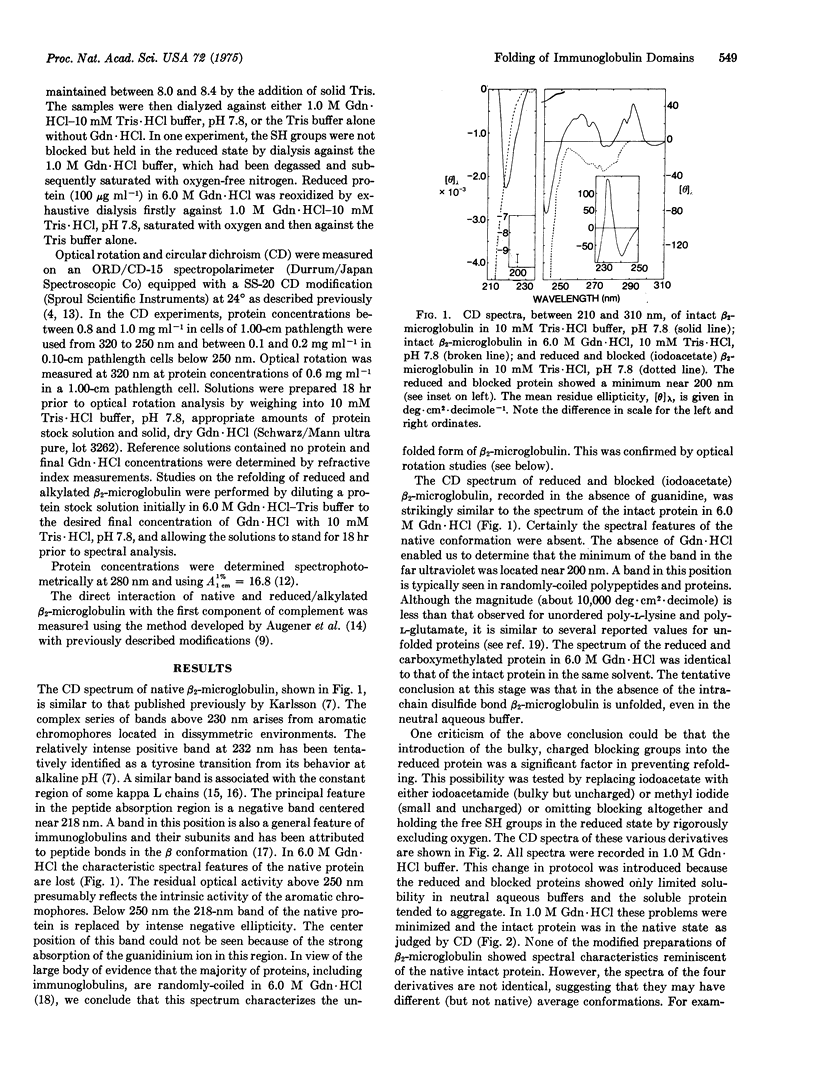
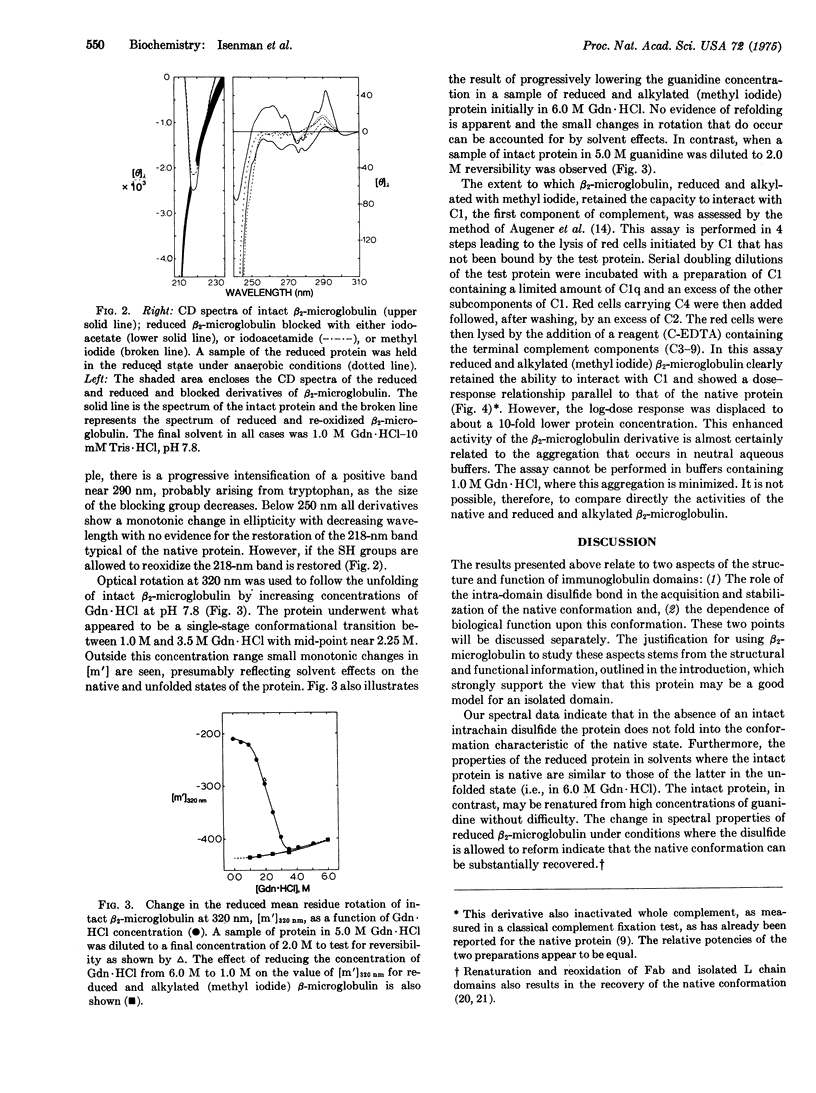
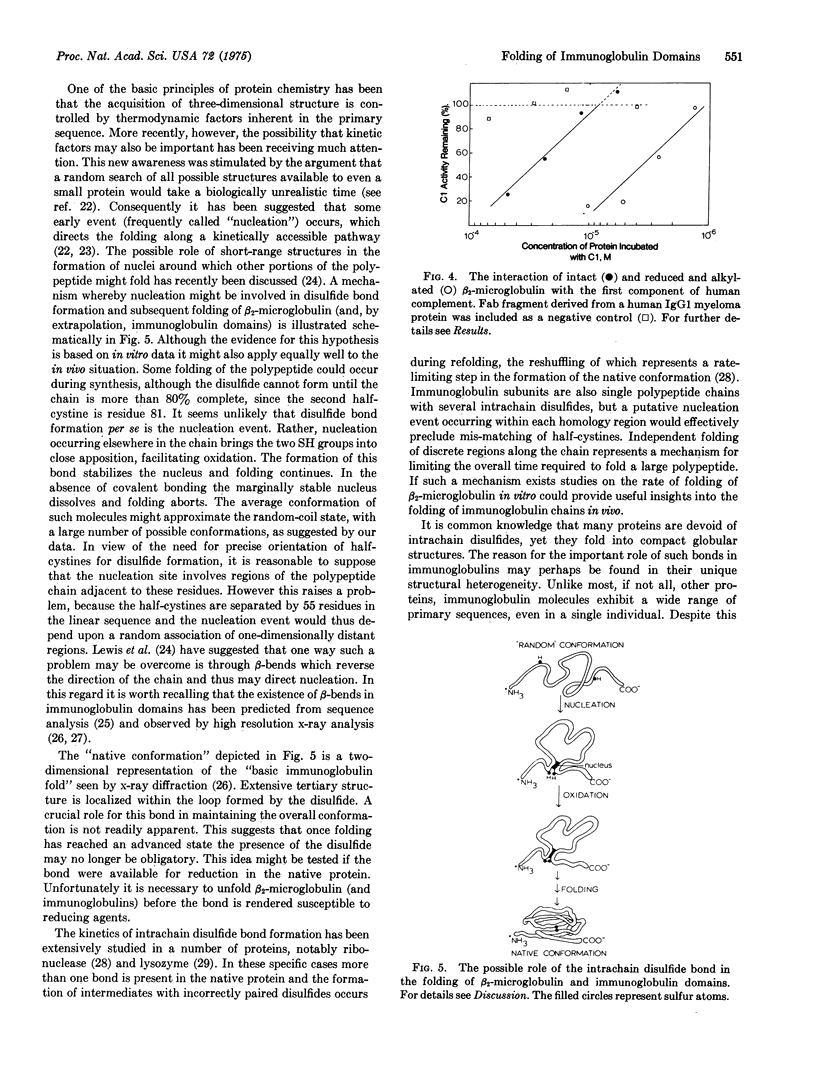
Abstract
Beta-Microglobulin, a low-molecular-weight protein structurally related to the homology regions of immunoglobulins, has been used to study the role of the intrachain disulfide bond in the unfolding of immunoglobulin domains. The intact protein could be reversibly unfolded in guanidine hydrochloride, as judged by circular dichroism and optical rotation. Similarly, reoxidation of the reduced protein, during transfer from high concentrations of guanidine to neutral aqueous buffer, yielded a product with spectral characteristics typical of the native protein. However, if the free SH groups were prevented from reoxidizing either by chemical modification or by holding them in the reduced state, the molecule appeared to be in the randomly coiled state even under conditions where the intact protein is in the native conformation, judged on the basis of chiroptical measurements. The complement-fixing activity exhibited by native beta-2-microglobulin was retained by the reduced and alkylated derivative, suggesting that the site may be formed from a linear array of amino acids. We suggest a model for the folding of beta-2-microglobulin (and immunoglobulin domains) in which one of the early folding events results in disulfide bond formation, the latter being an obligatory step for continued folding to the native state.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augener W., Grey H. M., Cooper N. R., Müller-Eberhard H. J. The reaction of monomeric and aggregated immunoglobulins with C1. Immunochemistry. 1971 Nov;8(11):1011–1020. doi: 10.1016/0019-2791(71)90489-7. [DOI] [PubMed] [Google Scholar]
- Berggård I., Bearn A. G. Isolation and properties of a low molecular weight beta-2-globulin occurring in human biological fluids. J Biol Chem. 1968 Aug 10;243(15):4095–4103. [PubMed] [Google Scholar]
- Bunting J. R., Athey T. W., Cathou R. E. Backbone folding of immunoglobulin light and heavy chains: a comparison of predicted -bend positions. Biochim Biophys Acta. 1972 Nov 28;285(1):60–71. doi: 10.1016/0005-2795(72)90180-8. [DOI] [PubMed] [Google Scholar]
- Cunningham B. A., Wang J. L., Berggård I., Peterson P. A. The complete amino acid sequence of beta 2-microglobulin. Biochemistry. 1973 Nov 20;12(24):4811–4822. doi: 10.1021/bi00748a001. [DOI] [PubMed] [Google Scholar]
- Dorrington K. J., Smith B. R. Conformational changes accompanying the dissociation and association of immunoglobulin-G subunits. Biochim Biophys Acta. 1972 Mar 15;263(1):70–81. doi: 10.1016/0005-2795(72)90160-2. [DOI] [PubMed] [Google Scholar]
- Dorrington K. J., Tanford C. Molecular size and conformation of immunoglobulins. Adv Immunol. 1970;12:333–381. doi: 10.1016/s0065-2776(08)60173-x. [DOI] [PubMed] [Google Scholar]
- Edelman G. M., Cunningham B. A., Gall W. E., Gottlieb P. D., Rutishauser U., Waxdal M. J. The covalent structure of an entire gammaG immunoglobulin molecule. Proc Natl Acad Sci U S A. 1969 May;63(1):78–85. doi: 10.1073/pnas.63.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M., Gall W. E. The antibody problem. Annu Rev Biochem. 1969;38:415–466. doi: 10.1146/annurev.bi.38.070169.002215. [DOI] [PubMed] [Google Scholar]
- Ellerson J. R., Yasmeen D., Painter R. H., Dorrington K. J. A fragment corresponding to the C(H)2 region of immunoglobulin G (IgG) with complement fixing activity. FEBS Lett. 1972 Aug 15;24(3):318–322. doi: 10.1016/0014-5793(72)80381-8. [DOI] [PubMed] [Google Scholar]
- Ghose A. C., Jirgensons B. Circular dichroism studies on the variable and constant halves of kappa-type Bence-Jones proteins. Biochim Biophys Acta. 1971 Oct;251(1):14–20. doi: 10.1016/0005-2795(71)90053-5. [DOI] [PubMed] [Google Scholar]
- Hantgan R. R., Hammes G. G., Scheraga H. A. Pathways of folding of reduced bovine pancreatic ribonuclease. Biochemistry. 1974 Aug 13;13(17):3421–3431. doi: 10.1021/bi00714a001. [DOI] [PubMed] [Google Scholar]
- Karlsson F. A., Peterson P. A., Berggard I. Properties of halves of immunoglobulin light chains. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1257–1263. doi: 10.1073/pnas.64.4.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson F. A. Physical-chemical properties of beta 2-microglobulin. Immunochemistry. 1974 Mar;11(3):111–114. doi: 10.1016/0019-2791(74)90207-9. [DOI] [PubMed] [Google Scholar]
- Kehoe J. M., Bourgois A., Capra J. D., Fougereau M. Amino acid sequence of a murine immunoglobulin fragment that possesses complement fixing activity. Biochemistry. 1974 Jun 4;13(12):2499–2504. doi: 10.1021/bi00709a005. [DOI] [PubMed] [Google Scholar]
- Kehoe J. M., Fougereau M. Immunoglobulin peptide with complement fixing activity. Nature. 1969 Dec 20;224(5225):1212–1213. doi: 10.1038/2241212a0. [DOI] [PubMed] [Google Scholar]
- Lapanje S., Dorrington K. J. Influence of subunit interaction and intersubunit disulphide bonds on the unfolding of immunoglobulin G by guanidine hydrochloride. Biochim Biophys Acta. 1973 Sep 21;322(1):45–52. doi: 10.1016/0005-2795(73)90173-6. [DOI] [PubMed] [Google Scholar]
- Lewis P. N., Momany F. A., Scheraga H. A. Folding of polypeptide chains in proteins: a proposed mechanism for folding. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2293–2297. doi: 10.1073/pnas.68.9.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter R. H., Yasmeen D., Assimeh S. N., Poulik M. D. Complement fixing and macrophage opsonizing activities associated with beta2 microglobulin. Immunol Commun. 1974;3(1):19–34. doi: 10.3109/08820137409055743. [DOI] [PubMed] [Google Scholar]
- Percy M. E., Dorrington K. J. An atypical human immunoglobulin G with deletions in both heavy and light chains. Studies of the conformation and the in vitro recombination of the isolated subunits. Can J Biochem. 1974 Jul;52(7):610–619. doi: 10.1139/o74-088. [DOI] [PubMed] [Google Scholar]
- Poljak R. J., Amzel L. M., Avey H. P., Chen B. L., Phizackerley R. P., Saul F. Three-dimensional structure of the Fab' fragment of a human immunoglobulin at 2,8-A resolution. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3305–3310. doi: 10.1073/pnas.70.12.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena V. P., Wetlaufer D. B. Formation of three-dimensional structure in proteins. I. Rapid nonenzymic reactivation of reduced lysozyme. Biochemistry. 1970 Dec 8;9(25):5015–5023. doi: 10.1021/bi00827a028. [DOI] [PubMed] [Google Scholar]
- Schiffer M., Girling R. L., Ely K. R., Edmundson A. B. Structure of a lambda-type Bence-Jones protein at 3.5-A resolution. Biochemistry. 1973 Nov 6;12(23):4620–4631. doi: 10.1021/bi00747a013. [DOI] [PubMed] [Google Scholar]
- Smithies O., Poulik M. D. Initiation of protein synthesis at an unusual position in an immunoglobulin gene? Science. 1972 Jan 14;175(4018):187–189. doi: 10.1126/science.175.4018.187. [DOI] [PubMed] [Google Scholar]
- Tanford C. Protein denaturation. Adv Protein Chem. 1968;23:121–282. doi: 10.1016/s0065-3233(08)60401-5. [DOI] [PubMed] [Google Scholar]
- WHITNEY P. L., TANFORD C. RECOVERY OF SPECIFIC ACTIVITY AFTER COMPLETE UNFOLDING AND REDUCTION OF AN ANTIBODY FRAGMENT. Proc Natl Acad Sci U S A. 1965 Mar;53:524–532. doi: 10.1073/pnas.53.3.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetlaufer D. B. Nucleation, rapid folding, and globular intrachain regions in proteins. Proc Natl Acad Sci U S A. 1973 Mar;70(3):697–701. doi: 10.1073/pnas.70.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetlaufer D. B., Ristow S. Acquisition of three-dimensional structure of proteins. Annu Rev Biochem. 1973;42:135–158. doi: 10.1146/annurev.bi.42.070173.001031. [DOI] [PubMed] [Google Scholar]


