, such as cetuximab or bevacizumab, have opened new perspectives in the treatment of resectable and unresectable liver metastases. In patients with KRAS wild-type tumors, chemotherapy with cetuximab yields high response rates compared with historical controls, and leads to significantly increased resectability[25-29]. The phase III NORDIC7 and COIN trials reported that first-line L-OHP-based chemotherapy plus cetuximab has no confirmed benefit[30,31]. However, in the NORDIC7 trial, patients received FLOX plus cetuximab, and in the COIN trial, patients received FOLFOX or XELOX (capecitabine + L-OHP) plus cetuximab. On the other hand, in the CELIM study[32], the response rates were significantly higher in patients whose tumors were wild type for KRAS (46 of 67 patients, 70%) than in those with KRAS mutations (11 of 27 patients, 41%). The R0 resection rates were 38% vs 30% (25 of 52 vs 18 of 44 patients) for FOLFOX plus cetuximab vs FOLFIRI plus cetuximab, respectively. Therefore, we selected FOLFOX plus cetuximab as the chemotherapy regimen.
It is quite difficult to predict the response to the tumor with chemotherapy. Negri et al[33] and Catalano et al[34] reported that mucinous histology predicts for poor response rate and overall survival in patients with colorectal cancer with fluorouracil-based or oxaliplatin-based chemotherapy. In our case, the histology of the original site was well-to-moderately differentiated adenocarcinoma. It might lead a good response with mFOLFOX6 plus cetuximab.
To secure the future remnant liver volume, PTPE may be considered as an option in selected patients[35-37]. The purpose of preoperative PTPE is to initiate compensatory hypertrophy in the future remnant liver in an attempt to counteract liver failure after major hepatectomy[38-40]. Nagino et al[41] underlined that indocyanine green clearance of the future liver remnant after PTPE should be more than 0.05 for major hepatectomy in patients with biliary cancers. However, liver function of our case was damaged due to 6 courses of FOLFOX. As an indication of PTPE for the patient with chemotherapy associated steatohepatitis remains controversial, it is to be elucidated.
There is no evidence on how many courses are the most effective for facilitating surgical resection in patients responsive to chemotherapy. It has been reported that the median duration of response to FOLFOX ranges from 4 to 6 courses[21,42]. Use of more than 6 courses of L-OHP-based chemotherapy is significantly associated with SOS[24]. Among patients undergoing a major hepatectomy, SOS was associated with significantly higher morbidity and longer hospital stays[24]. Therefore, preoperative chemotherapy was performed for total of 6 courses.
In conclusion, we herein report a case successfully treated with a multidisciplinary strategy, consisting of conversion chemotherapy using FOLFOX plus cetuximab and PTPE. This strategy may contribute to improve resectability for initially unresectable CRLM, thus leading to prolonged survival.
COMMENTS
Case characteristics
A 72-year-old female with a high level of carcinoembryonic antigen (CEA) was referred to our hospital.
Clinical diagnosis
The patient had no clinical symptoms.
Differential diagnosis
A high level of CEA are associated with adenocarcinoma; colon cancer, stomach cancer, lung cancer, pancreatic cancer, and so on.
Laboratory diagnosis
Laboratory workup showed that CEA level increased to 27.0 ng/mL.
Imaging diagnosis
Computed tomography revealed a synchronous colorectal liver metastasis, which involved the right hepatic vein and the inferior vena cava.
Pathological diagnosis
Histopathological analysis resulted in the diagnosis of a well-to-moderately differentiated adenocarcinoma, and the cancer cells were found to have wild-type KRAS.
Treatment
The patient underwent laparoscopic sigmoidectomy, followed by 5-fluorouracil, leucovorin, and oxaliplatin plus cetuximab, portal vein embolization for future remnant liver volume, and a right hemihepatectomy.
Term explanation
Conversion chemotherapy is a method that liver resection becomes possible by intensive chemotherapy, in patients with initially unresectable colorectal liver metastases (CRLM).
Experiences and lessons
In selected patients with unresectable metastases, CRLM may be down-staged by systemic chemotherapy with or without molecular targeted therapy, so that liver resection may be completed.
Peer-review
Conversion chemotherapy might lead to a successfully curative liver resection in a patient with initially unresectable synchronous CRLM. However, it depends on patients if systemic chemotherapy is effective.
Footnotes
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: May 26, 2014
First decision: June 18, 2014
Article in press: July 25, 2014
P- Reviewer: Bujanda L, Shao R, Sulkowski S S- Editor: Gou SX L- Editor: A E- Editor: Zhang DN
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.O’Connell JB, Maggard MA, Ko CY. Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst. 2004;96:1420–1425. doi: 10.1093/jnci/djh275. [DOI] [PubMed] [Google Scholar]
- 4.Adam R, Pascal G, Azoulay D, Tanaka K, Castaing D, Bismuth H. Liver resection for colorectal metastases: the third hepatectomy. Ann Surg. 2003;238:871–883; discussion 883-884. doi: 10.1097/01.sla.0000098112.04758.4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318; discussion 318-321. doi: 10.1097/00000658-199909000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minagawa M, Makuuchi M, Torzilli G, Takayama T, Kawasaki S, Kosuge T, Yamamoto J, Imamura H. Extension of the frontiers of surgical indications in the treatment of liver metastases from colorectal cancer: long-term results. Ann Surg. 2000;231:487–499. doi: 10.1097/00000658-200004000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaeck D, Bachellier P, Guiguet M, Boudjema K, Vaillant JC, Balladur P, Nordlinger B. Long-term survival following resection of colorectal hepatic metastases. Association Française de Chirurgie. Br J Surg. 1997;84:977–980. doi: 10.1002/bjs.1800840719. [DOI] [PubMed] [Google Scholar]
- 8.Steele G, Ravikumar TS. Resection of hepatic metastases from colorectal cancer. Biologic perspective. Ann Surg. 1989;210:127–138. doi: 10.1097/00000658-198908000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scheele J. Hepatectomy for liver metastases. Br J Surg. 1993;80:274–276. doi: 10.1002/bjs.1800800302. [DOI] [PubMed] [Google Scholar]
- 10.Adam R, Delvart V, Pascal G, Valeanu A, Castaing D, Azoulay D, Giacchetti S, Paule B, Kunstlinger F, Ghémard O, et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg. 2004;240:644–657; discussion 657-658. doi: 10.1097/01.sla.0000141198.92114.f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fowler WC, Eisenberg BL, Hoffman JP. Hepatic resection following systemic chemotherapy for metastatic colorectal carcinoma. J Surg Oncol. 1992;51:122–125. doi: 10.1002/jso.2930510212. [DOI] [PubMed] [Google Scholar]
- 12.Elias D, Lasser P, Rougier P, Ducreux M, Bognel C, Roche A. Frequency, technical aspects, results, and indications of major hepatectomy after prolonged intra-arterial hepatic chemotherapy for initially unresectable hepatic tumors. J Am Coll Surg. 1995;180:213–219. [PubMed] [Google Scholar]
- 13.Bismuth H, Adam R, Lévi F, Farabos C, Waechter F, Castaing D, Majno P, Engerran L. Resection of nonresectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Ann Surg. 1996;224:509–20; discussion 520-2. doi: 10.1097/00000658-199610000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meric F, Patt YZ, Curley SA, Chase J, Roh MS, Vauthey JN, Ellis LM. Surgery after downstaging of unresectable hepatic tumors with intra-arterial chemotherapy. Ann Surg Oncol. 2000;7:490–495. doi: 10.1007/s10434-000-0490-2. [DOI] [PubMed] [Google Scholar]
- 15.Shankar A, Leonard P, Renaut AJ, Lederman J, Lees WR, Gillams AR, Harrison E, Taylor I. Neo-adjuvant therapy improves resectability rates for colorectal liver metastases. Ann R Coll Surg Engl. 2001;83:85–88. [PMC free article] [PubMed] [Google Scholar]
- 16.Giacchetti S, Itzhaki M, Gruia G, Adam R, Zidani R, Kunstlinger F, Brienza S, Alafaci E, Bertheault-Cvitkovic F, Jasmin C, et al. Long-term survival of patients with unresectable colorectal cancer liver metastases following infusional chemotherapy with 5-fluorouracil, leucovorin, oxaliplatin and surgery. Ann Oncol. 1999;10:663–669. doi: 10.1023/a:1008347829017. [DOI] [PubMed] [Google Scholar]
- 17.Adam R, Wicherts DA, de Haas RJ, Ciacio O, Lévi F, Paule B, Ducreux M, Azoulay D, Bismuth H, Castaing D. Patients with initially unresectable colorectal liver metastases: is there a possibility of cure? J Clin Oncol. 2009;27:1829–1835. doi: 10.1200/JCO.2008.19.9273. [DOI] [PubMed] [Google Scholar]
- 18.Akgül Ö, Çetinkaya E, Ersöz Ş, Tez M. Role of surgery in colorectal cancer liver metastases. World J Gastroenterol. 2014;20:6113–6122. doi: 10.3748/wjg.v20.i20.6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hasegawa K, Takahashi M, Ohba M, Kaneko J, Aoki T, Sakamoto Y, Sugawara Y, Kokudo N. Perioperative chemotherapy and liver resection for hepatic metastases of colorectal cancer. J Hepatobiliary Pancreat Sci. 2012;19:503–508. doi: 10.1007/s00534-012-0509-7. [DOI] [PubMed] [Google Scholar]
- 20.Folprecht G, Grothey A, Alberts S, Raab HR, Köhne CH. Neoadjuvant treatment of unresectable colorectal liver metastases: correlation between tumour response and resection rates. Ann Oncol. 2005;16:1311–1319. doi: 10.1093/annonc/mdi246. [DOI] [PubMed] [Google Scholar]
- 21.Tournigand C, André T, Achille E, Lledo G, Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229–237. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]
- 22.Vauthey JN, Pawlik TM, Ribero D, Wu TT, Zorzi D, Hoff PM, Xiong HQ, Eng C, Lauwers GY, Mino-Kenudson M, et al. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol. 2006;24:2065–2072. doi: 10.1200/JCO.2005.05.3074. [DOI] [PubMed] [Google Scholar]
- 23.Rubbia-Brandt L, Audard V, Sartoretti P, Roth AD, Brezault C, Le Charpentier M, Dousset B, Morel P, Soubrane O, Chaussade S, et al. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol. 2004;15:460–466. doi: 10.1093/annonc/mdh095. [DOI] [PubMed] [Google Scholar]
- 24.Nakano H, Oussoultzoglou E, Rosso E, Casnedi S, Chenard-Neu MP, Dufour P, Bachellier P, Jaeck D. Sinusoidal injury increases morbidity after major hepatectomy in patients with colorectal liver metastases receiving preoperative chemotherapy. Ann Surg. 2008;247:118–124. doi: 10.1097/SLA.0b013e31815774de. [DOI] [PubMed] [Google Scholar]
- 25.Van Cutsem E, Köhne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D’Haens G, Pintér T, Lim R, Bodoky G, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 26.Stintzing S, Fischer von Weikersthal L, Decker T, Vehling-Kaiser U, Jäger E, Heintges T, Stoll C, Giessen C, Modest DP, Neumann J, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer-subgroup analysis of patients with KRAS: mutated tumours in the randomised German AIO study KRK-0306. Ann Oncol. 2012;23:1693–1699. doi: 10.1093/annonc/mdr571. [DOI] [PubMed] [Google Scholar]
- 27.Okines A, Puerto OD, Cunningham D, Chau I, Van Cutsem E, Saltz L, Cassidy J. Surgery with curative-intent in patients treated with first-line chemotherapy plus bevacizumab for metastatic colorectal cancer First BEAT and the randomised phase-III NO16966 trial. Br J Cancer. 2009;101:1033–1038. doi: 10.1038/sj.bjc.6605259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, de Braud F, Donea S, Ludwig H, Schuch G, Stroh C, et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2009;27:663–671. doi: 10.1200/JCO.2008.20.8397. [DOI] [PubMed] [Google Scholar]
- 29.Ocvirk J, Brodowicz T, Wrba F, Ciuleanu TE, Kurteva G, Beslija S, Koza I, Pápai Z, Messinger D, Yilmaz U, et al. Cetuximab plus FOLFOX6 or FOLFIRI in metastatic colorectal cancer: CECOG trial. World J Gastroenterol. 2010;16:3133–3143. doi: 10.3748/wjg.v16.i25.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tveit KM, Guren T, Glimelius B, Pfeiffer P, Sorbye H, Pyrhonen S, Sigurdsson F, Kure E, Ikdahl T, Skovlund E, et al. Phase III trial of cetuximab with continuous or intermittent fluorouracil, leucovorin, and oxaliplatin (Nordic FLOX) versus FLOX alone in first-line treatment of metastatic colorectal cancer: the NORDIC-VII study. J Clin Oncol. 2012;30:1755–1762. doi: 10.1200/JCO.2011.38.0915. [DOI] [PubMed] [Google Scholar]
- 31.Maughan TS, Adams RA, Smith CG, Meade AM, Seymour MT, Wilson RH, Idziaszczyk S, Harris R, Fisher D, Kenny SL, et al. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet. 2011;377:2103–2114. doi: 10.1016/S0140-6736(11)60613-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Folprecht G, Gruenberger T, Bechstein WO, Raab HR, Lordick F, Hartmann JT, Lang H, Frilling A, Stoehlmacher J, Weitz J, et al. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomised phase 2 trial. Lancet Oncol. 2010;11:38–47. doi: 10.1016/S1470-2045(09)70330-4. [DOI] [PubMed] [Google Scholar]
- 33.Negri FV, Wotherspoon A, Cunningham D, Norman AR, Chong G, Ross PJ. Mucinous histology predicts for reduced fluorouracil responsiveness and survival in advanced colorectal cancer. Ann Oncol. 2005;16:1305–1310. doi: 10.1093/annonc/mdi244. [DOI] [PubMed] [Google Scholar]
- 34.Catalano V, Loupakis F, Graziano F, Torresi U, Bisonni R, Mari D, Fornaro L, Baldelli AM, Giordani P, Rossi D, et al. Mucinous histology predicts for poor response rate and overall survival of patients with colorectal cancer and treated with first-line oxaliplatin- and/or irinotecan-based chemotherapy. Br J Cancer. 2009;100:881–887. doi: 10.1038/sj.bjc.6604955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kinoshita H, Sakai K, Hirohashi K, Igawa S, Yamasaki O, Kubo S. Preoperative portal vein embolization for hepatocellular carcinoma. World J Surg. 1986;10:803–808. doi: 10.1007/BF01655244. [DOI] [PubMed] [Google Scholar]
- 36.Honjo I, Suzuki T, Ozawa K, Takasan H, Kitamura O. Ligation of a branch of the portal vein for carcinoma of the liver. Am J Surg. 1975;130:296–302. doi: 10.1016/0002-9610(75)90389-x. [DOI] [PubMed] [Google Scholar]
- 37.Chun YS, Vauthey JN, Ribero D, Donadon M, Mullen JT, Eng C, Madoff DC, Chang DZ, Ho L, Kopetz S, et al. Systemic chemotherapy and two-stage hepatectomy for extensive bilateral colorectal liver metastases: perioperative safety and survival. J Gastrointest Surg. 2007;11:1498–1504; discussion 1504-1505. doi: 10.1007/s11605-007-0272-2. [DOI] [PubMed] [Google Scholar]
- 38.Makuuchi M, Thai BL, Takayasu K, Takayama T, Kosuge T, Gunvén P, Yamazaki S, Hasegawa H, Ozaki H. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery. 1990;107:521–527. [PubMed] [Google Scholar]
- 39.Nagino M, Nimura Y, Kamiya J, Kondo S, Uesaka K, Kin Y, Hayakawa N, Yamamoto H. Changes in hepatic lobe volume in biliary tract cancer patients after right portal vein embolization. Hepatology. 1995;21:434–439. [PubMed] [Google Scholar]
- 40.Ribero D, Chun YS, Vauthey JN. Standardized liver volumetry for portal vein embolization. Semin Intervent Radiol. 2008;25:104–109. doi: 10.1055/s-2008-1076681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagino M, Kamiya J, Nishio H, Ebata T, Arai T, Nimura Y. Two hundred forty consecutive portal vein embolizations before extended hepatectomy for biliary cancer: surgical outcome and long-term follow-up. Ann Surg. 2006;243:364–372. doi: 10.1097/01.sla.0000201482.11876.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–2947. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]


