Abstract
Cavernous hemangiomas were first reported in 1929 by Globus and Doshay, and are defined as benign vascular structures developed between the neural tissues occurring in the central nervous system, consisting of a dilated vascular bed. Cavernous hemangiomas comprise nearly 5-12% of all spinal vascular malformations; however, existence in the epidural space without bone involvement is rare. Only 4% of all cavernous hemangiomas (0.22/1.000.000) are purely epidural cavernous hemangiomas. In this case report, we removed a hemorrhagic thoracic mass presenting with progressive neurological deficits in a 55-year-old male patient. We found this case to be appropriate for presentation due to the rare occurrence of this type of cavernous hemangioma.
Keywords: Cavernous hemangioma, Extraosseous-epidural, Thoracic vertebrae, Hemangioma
INTRODUCTION
Cavernous hemangiomas (CHs) are defined as benign vascular structures inserted within the neural tissue, occurring in the central nervous system, and consisting of a dilated vascular bed5). They may be located in the cerebral cortex, cerebellum orspinal cord5). CHs create 5-12% of all spinal vascular malformations10). Existence in the epidural space without bone involvement is rare1,10), and only 4% of all CHs (0.22/1.000.000) are purely epidural1,10). Clinical symptoms are usually seen as slowly progressive paraparesis, myelopathy and localized pain1,5). Although magnetic resonance imaging (MRI) is the best diagnostic method for this lesion, it is not a definitive one8). We felt it appropriate to present this case because CHs in the epidural space have rarely been encountered.
CASE REPORT
A 55-year-old male patient was admitted to our clinic with complaints of back pain beginning two years previously. Additionally, he had progressive spastic paraparesis in both lower extremities, and an inability to stand without support for the previous 3 months. Upon neurological examination, the motor power in both lower extremities was II-III/V, deep tendon reflexes were hyperactive, and hypesthesia was present under the T10-11 level. The patient had no history ofurinary or fecal incontinence, and the laboratory tests were within normal limits.
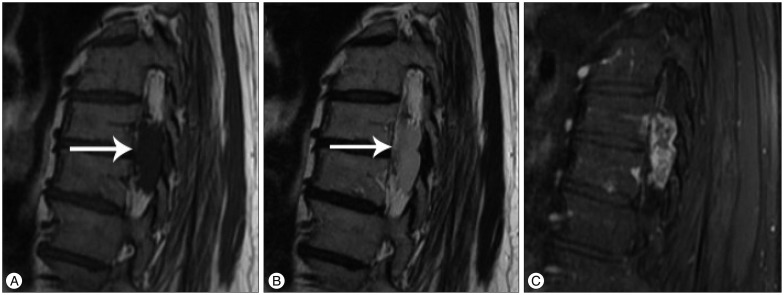
Routine X-rays were normal. However, inthe thoracic, lumbar, and cervical MRIs performed for further investigation, a mass lesion was seen along the T7-8 vertebral body levels. This lesion was hypointense upon the T1-weighted images (Fig. 1A), with a slightly lower signal than the cerebrospinal fluid (CSF) on the T2-weighted images (Fig. 1B), and slight heterogeneous staining on the gadolinium-enhanced T1-weighted images (Fig. 1C). Additionally, it was observed that the spinal cord was displaced to the right side due to the mass (Fig. 2).
Fig. 1.
Mass lesion with hypointensity in T1-weighted images (arrow) (A), with slightly lower signal than CSF in T2-weighted images (arrow) (B), and slightly heterogeneous gadolinium-enhanced T1-weighted images (C) upon sagittal thoracic spine MRI. CSF : cerebrospinal fluid.
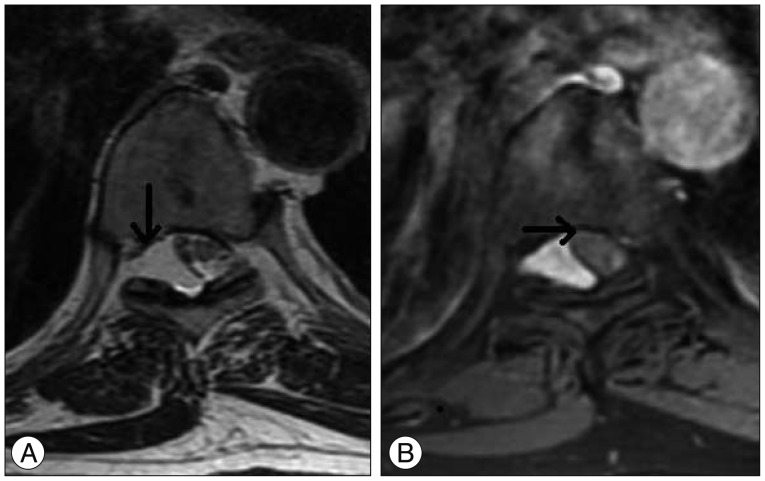
Fig. 2.
Mass displacing the spinal cord to the right side in the axial plane in the T2-weighted (A) and fat suppression T1-weighted enhanced (B) images uponthe MRI.
Under general anesthesia, a T8-9 total laminectomy was performed and an approximately 3×1.5 cm sized (Fig. 3A) dark-colored vascular mass with soft consistency was found in the epidural space without invasion of the dura (Fig. 3B), which was completely excised microsurgically.
Fig. 3.
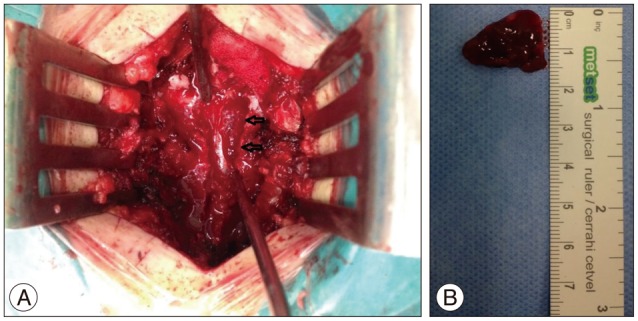
Extradural hemorrhagic mass located in the operating field (arrows) (A) and its gross finding after total removal (B).
The histopathological examination revealed a cavernous hemangioma, composed of large dilated blood-filled vessels lined with flattened endothelium (Fig. 4). Neurological deterioration was not found during the postoperative period. Ten days postoperatively, the patient could walk independently and reported reduction in the localized back pain.
Fig. 4.
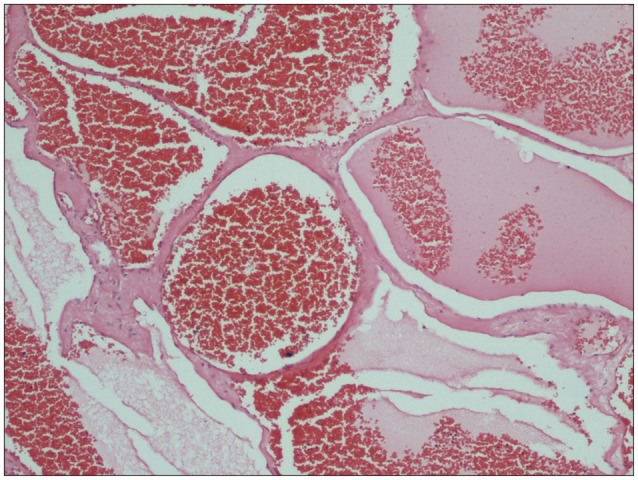
Flattened endothelium-coated large dilated blood vessels (H&E, ×40).
DISCUSSION
CHs were first reported in the literature in 1929 by Globus and Doshay4,6,8). CHs are collections of small capillaries covered with a single layer of endothelium, characterized by lobules, separated by fibrous connective tissue septa, and composed of irregular and dilated vascular channels6). CHs are not true neoplasms because they grow with hypertrophy without mitotic activity2). However, they create clinical signs with mass effects, fluid movement within vascular structure, hemorrhages, thromboses, and the formation of cysts or caverns6). These lesions can occur in every region of the body; however, the most common area of occurrence in the cranial region of the central nervous system is the supratentorial area8). CHs have rarely been seen in the spinal canal1,6,10), while only 4% of all cavernous hemangiomas (0.22/1.000.000) are purely epidural CHs1,10). Spinal CHs appear at an average age of 40 (30-60) and are more common in women (70%). According to frequency, the localization in the spinal vertebrae can be listed in order of occurrence as in the thoracic (54-60%), cervical (30%), and lumbar (10%) vertebral regions1,7,9); they are seen most commonly in the posterior part of the spinal canal1,9). There are two currently accepted hypotheses in the literature with regard to the frequent occurrence in the thoracic vertebrae; the first is that the thoracic epidural space is wider3,6), while the second is that the resistance in the posterior part of the thoracic spinal canal is lower3,6). Besides the sizes are very diverse3,6,7) the localization of the CH in our fifty-five-year-old male patient was consistent with the literature.
Although clinical symptoms may vary according to the localization, size and biological behavior, slow progressive paraparesis (71%) and radiculopathy (19%) are seen most commonly1,6,7,8,9,10). Acute presentation may occur due to either extradural hemorrhageor thrombotic occlusion within the cavernoma, by causing rapid increase in the bulk of the lesion6,8). In our case, the symptoms was presented as back pain and progressive paraparesis.
Spine MRI is the best method for the radiological diagnosis of CHs. Upon MRI, the demarcated lobular lesion appears isointensein the T1-weighted images, hyperintense in the T2-weighted images, and intensely homogeneous with contrast enhancement in the contrast-enhanced T1-weighted images3,9). In case of intramedullary CHs, a hypointense ring due to peripheral hemosiderin deposition and heterogeneous intensity in the center are seen, but these are not observed in epidural hemangiomas3,5,7,9). Although differential diagnosis is most commonly required with schwannoma in MRIs, it may be confused with other neurogenic tumors, metastasis, lymphoma, meningioma, multiple myeloma, extraosseous Ewing's sarcoma, disc fragments or epidural angiolipoma6,8). In our case, the absence of a hemosiderin ring, as well as the displacement of the spinal cord, slightly lower signal than the CSF signal in the T2-weighted images, and slightly heterogeneous significant contrast involvement suggested epidural hemangioma6).
CHs have the tendency to bleed and grow6). Because of these behaviors, early surgical excision is recommended as soon as possible. Aim of the surgery should be total removal at first operation6); however, intraoperative bleeding and intramedullary placement may impede the total removal. Although hemorrhage is rare in intramedullary CHs, massive bleeding may be seen in epidural CHs1,2,6,8,10). In cases of incomplete removal due to bleeding and its localization, adjuvant radiotherapy may be required6,8). In our case, the bleeding in the perioperative period could be controlled surgically and adjuvant radiotheraphy was not needed.
CONCLUSION
CHs are non-neoplastic vascular malformations rarely seen in the spinal region, leading to a variety of clinical symptoms. In MRIs, it is very difficult to distinguish these from schwannomas. In this case with this kind of spinal epidural CH, despite wrong diagnosis is likely to occur, the author could perform complete reomval of this vascular malformation microsurgically.
References
- 1.A L H, T R, Chamarthy NP, Puri K. A pure epidural spinal cavernous hemangioma-with an innocuous face but a perilous behaviour!! J Clin Diagn Res. 2013;7:1434–1435. doi: 10.7860/JCDR/2013/6030.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babu R, Owens TR, Karikari IO, Moreno J, Cummings TJ, Gottfried ON, et al. Spinal cavernous and capillary hemangiomas in adults. Spine (Phila Pa 1976) 2013;38:E423–E430. doi: 10.1097/BRS.0b013e318287fef7. [DOI] [PubMed] [Google Scholar]
- 3.Feng J, Xu YK, Li L, Yang RM, Ye XH, Zhang N, et al. MRI diagnosis and preoperative evaluation for pure epidural cavernous hemangiomas. Neuroradiology. 2009;51:741–747. doi: 10.1007/s00234-009-0555-2. [DOI] [PubMed] [Google Scholar]
- 4.Globus JH, Doshay LJ. Venous dilatations and other intraspinal vessel alterations, including true angiomata, with signsand symtoms of cord compression. A report of four cases with areview of the literature. Surg Gynecol Obstet. 1929;48:345–366. [Google Scholar]
- 5.Hegde A, Mohan S, Tan KK, Lim CC. Spinal cavernous malformations : magnetic resonance imaging and associated findings. Singapore Med J. 2012;53:582–586. [PubMed] [Google Scholar]
- 6.Khalatbari MR, Abbassioun K, Amirjmshidi A. Solitary spinal epidural cavernous angioma : report of nine surgically treated cases and review of the literature. Eur Spine J. 2013;22:542–547. doi: 10.1007/s00586-012-2526-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park ES, Park JS, Kim E. Spinal epidural cavernous angioma : a case report. Korean J Spine. 2008;5:99–101. [Google Scholar]
- 8.Sharma MS, Borkar SA, Kumar A, Sharma MC, Sharma BS, Mahapatra AK. Thoracic extraosseous, epidural, cavernous hemangioma : case report and review of literature. J Neurosci Rural Pract. 2013;4:309–312. doi: 10.4103/0976-3147.118772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shin JH, Lee HK, Rhim SC, Park SH, Choi CG, Suh DC. Spinal epidural cavernous hemangioma : MR findings. J Comput Assist Tomogr. 2001;25:257–261. doi: 10.1097/00004728-200103000-00019. [DOI] [PubMed] [Google Scholar]
- 10.Zhong W, Huang S, Chen H, Sun H, Cai B, Liu Y, et al. Pure spinal epidural cavernous hemangioma. Acta Neurochir (Wien) 2012;154:739–745. doi: 10.1007/s00701-012-1295-3. [DOI] [PubMed] [Google Scholar]