Abstract
Objective:
To evaluate the safety and effectiveness of different bipolar radiofrequency system algorithms in interrupting the renal sympathetic nerves and reducing renal norepinephrine in a healthy porcine model.
Methods:
A porcine model (N = 46) was used to investigate renal norepinephrine levels and changes to renal artery tissues and nerves following percutaneous renal denervation with radiofrequency bipolar electrodes mounted on a balloon catheter. Parameters of the radiofrequency system (i.e. electrode length and energy delivery algorithm), and the effects of single and longitudinal treatments along the artery were studied with a 7-day model in which swine received unilateral radiofrequency treatments. Additional sets of animals were used to examine norepinephrine and histological changes 28 days following bilateral percutaneous radiofrequency treatment or surgical denervation; untreated swine were used for comparison of renal norepinephrine levels.
Results:
Seven days postprocedure, norepinephrine concentrations decreased proportionally to electrode length, with 81, 60 and 38% reductions (vs. contralateral control) using 16, 4 and 2-mm electrodes, respectively. Applying a temperature-control algorithm with the 4-mm electrodes increased efficacy, with a mean 89.5% norepinephrine reduction following a 30-s treatment at 68°C. Applying this treatment along the entire artery length affected more nerves vs. a single treatment, resulting in superior norepinephrine reduction 28 days following bilateral treatment.
Conclusion:
Percutaneous renal artery application of bipolar radiofrequency energy demonstrated safety and resulted in a significant renal norepinephrine content reduction and renal nerve injury compared with untreated controls in porcine models.
Keywords: catheter ablation, renal artery, renal denervation, sympathetic nervous system
INTRODUCTION
Hyperactivation of the sympathetic nervous system plays a major role in initiating and maintaining hypertension [1]. Elevated levels of catecholamines are found in hypertensive individuals [2], and renal sympathetic nerve activation enhances norepinephrine spillover and norepinephrine production at nerve endings [3–5]. In addition, in patients with untreated essential hypertension, a direct relationship between blood pressure and sympathetic activity has been reported [6]. Although renal sympathetic nerve activation enhances norepinephrine spillover [3–5], interruption of renal sympathetic fibers results in a marked decrease (up to 95% [7]). These findings suggest that hyperactivity of the renal sympathetic nerves could be mitigated with selective renal denervation strategies.
Renal sympathetic efferent and afferent nerves run through the arterial adventitia in a mesh-like pattern. An approach to percutaneous renal denervation involves the application of radiofrequency energy by contact of an electrode with the renal artery intima. A human postmortem histologic study described the microanatomy of the renal sympathetic nervous system and observed that 90.5% of all renal nerves are located within 2.0 mm of the renal artery lumen [8].
Investigators using a porcine model have reported that monopolar radiofrequency energy delivered via a single-electrode catheter (Symplicity; Medtronic, Palo Alto, California, USA) at a sufficient wattage (6–8 W) and duration at an optimal number of sites along the renal artery results in selective ablation of renal nerves with minimum damage to the vessel wall [9]. The monopolar radiofrequency system delivers electrical energy to the targeted tissue via a single electrode, with the return path for the electrical current provided by a grounding pad usually placed on the patient's back or thigh. Therefore, electrical current travels from the electrode to the grounding pad through a significant and variable portion of the body mass.
Distinct from monopolar systems, bipolar radiofrequency systems deliver energy using the same electrical phenomenon, namely, ohmic resistive heating of adjacent tissues. However, the electrical energy field delivered in a bipolar system is confined to the electrode pair (Fig. 1), the poles of which are in close proximity to one another (i.e. a few millimeters). In consequence, the bipolar system obviates the need for a grounding pad. Furthermore, because bipolar systems dissipate energy into a confined area determined by spacing of the electrode pair, they use energy more efficiently and create a well defined and predictable field of injury (Fig. 1d).
FIGURE 1.
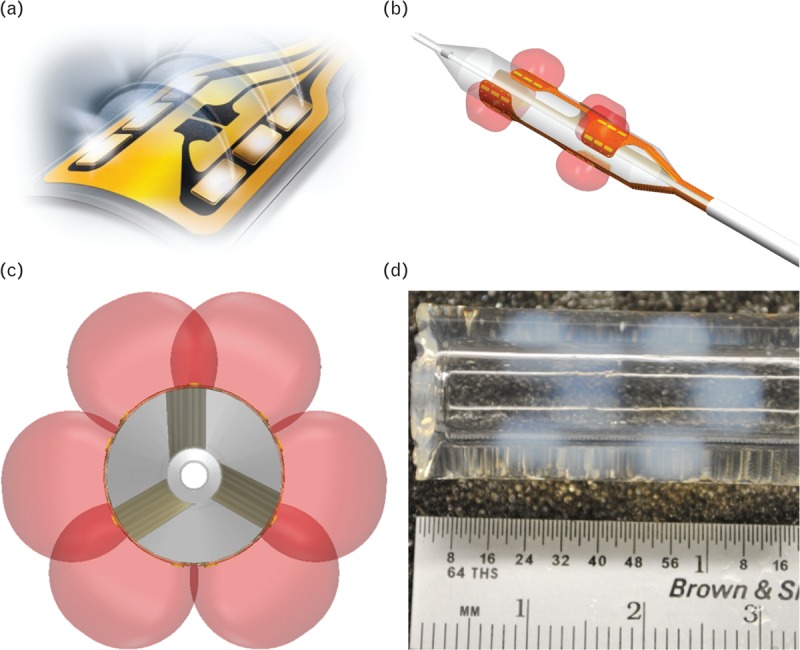
Balloon-based bipolar radiofrequency energy delivery. (a) Illustration depicting bipolar radiofrequency energy delivery by an electrode. Bipolar radiofrequency energy travels only from a positive to negative pole located on each electrode thereby localizing the energy and eliminating the necessity of a grounding pad. (b) Illustration of the forms of the radiofrequency energy delivered by all bipolar electrodes mounted on the surface of the balloon. (c) Illustration of the circumferential coverage of the radiofrequency energy around the balloon from a front angle of the balloon. (d) The pattern of energy delivered by the balloon-based bipolar radiofrequency electrodes was demonstrated by activating the electrodes within a polyacrylamide gel containing bovine serum albumin, glycerol and citrate buffer, which had a thermal coagulation temperature (i.e. temperature of color change) of approximately 60°C.
The aims of this study were to evaluate the safety and effectiveness of different bipolar radiofrequency system algorithms in interrupting the renal sympathetic nerves and reducing renal norepinephrine in a healthy porcine model. Findings regarding the optimal combination of parameters, including the electrode length and energy delivery algorithm variables, such as temperature and treatment duration, that resulted in significant norepinephrine reductions while minimizing injury to renal artery tissue, were then applied to the design of a bipolar radiofrequency renal denervation balloon device.
METHODS
Overview of experiments
A porcine model was used in a series of studies (see Table 1) to investigate renal norepinephrine levels and changes to renal artery tissues and nerves following percutaneous renal denervation with radiofrequency bipolar electrodes mounted on a balloon catheter. The results of each study were used to determine adjustments to device design or treatment parameters to be subsequently tested. The effects of varying parameters of the radiofrequency system (i.e. electrode length and energy delivery algorithm), and the effects of single and longitudinal treatments along the artery were studied with a 7-day model in which swine received unilateral radiofrequency treatments. Additional sets of animals were used to examine norepinephrine and histological changes 28 days following bilateral percutaneous radiofrequency treatment or surgical denervation; untreated swine were used for comparison of kidney norepinephrine levels.
TABLE 1.
Overview of 7 and 28-day study conditions
| Radiofrequency system treatment parameters | |||||||||
| Study | Energy delivery | Electrode length | Target tissue temperature | Duration of treatment | Electrode activation sequence | Number of treatments | Control | N | |
| Unilateral radiofrequency treatments, 7-day follow-up | |||||||||
| 1 | Bipolar electrode length | Constant impedance (no thermistor) | 16 mm | 75°Ca | 15 sb | Sequential | 1 | Contralateral artery and kidney | 9 (3/group) |
| 4 mm | 65°Ca | 30 sb | |||||||
| 2 mm | 65°Ca | 60 sb | |||||||
| 2 | Temperature | Temperature-controlled | 4 mm | 64°C, 68°C and 74°C | 30 sc | Sequential | 1 | Contralateral artery and kidney | 9 (3/group) |
| 3 | Electrode control | Temperature-controlled | 4 mm | 68°C | 30 sc | Simultaneous | 1 | Contralateral artery and kidney | 5 |
| 4 | Full artery-length treatment | Temperature-controlled | 4 mm | 68°C | 30 sc | Simultaneous | Full artery-lengthd | Contralateral artery and kidney | 5 |
| Bilateral radiofrequency treatments, 28-day follow-up | |||||||||
| 5 | Long-term follow-up | Temperature-controlled | 4 mm | 68°C | 30 sc | Simultaneous | 1 vs. full artery-lengthd | Surgical denervation (n=3) and untreated (n = 3) | 12 radiofrequency treatment (6/group) |
aTissue temperatures were analyzed in vitro during bench-top testing using thermocouples and were not measured in vivo during the studies.
bTarget tissue temperatures were reached within 1 s and then held constant for the remainder of the treatment period.
cTemperature rose gradually over 12 s and was then held constant for the remainder of the treatment period.
dUp to 2 treatments per artery.
Electrode length
For the studies of electrode length (Table 1), a constant impedance energy delivery algorithm was applied with a bipolar radiofrequency generator, which controls energy output by calculating impedance. In the initial experiment, 16-mm long electrode pairs mounted on the surface of a 20-mm long balloon were tested (Fig. 2a). These circumferentially positioned electrode pairs were designed to treat most of the artery wall surface in order to determine maximum efficacy of bipolar radiofrequency energy application, assuming most of the renal nerves would be affected. Balloon diameters of 4, 5 and 6 mm were mounted with four, five and six electrode pairs, respectively. The generator activated the electrode pairs in a sequential manner (pair one followed by pair two and so forth).
FIGURE 2.
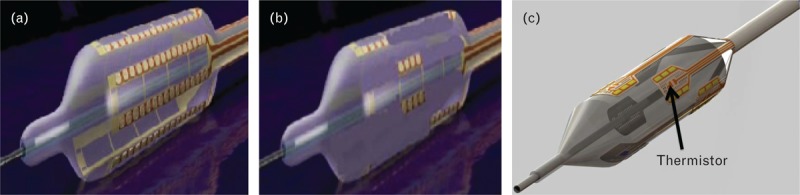
Bipolar electrode configurations used in the studies. (a) An illustration of the balloon catheter, with 16-mm long electrode pairs mounted on its surface. Each electrode pair was activated one at a time in a sequential pattern. During treatment, the electrodes deliver a preset amount of radiofrequency energy (watts) titrated by measurement of impedance. (b) An illustration of the modified balloon catheter with 4-mm long gold electrodes mounted on its surface in an offset pattern. (c) Illustration of the prototype balloon catheter with 4-mm long gold electrodes mounted on its surface. A thermistor was added at the center of each electrode pair to measure temperature during treatment. This allows the generator to control and titrate the amount of radiofrequency energy needed in order to reach a certain temperature for any duration specified.
The tissue temperatures generated for these treatments were analyzed in vitro during bench-top testing using thermocouples and were not measured in vivo during this animal study. For the treatments employing the 16-mm-sized electrode, the target temperature chosen was 75°C for a duration of 15 s, the combination of which was determined to enable significant in-vivo tissue depth penetration (up to 10 mm, data not shown). Using this constant impedance algorithm, the target tissue temperature was reached within 1 s.
With the results from the study of the 16-mm electrodes as a benchmark, subsequent studies were conducted to refine the electrode arrangement and treatment algorithm. Shorter electrodes were used to assess whether reducing the circumferential treatment zone (i.e. by increasing electrode spacing and decreasing overlap along the length of the balloon) would yield norepinephrine reductions similar to those achieved with the 16-mm electrodes but with less histologic evidence of necrosis. The same radiofrequency constant-impedance balloon catheter as described above was used, with the exceptions that the electrodes were 2 or 4 mm in length and were positioned in an offset pattern (Fig. 2b). The target temperature was decreased to 65°C, with treatment duration of 60 s for the balloons with 2-mm electrodes and 30 s for the balloons with 4-mm electrodes in order to ensure similar depth of energy penetration into the tissue.
Temperature
In the next series of studies (Table 1), thermistors were mounted within each electrode pair on the radiofrequency balloon catheter (Fig. 2c), providing the ability to accurately measure the balloon surface temperature (± 2C) during treatment. This iteration enabled the radiofrequency generator to titrate the amount of energy required to reach and maintain the target temperature independently for each electrode pair (i.e. temperature control). In temperature control treatments, the temperature rose gradually over 12 s until the target was reached and was then held constant for the remainder of the treatment period.
Simultaneous electrode control
Five animals received a single unilateral treatment with 68°C (± 0.5°C) for 30 s; in these experiments, all electrode pairs were activated simultaneously rather than sequentially as in previous experiments. Norepinephrine reductions, histopathology and nerve assessments of these animals which received a single treatment were compared with findings from animals that received longitudinal treatments along the full length of the artery.
Longitudinal treatment along the renal artery
Impedance reading by the electrode distinguishing between blood and renal artery tissue enabled comparisons between single treatments per artery vs. full artery-length treatment. Five animals were treated with a treatment algorithm identical to the simultaneous electrode control algorithm with the exception that up to two treatments per artery were applied (i.e. one distal and one proximal treatment), thus covering the entire length of the artery (Table 1); these animals were euthanized 7 days posttreatment.
Bilateral radiofrequency treatment with 28-day follow-up
To determine whether full artery-length radiofrequency treatment affected long-term efficacy, additional sets of animals were euthanized 28 days postprocedure. Animals in this study were treated bilaterally with one (n = 6) or up to two radiofrequency treatments (n = 6). The kidney norepinephrine levels of the radiofrequency-treated animals were compared with endogenous norepinephrine levels of untreated animals (i.e. angiography of the renal artery without delivery of radiofrequency energy; n = 3), and with animals 28 days after bilateral surgical denervation (n = 3). Blood samples for serum chemistry were collected on day 0 prior to the procedure and on the day of necropsy. Serum creatinine levels and urea nitrogen were used to assess the effect of treatment on kidney function. In addition, blood pressure and heart rate were measured in a subset of the radiofrequency-treated animals.
Animal model and surgical procedures
Animal experiments adhered to the Guide for the Care and Use of Laboratory Animals [10], an approved Institutional Animal Care and Use Committee protocol, and were in compliance with the Animal Welfare Act and the Food and Drug Administration Regulations and their amendments. Male (castrated) or female (nulliparous) Yorkshire swine (N = 46), weighing between 35 and 50 kg were used in these studies.
For all surgical procedures, intramuscular buprenorphine (0.01 mg/kg) was administered at the time of anesthetic induction with telazol (4–6 mg/kg intramuscular). General anesthesia was maintained with isoflurane delivered in 100% oxygen. ECG activity, heart rate, respiratory rate, oxygen saturation and temperature were monitored at regular intervals throughout the procedure. Aspirin (650 mg, per os) and clopidogrel (300 mg, per os) were administered 1 or 2 days prior to treatment. Aspirin (81 mg, per os) and clopidogrel (75 mg, per os) were administered daily thereafter for the remainder of the study.
Animals were euthanized under anesthesia via an overdose of intravenous potassium chloride solution at the assigned 7 or 28-day timepoint.
Percutaneous radiofrequency renal denervation
Forty animals underwent percutaneous renal denervation with bipolar radiofrequency electrode pairs mounted on the surface of a low-pressure (3 atm) noncompliant angioplasty balloon. Electrodes were composed of elemental gold because of its ideal thermal and electrical conductivity, as well as excellent radiopacity.
Following the insertion of an introducer sheath through the common femoral artery, intraarterial heparin (50–200 U/kg) was administered to achieve an activated clotting time of 275 s. Under fluoroscopic guidance, an 8F guide sheath was placed over a 0.035“ J-guidewire into the descending aorta. Nitroglycerin (200–400 μg, intraarterial) was administered before contrast angiography. Renal artery diameter and length were quantitatively measured via angiography to determine the appropriate balloon diameter for radiofrequency treatment. Renal arteries ranging from 3 to 7 mm in diameter were treated. The radiofrequency catheter system was introduced over a 0.018” guidewire and, after positioning within the renal artery, the balloon catheter was inflated to nominal pressure and activated to provide low-power radiofrequency energy treatments (∼ 1 W). Impedance was measured to determine whether apposition of the balloon catheter electrodes against the renal artery wall had been achieved. Radiofrequency energy was delivered according to predetermined parameters (Table 1). Nitroglycerin (50–200 μg, intraarterial) was administered to treat arterial vasospasm, as needed. Contrast angiography was conducted immediately posttreatment and prior to necropsy on all animals.
Surgical denervation
Three animals underwent bilateral surgical renal denervation. A bilateral retroperitoneal approach was used to access the renal arteries. A skin incision was made along the right lateral abdominal wall, and the muscle separated to access the right renal artery. The adventitia was gently separated along the entire length of the renal artery. Following denervation, the right lateral incision was closed in layers with appropriate suture techniques and materials. The procedure was repeated for the left renal artery. Bupivicaine was infused into both incision sites.
Tissue preparation for histology
Both renal arteries were removed en bloc with approximately 4–6 cm of aorta and perirenal tissue on either end to maintain orientation. The tissue block of renal arteries, aorta, and perirenal tissue was fixed in 10% neutral buffered formalin and then trimmed at intervals of 3–5 mm along the length of the artery, from ostium to hilum, to yield cross-sections. Trimmed specimens were processed for paraffin embedding, mounted on slides, and stained with hematoxylin and eosin for light microscopy evaluation.
Histopathology evaluation and nerve assessment
Renal artery and renal parenchyma sections were assessed under light microscopy by a board-certified veterinary pathologist. Renal parenchyma sections were assessed for downstream effects of treatment, including thromboemboli, infarction and inflammation. Renal artery sections were examined to evaluate the tissue response to treatment, including mural injury (e.g. disruption of vascular layers, dissection and perforation), thrombosis, mural and adventitial inflammation, necrosis, fibroplasia, dystrophic mineralization and endothelialization.
For arterial sections corresponding to areas of radiofrequency treatment (i.e. zones with localized coagulative changes in the renal artery and adjacent adventitia), the number of nerves within the treatment zone (i.e. ‘susceptible nerves’) and outside the treatment zone (i.e. ‘nonsusceptible nerves’) was quantified. Changes to nerve structures induced by radiofrequency energy were further characterized by the type of change exhibited: none, primarily degenerative, primarily necrotic or primarily chronic/reactive. Nerves outside the treatment zone (i.e. nonsusceptible nerves) were further classified as affected or nonaffected based on these types of morphologic changes.
Renal tissue norepinephrine assay
Norepinephrine levels in porcine kidney tissue were measured by high-performance liquid chromatography-mass spectrometry (HPLC-MS/MS) assay, based on methods described previously [11]. Following euthanization, the entire kidney was rapidly harvested and immediately minced on dry ice. Tissue subsamples were frozen in triplicate at −80°C. These subsamples were subsequently homogenized with a hand-held Omni Tip Homogenizer (OMNI International, Kennesaw, Georgia at a ratio of 1 : 9 (w : v) in a solution containing 1.0 mg/ml cysteine and 10 mg/ml sodium metabisulfite. Stable isotopes of norepinephrine (norepinephrine-d6) and epinephrine (epinephrine-d6) were added to the kidney subsamples to serve as a surrogate reference standard and an internal reference standard, respectively. Norepinephrine was extracted from the tissue homogenate by protein precipitation and was derivatized by a reductive amination reaction with d4-acetaldehyde. Chromatographic separation was achieved on a Waters Xbridge Phenyl analytical column (2.1 × 50 mm, 2.5 μm). All HPLC-MS/MS analysis was performed on an AB Sciex API-5500 coupled to a Thermo Aria System with an Agilent 1200 binary pump and a LEAP autosampler using TurboIonspray ionization in the positive ion mode. Analytical run times were 2 min.
The final quantification method was validated using duplicate bracketing surrogate calibration curves within a range of 1.00 ng/ml through 250 ng/ml in tissue homogenates (10–2500 ng/g of tissue). Independently prepared quality control samples were assayed with each analytical run at three levels; assay acceptance criteria were defined to be ± 15% of nominal concentrations (±20% at the lower limit of quantitation) for standards and quality controls. Analyst, version 1.5 (Applied Biosystems, Foster City, California, USA) and Aria, version 1.6.2 (Thermo-Fisher, Waltham, Massachusetts, USA) software programs were used for data acquisition and processing. Calibration curves were constructed based on the peak area ratios vs. concentrations of the surrogate reference standards and fitted to a least squares linear regression. Endogenous norepinephrine levels were calculated using regression constants from surrogate calibration curves and peak area ratios of samples.
For 7-day experiments, the percentage reduction in renal norepinephrine concentration was determined by comparing the norepinephrine level in the kidney of the radiofrequency-treated artery with that of the contralateral untreated kidney. For 28-day experiments (bilateral radiofrequency treatments), the norepinephrine concentrations of radiofrequency-treated kidneys were compared with levels from kidneys of animals 28 days following surgical denervation and with endogenous norepinephrine levels in untreated control animals.
Tyrosine hydroxylase immunohistochemistry
For the 7-day experiment in which animals received longitudinal treatment along the full artery length, nerves were further analyzed for the intensity of tyrosine hydroxylase as a proxy for efferent nerve activity and/or function. Because tyrosine hydroxylase is a rate-limiting enzyme in the biosynthesis of catecholamines (e.g. dopamine, norepinephrine and epinephrine), it can be used to indirectly assess changes in renal sympathetic nerve catecholamine production that are associated with neural injury. Thus, immunochemical staining for tyrosine hydroxylase was performed on select renal artery and nerve tissue blocks. These blocks were deparaffinized, rehydrated, subjected to antigen retrieval at 90°C, rinsed and peroxidase blocked. Sections were incubated for 45 min with polyclonal rabbit antityrosine hydroxylase (Abcam Inc., Cambridge, Massachusetts, USA). A nonspecific Rabbit IgG was used as a negative control. Rabbit-on-Farma HRP-Polymer detection solution was used for detection, and color was developed using betazoid diaminobenzidine substrate-chromogen (Biocare Medical LLC, Concord, California, USA). All slides were water-rinsed, counterstained with Mayer's hematoxylin, dehydrated and mounted. The intensity of tyrosine hydroxylase staining was scored as: none (0), primarily nearly imperceptible to light staining (1), primarily variable (i.e. light to dark) (2); primarily uniform moderate staining (3), primarily uniform dark staining (4).
Statistical analysis
Nerve counts and percentage reductions of renal norepinephrine are reported descriptively as mean ± SD. Percentage reductions of renal norepinephrine concentrations were calculated from the concentration for radiofrequency-treated arteries relative to the untreated side (7-day studies) and relative to untreated animals for studies of bilateral radiofrequency treatment or surgical denervation (28-day studies). T-test analyses were used to determine the statistical significance of the percentage reduction in renal norepinephrine, the difference between endogenous and denervated renal norepinephrine concentrations and differences between nerve counts for arteries with one vs. full-length treatments; differences were considered significant when P < 0.05. Angiographic images were analyzed quantitatively with Centricity Cardiology CA1000 Cardiac Review 2.0 QCA Software (GE Healthcare, USA). Angiographic percentage stenosis was calculated as [(baseline diameter – prenecropsy diameter)/baseline diameter] multiplied by 100.
RESULTS
Electrode length studies
Histopathology and nerve assessment
Seven days posttreatment with 16-mm bipolar radiofrequency electrodes (refer to Table 1 for additional treatment conditions), representative subacute morphologic changes within the renal artery area were consistent with those shown previously [12], including coagulative changes of the treated arterial wall and adjacent adventitia (typically > 75% circumferential ‘treatment zone;’ Fig. 3a) and partial reendothelialization of the luminal surface (overall ∼25–75% circumference), but with no evidence of thrombus formation or significant mural injury. There was also no evidence of adverse inflammation (i.e. exuberant neutrophilic or granulomatous). Inflammation was typically mononuclear (i.e. lymphohisticytic) and minimal in the arterial wall and mild-to-moderate in the adventitia. No early reparative response (i.e. a fibromuscular proliferation) was observed at this time within the injured arterial wall. Nerves within or adjacent to the treatment zone (i.e. susceptible nerves) exhibited radiofrequency-related injury (i.e. significant degeneration and necrosis) as did, to a lesser extent, nerves outside the treatment zone (i.e. nonsusceptible nerves).
FIGURE 3.
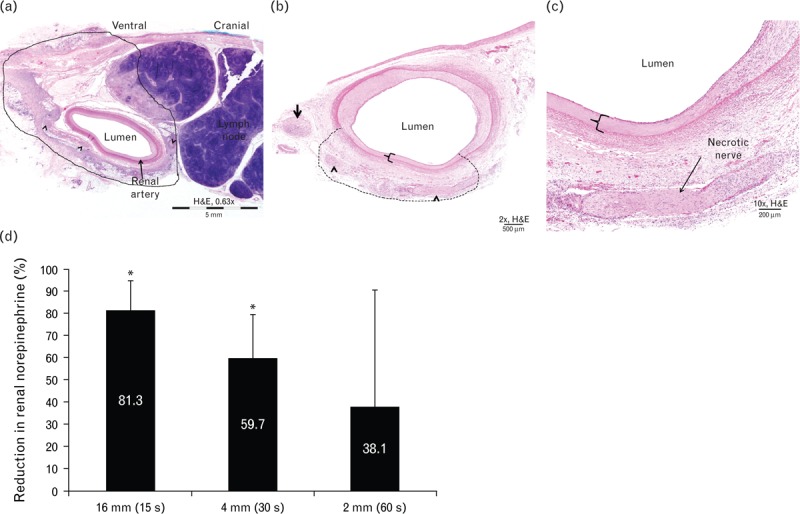
Electrode length studies. (a) Representative image of renal artery and surrounding tissues 7 days following 16-mm constant impedance radiofrequency treatment (75°C for 15 s). Note circumferential radiofrequency treatment of artery, as evidenced by transmural coagulative necrosis of the arterial wall and extension of thermal injury into the adjacent adventitia (i.e. zone of treatment within the black line) with affected nerves (arrowheads). (b) Representative image of renal artery and surrounding tissues 7 days following 4-mm constant impedance radiofrequency treatment (65°C for 30 s). Note localized radiofrequency treatment of artery, as evidenced by transmural coagulative necrosis of the arterial wall [in bracket and (c)] and extension of thermal injury into the adjacent adventitia (i.e. zone of treatment within dashed line) with affected nerves (arrowheads). (d) Mean (SD) percentage reduction of renal norepinephrine levels in the treated vs. untreated contralateral kidney 7 days following treatment with bipolar electrodes of length 16, 4, or 2 mm. ∗Statistically significant difference at P < 0.05 between RF-treated artery vs. untreated contralateral control. RF, radiofrequency.
Treatment with the shorter (2 and 4 mm) electrodes was typically associated with a smaller treatment zone relative to the longer 16-mm electrodes, with treatment zones 25–75% of the artery circumference (Fig. 3b), as well as more circumferential reendothelialization of the luminal surface (∼75–100% circumference) with no evidence of thrombus formation or significant transmural injury. Evidence of an early reparative response was observed in day 7 arterial sections treated with short electrodes, unlike renal artery sections treated with the 16-mm electrode. Inflammation was primarily lymphohistiocytic and minimal, regardless of mural or adventitial location. Morphological changes in susceptible and nonsusceptible renal nerves were consistent with the previous experiment (Fig. 3c).
Norepinephrine reduction
At 7 days posttreatment, renal tissue associated with treatment with the 16-mm electrode demonstrated a significant 81.3% reduction in mean norepinephrine concentration relative to the untreated contralateral kidney (P = 0.007, Fig. 3d), demonstrating that reduction in tissue norepinephrine can be detected as early as 7 days following bipolar radiofrequency renal denervation.
Shorter electrodes were used to assess whether norepinephrine reduction similar to that of the 16-mm electrodes could be achieved. The renal norepinephrine concentrations ipsilateral to renal arteries treated with the shorter electrodes appeared to decrease directly proportional to the electrode length (Fig. 3d): the reduction in mean renal norepinephrine concentrations with the 4-mm electrodes was statistically significant at 59.7% (P = 0.007), and the reduction with 2-mm electrodes was 38.1% (P = 0.17). Reductions in renal norepinephrine concentrations using 4-mm electrode pairs at approximately 65°C for 30 s were more consistent (i.e. smaller standard deviation) than norepinephrine reductions associated with the 2-mm electrodes at 65°C for 60 s, and norepinephrine levels were significantly lowered compared with the untreated side. As the reduction in norepinephrine tissue concentrations was balanced with minimal tissue injury for swine treated with the 4-mm electrode system, all subsequent experiments were conducted using 4-mm electrodes only.
Temperature studies
Histopathology and nerve assessment
Similar to the constant impedance method, all temperature-controlled radiofrequency treatments resulted in localized zones of treatment (i.e. localized radiofrequency-associated coagulative changes in the renal artery and adjacent adventitia), regardless of temperature. There was no significant mural injury or evidence of thrombus formation, and minimal lymphohistiocytic infiltrates, but reendothelialization of the luminal surface was complete 7 days posttreatment (Fig. 4a). Morphological changes in susceptible renal and nonsusceptible renal nerves were consistent with the constant impedance/electrode length experiments.
FIGURE 4.
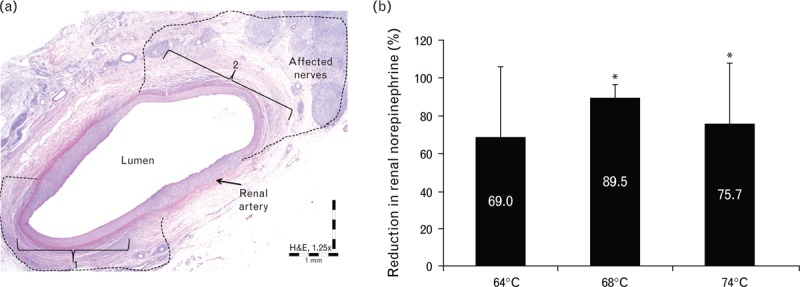
Target temperature studies. (a) Representative images of renal artery 7 days following a single treatment with 4-mm long electrodes, at 68°C for 30 s. Note two localized radiofrequency treatment zones (1 and 2), as evidenced by transmural coagulative necrosis of the arterial wall (e.g. in bracket) and extension of thermal injury into the adjacent adventitia (i.e. zones of treatment within dashed lines) with affected nerves. (b) Mean (SD) percentage reduction of renal norepinephrine levels in the treated vs. untreated contralateral kidney 7 days following temperature-controlled radiofrequency treatment with target temperatures of 64, 68, or 74°C. ∗Statistically significant difference at P < 0.05 between RF-treated artery vs. untreated contralateral control. RF, radiofrequency.
Norepinephrine reduction
The animals treated at 64 and 74°C had 69.0% (P = 0.05) and 75.7% (P = 0.008) reductions in mean renal norepinephrine concentrations, respectively, vs. untreated contralateral control (Fig. 4b); however, treatment at 68°C yielded an 89.5% reduction (P < 0.001) in the mean renal norepinephrine concentration, which was similar to results obtained using the 16-mm electrodes. Importantly, the reduction in renal norepinephrine concentration was at least 50% compared with the untreated contralateral kidney in all animals treated with the temperature-control algorithm and assessed at 7 days (n = 8), demonstrating that the temperature-control algorithm produced more efficacious and predictable norepinephrine reductions than the constant impedance treatment algorithm.
Given the acceptable histopathological findings and large norepinephrine reduction associated with treatment at 68°C, this temperature was applied in all subsequent studies described here (see Table 1).
Safety observations
One animal treated at 64°C was euthanized 2 days postprocedure because of notable abdominal distention. Upon opening the abdominal cavity, the stomach and large intestine were markedly distended. The treatment site (left renal artery) and left kidney were unremarkable. There were no other macroscopic observations.
Simultaneous electrode activation (one treatment per artery)
Histopathology and nerve assessment
When all electrodes were activated simultaneously rather than sequentially, no complications were noted postprocedure or through 7 days posttreatment (n = 5). Tissue responses (i.e. arterial, adventitial and neural) were consistent with those described in the previous experiments.
Norepinephrine reduction
Animals with a single unilateral temperature-controlled 30-s treatment at 68°C and simultaneous electrode activation had a significant reduction (78.3%, P < 0.0001) in mean norepinephrine concentration (Fig. 5).
FIGURE 5.
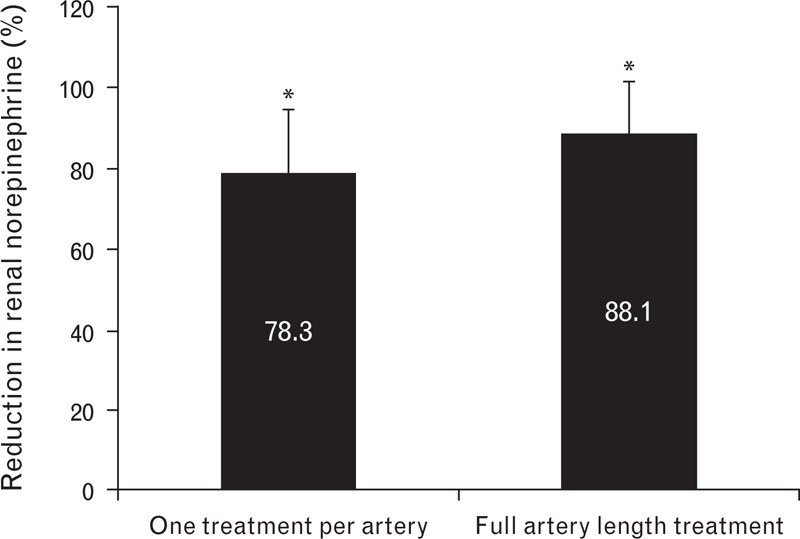
Mean (SD) percentage reduction of renal norepinephrine levels in the treated vs. untreated contralateral kidney 7 days following temperature-controlled treatment with simultaneous activation of all electrode pairs. Animals received either a single treatment or full artery-length treatment. ∗Statistically significant difference at P < 0.05 between RF-treated artery vs. untreated contralateral control. RF, radiofrequency.
Full artery-length treatment
Contrast angiography
No evidence of renal artery stenosis, thrombus, dissection, perforation or spasm was apparent based on angiography immediately postprocedure or at 7 days following artery-length treatment (Fig. 6). The overall mean angiographic percentage stenosis in the day 7 full artery-length radiofrequency treatment group was –0.09 ± 3% (n = 5 arteries).
FIGURE 6.

Representative angiography images of a porcine renal artery pretreatment (a) and 7 days following artery-length treatment (b) of 68°C for 30 s. The balloon locations are shown in panels (c) and (d).
Histopathology and nerve assessment
A representative image of renal artery 7 days following full artery-length treatment with 4-mm long electrodes, 68°C for 30 s, is shown in Fig. 7a. Nerves with degenerative changes were generally intact but exhibited a variety of pathologic processes, including hypercellularity, intrafascicle/perifascicle fibrosis, inflammatory infiltrates (e.g. eosinophils, lymphocytes and histiocytes) and/or single cell necrosis (Fig. 7b). Necrotic changes ranged from variable devitalization of cellular elements and dissolution of fibers to generalized coagulative necrosis and/or coagulation of the nerve bundle (Fig. 7c). Chronic reactive changes were similar to degenerative changes in variability but generally exhibited prominent intrafascicular/perifascicular fibrosis (Fig. 7d).
FIGURE 7.
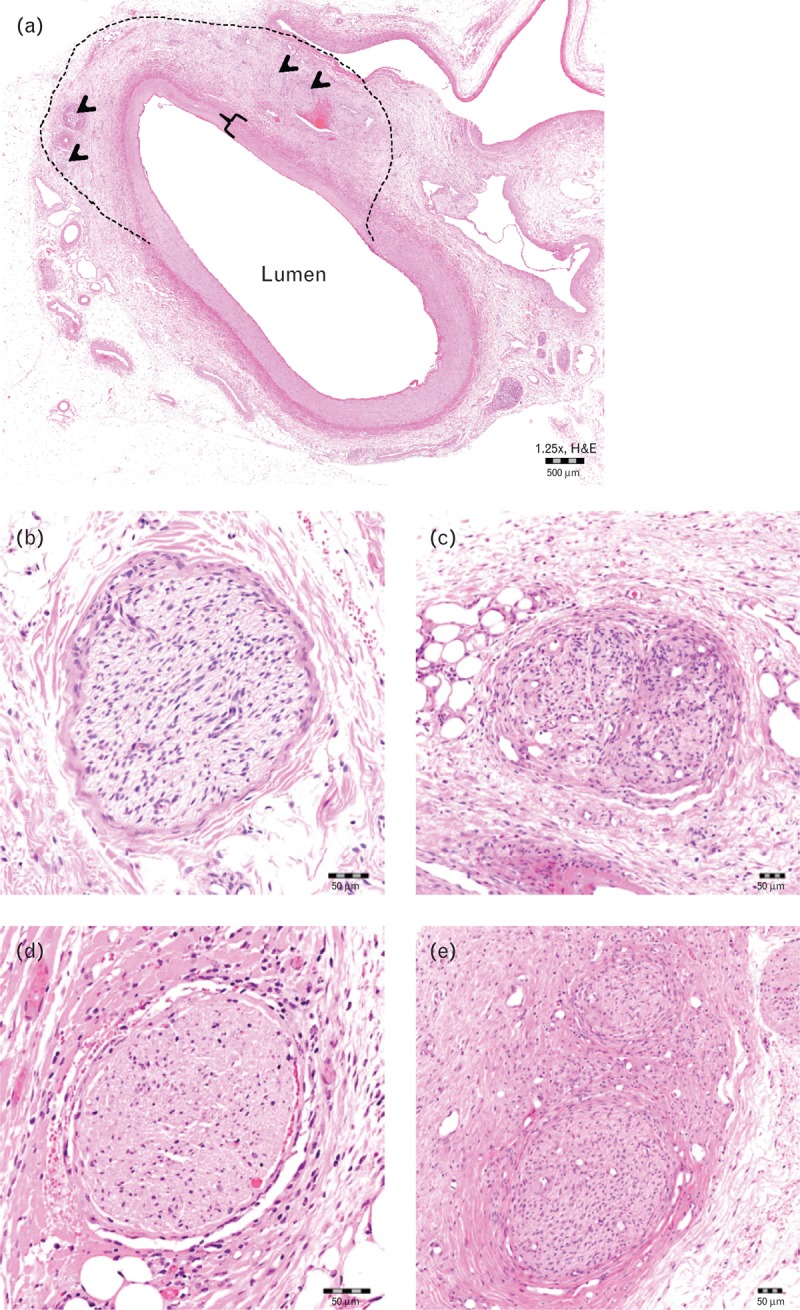
Histology 7 days following full artery-length treatments. (a) Representative images of renal artery 7 days following full artery-length treatment with 4-mm long electrodes, 68°C for 30 s. Note localized radiofrequency treatment of artery, as evidenced by transmural coagulative necrosis of the arterial wall (in bracket) and extension of thermal injury into the adjacent adventitia (i.e. zone of treatment within dashed line) with affected nerves (arrowheads). Scale bar represents 500 μm. Representative images of nerve change scores: (b) no changes; (c) primarily degenerative; (d) primarily necrotic; (e) primarily chronic/reactive. Scale bars in panels b–e represent 50 μm.
Histologic analysis of nerve fibers in planes with treated zones demonstrated that arteries that had bipolar radiofrequency energy applied along their entire length were associated with a significantly greater total number of nerves than were arteries that had received only one treatment (Fig. 8a): sections from arteries that received one treatment had a mean (± SD) of 24 ± 7 nerves vs. 36 ± 9 nerves when the entire length of the artery was treated (P < 0.05). In addition, treatments along the length of the artery were associated with an increase in the mean number of susceptible nerves per histologic plane (3.00 vs. 2.68) and overall number of susceptible nerves per treatment (17.2 ± 5 vs. 11.8 ± 5) compared with a single treatment. The percentage of all nerves located within treatment zones (i.e. susceptible nerves) was similar for single and artery-length treatments (approximately 48% for each). However, artery-length treatments were associated with a notable increase in the percentage of susceptible nerves undergoing irreversible necrosis vs. only degenerative changes (Fig. 8b). The percentage of susceptible nerves exhibiting primarily chronic/reactive changes was similar for each group (Fig. 8b).
FIGURE 8.
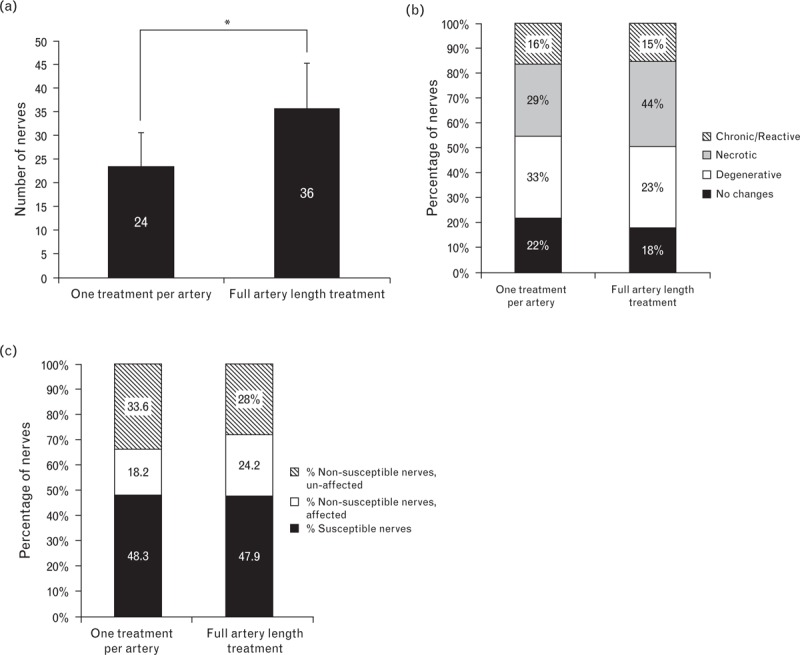
Nerve assessments following single or full artery-length radiofrequency treatment. (a) Mean (SD) number of nerves per artery counted in planes of the arteries with treatment zones (i.e. zones wherein localized radiofrequency energy-induced coagulative changes in the renal artery and adjacent adventitia were evident) at 7 days posttreatment. ∗Statistically significant difference at P < 0.05 between the two groups. (b) Percentages of susceptible nerves that appeared normal or exhibited primarily degenerative, necrotic or chronic changes. (c) Percentages of nerves within treatment zones (i.e. susceptible nerves) and outside treatment zones (with and without morphologic changes; i.e. affected and unaffected, respectively) from slides with arterial changes indicative of treatment zones.
Relative to a single treatment, artery-length treatments were associated with a greater percentage of nerves which showed morphologic changes but were not in the treatment zone (Fig. 8c), suggestive of radiofrequency-related injury along their length. Susceptible nerves (i.e. those within the treatment zone) and affected nerves located outside the treatment zone together comprised approximately 66 and 72% of nerves in sections from animals receiving a single radiofrequency treatment or full artery-length treatment, respectively (Fig. 8c).
Norepinephrine reduction
Although histologic nerve analysis demonstrated that application of bipolar radiofrequency energy along the entire length of the artery was associated with a significantly greater number of nerve fibers in the treatment area than were single treatments per artery (Fig. 8a), the mean percentage reduction of norepinephrine did not differ significantly from the reduction observed with single treatments (Fig. 5). Full-length treatment resulted in a mean percentage norepinephrine reduction of 88.1% after 7 days (Fig. 5).
Tyrosine hydroxylase
The intensity of tyrosine hydroxylase staining was assessed in sections from animals receiving full-length treatments. Tyrosine hydroxylase staining was generally decreased at day 7 regardless of the location of the nerve (i.e. susceptible or nonsusceptible). The most notable decreases in tyrosine hydroxylase staining correlated with those nerves that were within or adjacent to the radiofrequency zone of treatment (i.e. susceptible nerves); representative examples of tyrosine hydroxylase staining of susceptible sympathetic nerves in the renal artery adventitia are shown in Fig. 9. Interestingly, all nerves with no evidence of radiofrequency-related changes had reduction in the intensity of tyrosine hydroxylase, with 30% of these nerves showing no tyrosine hydroxylase staining (Fig. 9d). Among nerves with increased severity of radiofrequency-related changes (e.g. degeneration or necrosis), tyrosine hydroxylase staining intensity ranged between nearly imperceptible to primarily uniform moderate staining, with few showing dark staining or no staining (Fig. 9d). Seventy percent of nerves exhibiting chronic/reactive changes had no tyrosine hydroxylase staining and 30% showed primarily variable (light to dark) tyrosine hydroxylase staining (Fig. 9d).
FIGURE 9.
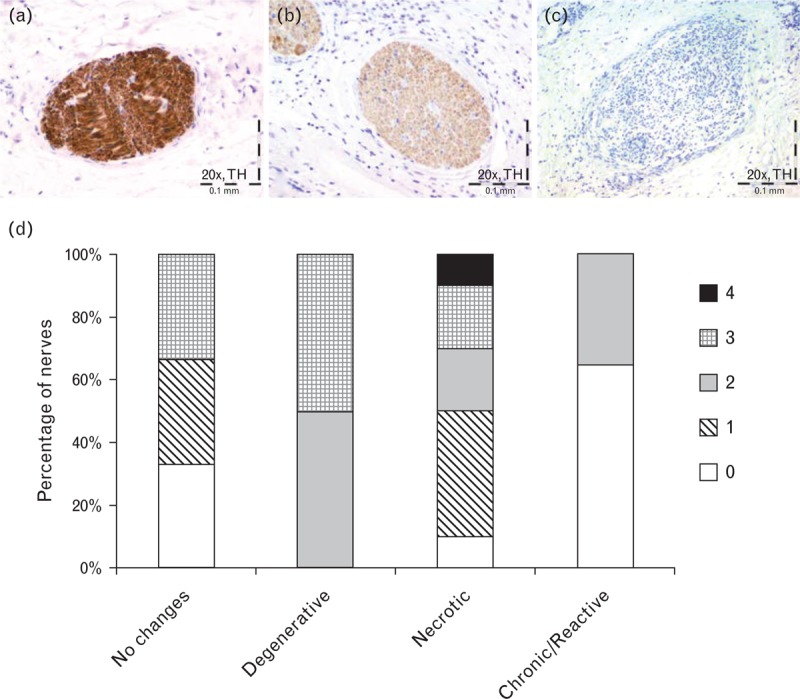
Tyrosine hydroxylase immunostaining 7 days following full artery-length treatments. (a) Representative example of an unaffected sympathetic nerve in the renal artery adventitia with uniform dark staining with TH (intensity score of 4). (b) Representative example of a sympathetic nerve in the renal artery adventitia affected by radiofrequency energy with notable loss of TH staining (intensity score of 3). (c) Representative example of a sympathetic nerve in the renal artery adventitia affected by radiofrequency energy with complete loss of TH staining (intensity score of 0). (d) Distribution of tyrosine hydroxylase stain intensity scores across nerves in the renal artery adventitia. 0 = no staining; 1 = primarily nearly imperceptible to light staining; 2 = primarily variable (light to dark) staining; 3 = primarily uniform moderate staining; 4 = primarily uniform dark staining. TH, tyrosine hydroxylase.
28-day assessments
Contrast angiography
Prenecropsy angiography 28 days following full artery-length treatment showed no evidence of renal artery stenosis, thrombus, dissection, perforation or spasm. The overall mean angiographic percentage stenosis in the day 28 full artery-length treatment group was 4 ± 11% (n = 12 arteries). No change in mean arterial diameter was detected at 7 or 28 days posttreatment (Fig. 10).
FIGURE 10.
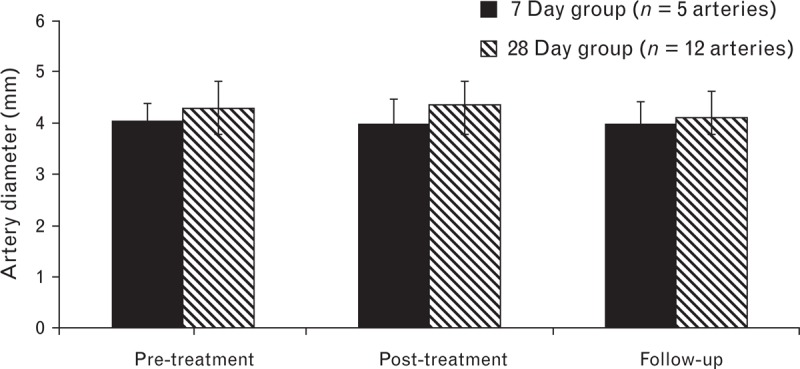
Renal artery diameter. Mean (SD) renal artery diameter prior to treatment, immediately following radiofrequency treatment, and prior to necropsy.
Histopathology and nerve assessment
Whereas at 7 days, morphologic changes in susceptible nerves included degenerative, necrotic or chronic changes (Fig. 8b), only chronic changes were identified at 28 days (Fig. 11a). Twenty-nine percent of nerves were unaffected following one treatment, whereas only 15% of nerves were unaffected following full artery-length treatment (Fig. 11a).
FIGURE 11.

Histology and renal norepinephrine changes at 28 days. (a) Percentages of susceptible nerves that appeared normal or exhibited morphological changes (as represented in Fig. 7b–e). (b) Representative image of a radiofrequency-treated artery 28 days posttreatment. Note minimal-to-mild/moderate neointima formation, and an open arterial lumen with no evidence of thrombosis. (c) Mean (SD) percentage reduction of renal norepinephrine levels in the kidneys of bilaterally treated (single radiofrequency treatment, full artery-length radiofrequency treatment or surgical renal denervation) vs. untreated swine 28 days following treatment. The levels in the renal denervation groups did not differ significantly between each other (P = 0.1).
At 28 days, treated arterial walls remained intact with no evidence of dissections or perforations and exhibited a robust reactive/reparative (i.e. healing) response over time characterized by notable, segmental fibromuscular (smooth) remodeling (hyperplasia/hypertrophy) and chronic inflammation. Remodeling of the arterial wall typically resulted in minimal to mild/moderate neointima formation, and all arterial lumens remained open with no evidence of thrombosis (Fig. 11b).
Balloon inflation within the renal artery without the delivery of radiofrequency energy did not affect the integrity of the arterial wall (data not shown).
Norepinephrine quantification
Mean renal norepinephrine concentrations 28 days following a single radiofrequency treatment per artery (359.5 ± 272.4 ng/g), full artery-length radiofrequency treatment (247.4 ± 188.6 ng/g) and surgical renal denervation (149.8 ± 288.4 ng/g) were each significantly less than concentrations in untreated animals (601.2 ± 60.5 ng/g; P < 0.05 for each comparison). These concentrations translate into reductions of 40, 59, and 75%, respectively, compared with untreated control animals (Fig. 11c).
Renal function
Serum creatinine levels and urea nitrogen were used to investigate the effect of treatment on renal function 28 days following bilateral treatment (n = 5). Baseline urea nitrogen was 12.2 ± 3.8 mg/dl, and creatinine was 1.4 ± 0.3 mg/dl. At 28 days postprocedure, urea nitrogen was 9.7 ± 2.2 mg/dl (P = 0.22 vs. baseline), and creatinine was 1.6 ± 0.2 mg/dl (P = 0.15 vs. baseline).
Blood pressure and heart rate
Blood pressure and heart rate were measured on the day of the procedure and 28 days postprocedure in five animals that received bilateral radiofrequency treatments. In these healthy swine, baseline blood pressure was 116 ± 9/60 ± 9 mmHg, and heart rate was 100 ± 16 beats per min. At 28 days postprocedure, blood pressure was 113 ± 20/63 ± 10 mmHg (P = 0.7 vs. baseline), and heart rate was 104 ± 12 beats per min (P = 0.2 vs. baseline).
DISCUSSION
Our findings demonstrate that the percutaneous application of bipolar radiofrequency energy to the renal artery, for a specific duration and temperature, results in renal nerve injury and a significant reduction in kidney norepinephrine content at both 7 and 28 days postprocedure in a preclinical porcine model. By testing different electrode lengths, target tissue temperatures and energy delivery algorithms, we refined the device design to balance the reduction in renal norepinephrine against evidence of histopathology. In addition, these studies showed that applying treatments along the renal artery length (i.e. up to two radiofrequency treatments with the investigational balloon catheter) further reduced renal norepinephrine but was not associated with notably different histological findings compared with a single treatment per artery.
Previous studies showing greater renal norepinephrine spillover in hypertensive patients [13] and reductions in renal norepinephrine among hypertensive patients who received renal denervation treatment [14] support the relevance of using renal norepinephrine as a biomarker to gauge denervation effectiveness. However, the relationship between renal tissue norepinephrine levels in normotensive swine and renal norepinephrine spillover in hypertensive patients is unknown. In addition, although reductions in both renal norepinephrine and blood pressure have been observed among hypertensive patients who received renal denervation treatment [14], the threshold of norepinephrine reduction that corresponds with a clinically significant magnitude of blood pressure reduction for a hypertensive patient is not known.
This bipolar radiofrequency system applies low power (0.5–2 W) for a short duration (<1 min), which is in contrast to monopolar electrode radiofrequency systems that require the application of higher power (6–8 W) in order to overcome the cooling effect of blood flow over the exposed electrodes [9,15]. However, careful titration of the radiofrequency dose (temperature profile and duration) is required to optimize safety and effectiveness. Our results demonstrated that bipolar radiofrequency application using the 16-mm electrodes resulted in an 81% decrease in norepinephrine levels, but induced significantly more vessel wall injury than did shorter 4-mm long electrodes, which were associated with a 60% decline in norepinephrine tissue levels. The 16-mm electrodes, which covered the entire vessel circumference, caused more vessel wall injury that was slower to heal. Reducing the electrode length to 4 mm enabled us to stagger the electrodes along the balloon surface, which limited the radiofrequency treatment zone, leaving most of the arterial wall circumference untreated and enabling faster healing. We believe that the lack of thrombotic material observed may be attributed to the apposition of the inflated balloon against the artery wall, which prevents direct contact between the electrodes and blood during radiofrequency energy delivery.
A temperature control radiofrequency algorithm was applied to the 4-mm electrodes to maximize radiofrequency penetration into the adventitia and cause more sympathetic nerve injury, resulting in an optimal decline in norepinephrine levels. A thermistor was mounted in the center of each electrode pair, which provided the ability to accurately measure the surface temperature of the balloon during treatment. This enabled the generator to independently titrate the exact amount of energy necessary to reach and maintain a target temperature for each electrode pair. In addition, the temperature control algorithm provided a temperature feedback mechanism, providing the user with an exact temperature reading during radiofrequency treatments.
Three target temperatures were evaluated using this temperature control algorithm: 64, 68 and 74°C. The algorithm allowed the temperature to gradually increase until reaching the target temperature, which was maintained for the remainder of the 30-s treatment time. The gradual heating of the tissue enabled the radiofrequency energy to penetrate deeper into the adventitia than when temperature was raised abruptly (data not shown). All three temperatures were found to be safe and effective; however, the most effective temperature was 68°C, which resulted in a norepinephrine reduction of nearly 90% relative to the untreated contralateral kidney, similar to the reduction achieved with the 16-mm electrodes. As with abrupt temperature increases, higher treatment temperatures were associated with poorer penetration and tissue charring, which may explain why a greater norepinephrine reduction was observed with treatment at 68°C than at 74°C. The electrodes were activated sequentially in the temperature experiments, but by simultaneously activating all electrodes we were able to reduce the entire treatment time to 30 s. This, in addition to treating the entire artery length did not adversely impact the safety or efficacy of the procedure.
An important additional consequence of the significant heat loss in certain monopolar systems is that longer treatment times (1.5–2.0 min) are required for heat to penetrate to the depth of the adventitia [9]. As renal denervation is known to cause visceral pain [15], which is likely due to afferent nerve damage, a bipolar radiofrequency system, by requiring less energy and a shorter treatment time, may reduce procedure-related pain. In addition, because blood perfusion is not needed to cool the catheter, small diameter balloons allow treatment in smaller renal arteries.
Interestingly, comparisons between one renal artery radiofrequency treatment and full artery-length treatments at 7 days resulted in similar norepinephrine reductions and similar proportions of nerves affected by bipolar radiofrequency energy. This may reflect an acute ‘threshold’ effect, as detailed histologic nerve analysis suggests that treatments extending along the length of the artery may actually increase the duration of the intended radiofrequency effect (i.e. decreased renal norepinephrine response). Long-term effects of radiofrequency treatment might be achieved through impaired nerve healing, by damaging the nerve fibers at multiple sites along their length and exposing them to increasingly irreversible injury (i.e. necrosis). Nerve functionality, as assessed by tyrosine hydroxylase staining of arteries undergoing full-length treatment, showed that nerves without pathologic changes had low tyrosine hydroxylase staining intensity, suggesting that these nerves may be affected at other sites along their length; indeed, most nerves had a reduction in the intensity of the enzyme despite the fact that only approximately 70% of nerves from animals receiving full-length treatment demonstrated pathologic changes. Long-term impairment of nerve functionality is also supported by the fact that norepinephrine levels were 31% lower following full artery-length treatment vs. single treatment at 28 days. Regardless of the number of treatments, there was a tendency for the renal norepinephrine concentrations in the kidneys of treated renal arteries to notably increase over time (i.e. by day 28), relative to their respective day 7 concentrations, leading to a lower percentage reduction in norepinephrine at 28 days than at 7 days. Although the exact cause of the increase in renal norepinephrine over time is unknown, it is presumed likely due to the subsiding initial inflammatory response as well as possible healing of degenerative nerve bundles.
We conclude that the application of bipolar radiofrequency energy along the entire length of the renal artery using 4-mm staggered electrodes with a temperature-controlled algorithm leads to sufficient renal nerve injury to reduce sympathetic function, as shown by changes in renal norepinephrine and tyrosine hydroxylase immunostaining. The elucidation of these treatment parameters, which result in safe, effective and rapid renal denervation, may have important implications in the treatment of patients with severe treatment-resistant hypertension.
ACKNOWLEDGEMENTS
We thank Andres Dandler for his help with graphic design (Fig. 1b and Fig. 1c) and Elizabeth J. Davis, PhD (Boston Scientific Corporation) for editorial assistance.
Previous presentations: M.M. Efficacy of renal denervation is positively impacted by longitudinal treatments. TCT 2012, October 22–26, Miami, FL; and M.M, B.R., S.J.R.L. Evaluation of acute, subacute and chronic renal nerve morphological changes following bipolar radiofrequency renal denervation treatment in the porcine model. TCT 2013, October 27–November 1, San Francisco, CA.
Source of funding: All these studies were funded by Vessix Vascular.
Conflicts of interest
Authors M.C.M., P.M., and H.L. are employees and stockholders of Vessix Vascular/Boston Scientific Corporation. Authors K.R.S. and F.O.M. are consultants for Vessix/Boston Scientific. Authors J.R.L.S., R.B., B.G.Z., and P.M.M. have nothing to disclose.
Reviewer's Summary Evaluation
Reviewer 1
This carefully performed study showed that bipolar radiofrequency energy application to the renal arteries is safe and accompanied by reduction of renal norepinephrine and significant sympathetic nerve interruption in swines. The strengths of the study are the sufficient number of animals, the variety of electrode length and energy delivery algorithms studied, the comparison of unilateral and bilateral treatments as well as the inclusion of surgical denervation and sham-ablation arms. The main weakness of the study is that changes in renal norepinephrine and sympathetic nerves could not be related to blood pressure alterations in this normotensive animal model.
Reviewer 2
The manuscript by Cohen-Mazor et al., is describing a novel percutaneous renal denervation technique involving a bipolar radiofrequency delivery system. Using a meticulous stepwise approach the authors carefully identified the optimal combination (length of the catheter, number of electrode pairs, temperature, duration of the radio-frequency treatment, single vs. entire length of the treatment) to induce an efficient and localized denervation with a well defined and predictable field of injury. The next step of this study would consist in determining whether this novel technique is efficient at reducing blood pressure in hypertensive animals or patients.
Reviewer 3
This paper reports the results obtained by applying a percutaneous approach to renal denervation in a porcine model. The data are interesting and the paper is clearly written, with a correct analysis of the observations made. Percutaneous approach to renal denervation is an obviously exciting possibility, although recent negative findings on the ability of real denervation to lower blood pressure should not be forgotten.
Footnotes
Abbreviation: HPLC-MS/MS, high-performance liquid chromatography tandem mass spectrometry
REFERENCES
- 1.Schlaich MP, Sobotka PA, Krum H, Whitbourn R, Walton A, Esler MD. Renal denervation as a therapeutic approach for hypertension: novel implications for an old concept. Hypertension 2009; 54:1195–1201. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein DS. Plasma catecholamines and essential hypertension. An analytical review. Hypertension 1983; 5:86–99. [DOI] [PubMed] [Google Scholar]
- 3.Barajas L, Powers K, Wang P. Innervation of the renal cortical tubules: a quantitative study. Am J Physiol 1984; 247:F50–60. [DOI] [PubMed] [Google Scholar]
- 4.Esler M. The sympathetic system and hypertension. Am J Hypertens 2000; 13:99S–105S. [DOI] [PubMed] [Google Scholar]
- 5.Esler M, Rumantir M, Kaye D, Jennings G, Hastings J, Socratous F, Lambert G. Sympathetic nerve biology in essential hypertension. Clin Exp Pharmacol Physiol 2001; 28:986–989. [DOI] [PubMed] [Google Scholar]
- 6.Smith PA, Graham LN, Mackintosh AF, Stoker JB, Mary DA. Relationship between central sympathetic activity and stages of human hypertension. Am J Hypertens 2004; 17:217–222. [DOI] [PubMed] [Google Scholar]
- 7.DiBona GF, Kopp UC. Neural control of renal function. Physiol Rev 1997; 77:75–197. [DOI] [PubMed] [Google Scholar]
- 8.Atherton DS, Deep NL, Mendelsohn FO. Micro-anatomy of the renal sympathetic nervous system: a human postmortem histologic study. Clin Anat 2012; 25:628–633. [DOI] [PubMed] [Google Scholar]
- 9.Rippy MK, Zarins D, Barman NC, Wu A, Duncan KL, Zarins CK. Catheter-based renal sympathetic denervation: chronic preclinical evidence for renal artery safety. Clin Res Cardiol 2011; 100:1095–1101. [DOI] [PubMed] [Google Scholar]
- 10.Institute of Laboratory Animal Resources (US) Guide for the care and use of laboratory animals. 7 ed.1996; Washington, D.C:National Academy Press, http://books.nap.edu/openbook.php?isbn=0309053773http://books.nap.edu/openbook.php?isbn=0309053773 [Accessed 24 September 2013]. [Google Scholar]
- 11.Ji C, Walton J, Su Y, Tella M. Simultaneous determination of plasma epinephrine and norepinephrine using an integrated strategy of a fully automated protein precipitation technique, reductive ethylation labeling and UPLC-MS/MS. Anal Chim Acta 2010; 670:84–91. [DOI] [PubMed] [Google Scholar]
- 12.Steigerwald K, Titova A, Malle C, Kennerknecht E, Jilek C, Hausleiter J, et al. Morphological assessment of renal arteries after radiofrequency catheter-based sympathetic denervation in a porcine model. J Hypertens 2012; 30:2230–2239. [DOI] [PubMed] [Google Scholar]
- 13.Schlaich MP, Lambert E, Kaye DM, Krozowski Z, Campbell DJ, Lambert G, et al. Sympathetic augmentation in hypertension: role of nerve firing, norepinephrine reuptake, and angiotensin neuromodulation. Hypertension 2004; 43:169–175. [DOI] [PubMed] [Google Scholar]
- 14.Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartuc K, et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet 2009; 373:1275–1281. [DOI] [PubMed] [Google Scholar]
- 15.Schlaich MP, Schmieder RE, Bakris G, et al. International Expert Consensus Statement: percutaneous transluminal renal denervation for the treatment of resistant hypertension. J Am Coll Cardiol 2013; 62:2031–2045. [DOI] [PubMed] [Google Scholar]


