Supplemental digital content is available in the text.
KEY WORDS: DAMPs, SIRS, mtDNA, trauma
Abstract
BACKGROUND
Mitochondrial DNA (mtDNA), a potent proinflammatory damage-associated molecular pattern, is released in large titers following trauma. The effect of trauma surgery on mtDNA concentration is unknown. We hypothesized that mtDNA and nuclear DNA (nDNA) levels would increase proportionately with the magnitude of surgery and both would then decrease rapidly.
METHODS
In this prospective pilot, plasma was sampled from 35 trauma patients requiring orthopedic surgical intervention at six perioperative time points. Healthy control subjects (n = 20) were sampled. DNA was extracted, and the mtDNA and nDNA were assessed using quantitative polymerase chain reaction. Markers of cell necrosis were also assayed (creatine kinase, lactate dehydrogenase, and aspartate aminotransferase).
RESULTS
The free plasma mtDNA and nDNA levels (ng/mL) were increased in trauma patients compared with healthy controls at all time points (mtDNA: preoperative period, 108 [46–284]; postoperative period, 96 [29–200]; 7 hours postoperatively, 88 [43–178]; 24 hours, 79 [36–172]; 3 days, 136 [65–263]; 5 days, 166 [101–434] [healthy controls, 11 (5–19)]) (nDNA: preoperative period, 52 [25–130]; postoperative period, 100 [35–208]; 7 hours postoperatively, 75 [36–139]; 24 hours postoperatively, 85 [47–133]; 3 days, 79 [48–117]; 5 days, 99 [41–154] [healthy controls, 29 (16–54)]). Elevated DNA levels did not correlate with markers of cellular necrosis. mtDNA was significantly elevated compared with nDNA at preoperative period (p = 0.003), 3 days (p = 0.003), and 5 days (p = 0.0014). Preoperative mtDNA levels were greater with shorter time from injury to surgery (p = 0.0085). Postoperative mtDNA level negatively correlated with intraoperative crystalloid infusion (p = 0.0017). Major pelvic surgery (vs. minor) was associated with greater mtDNA release 5 days postoperatively (p < 0.05).
CONCLUSION
This pilot of heterogeneous orthopedic trauma patients showed that the release of mtDNA and nDNA is sustained for 5 days following orthopedic trauma surgery. Postoperative, circulating DNA is not associated with markers of tissue necrosis but is associated with surgical invasiveness and is inversely related to intraoperative fluid administration. Sustained elevation of mtDNA levels could be of inflammatory origin and may contribute to postinjury dysfunctional inflammation.
LEVEL OF EVIDENCE
Prospective study, level III.
The release of mitochondrial DNA (mtDNA) following trauma has been documented in both humans and animal models.1,2 High titers of mtDNA have also been found in reaming material collected during the intramedullary nailing of fractured femora with subsequent rises in patient plasma levels.3 mtDNA4 released during cell necrosis stimulates the innate immune system and is associated with postinjury sterile systemic inflammatory response syndrome (SIRS)1,2 and acute lung injury.1
The origin of the eukaryotic cell is a debated topic, but one of the widely accepted theories is that during evolution, archaebacteria internalized α-proteobacteria,5,6 which became the energy producing endosymbiont (mitochondrion) of the eukaryotic cell.6 In contrast to nuclear DNA (nDNA), bacterial DNA (bDNA) is circular and contains unmethylated CpG repeats.7 Mitochondria possess several copies of their genome (between 2 and 10 per mitochondrion), which resembles bDNA containing unmethylated CpG repeats.8
These mtDNA and other cellular derivatives have been termed damage-associated molecular patterns (DAMPs) or alarmins.4 Notably, alarmins can induce an inflammatory response at physiologic concentration.4 Human neutrophils have been shown to be activated by exposure to mtDNA through activation of TLR4,3 TLR9,2 and FPR1.3 Acute inflammatory lung injury was induced by injecting mitochondrial DAMPs in a healthy rat model.3
The tissue injury at the time of trauma is an important but nonmodifiable factor for postinjury inflammation-associated complications. Surgical interventions on trauma patients cause secondary tissue injury, releasing DAMPs, and can potentially act as a deleterious second hit to negatively influence the clinical outcome.9 More importantly, the timing and the magnitude of these procedures are potentially modifiable predictors of multiple-organ failure (MOF). The perioperative profile of mtDNA plasma concentrations of trauma patients is unknown. It has been suggested to that the initial high titers rapidly decrease within hours after injury.10 mtDNA plasma concentrations are recently reported in isolation without addressing the simultaneous release of nDNA.10 nDNA has been correlated with injury severity.11
We aimed to describe the natural history of mtDNA and nDNA release after surgery and the potential association between DNA release and resuscitation and the invasiveness of the surgical intervention.
We hypothesized that the mtDNA levels (and similarly nDNA levels) would increase in a dose-dependent fashion directly related to the degree of tissue damage sustained in surgery. Levels are then anticipated to rapidly decrease after the surgical intervention.
PATIENTS AND METHODS
Research Compliance
Ethical approval for the study was obtained from Hunter New England Human Research Ethics Committee (HNEHREC). All plasma samples were obtained with informed consent.
Patients and Blood Samples
Blood was sampled from 35 trauma patients with informed consent at six time points in the perioperative period (immediate preoperative and postoperative periods and then 7 hours, 24 hours, 3 days, and 5 days postoperatively). The rationale for the sampling time points was based on previous publications examining immune responses to orthopedic trauma interventions.12,13 Consecutive trauma patients older than 17 years with skeletal injuries, requiring standardized orthopedic trauma surgical fixation including pelvic (symphyseal plating, iliosacral screw fixation, or open reduction and internal fixation of the sacrum or sacroiliac joint), acetabulum (open reduction and internal fixation from ilioinguinal and/or Kocher-Langenbeck approaches), femoral (intramedullary nailing of the femur), or tibia/fibula fractures (intramedullary nailing of the tibia), were included between January 2013 and June 2013. Patients operated on between Friday and Sunday have not been included because of the potential logistic problems with sample collection and procession during the weekends. There were no refusals or withdrawals during the 6-month recruitment period. Pelvic and acetabulum surgery cases were further categorized as major open surgery or percutaneous/minimally invasive surgery. Patients were excluded if they had underlying autoimmune or any chronic inflammatory condition. SIRS was defined based on consensus; for the definition of MOF, the Denver score was used.14,15 Healthy control subjects who were age and sex matched with the study cohort (n = 20) were recruited through the Hunter Medical Research Institute registry. The registry tends to have more senior and more female volunteers than the usual trauma populations. The primary goal was better matching for age, and the secondary goal was to match sex. Plasma was separated from 5 mL of whole blood and frozen at −80°C before analysis.
mtDNA and nDNA Extraction From Plasma
Plasma samples were thawed on ice, pulse vortexed for 15 seconds, and then centrifuged at 12,000 G for 10 minutes to pellet cell debris. Cell free plasma sample (200 μL) was aspirated and used for DNA extraction16 using a blood DNAeasy extraction kit (Qiagen Chadstone, Victoria, Australia) according to the manufacturer’s instructions and using a 200-μL elution volume.
Real-Time quantitative polymerase chain reaction (qPCR) Protocols
DNA eluates were diluted 10-fold with nuclease free water, and 5 μL of the diluent was then used for each qPCR reaction. The 5 μL of diluted DNA was added to 7 μL of SYBR green master mix (SensiFast, Biolines, Alexandria, New South Wales, Australia). The real-time qPCR analysis was performed using Applied Biosystems Real-Time 7500 analyser (Applied Biosystems, Life Technologies, Foster City, CA). mtDNA primers were designed and synthesized for COX3 and ND3; and the nDNA primer, for GAPDH (Geneworks Hindmarsh, South Australia, Australia) (Supplementary Digital Content 1, http://links.lww.com/TA/A504). Standard curves were constructed using highly purified mtDNA/nDNA to enable calculation mtDNA and nDNA sample concentrations in nanogram per milliliter. All samples were screened for bDNA, the presence of which could indicate sepsis.
Data Analysis
Data are presented as mean (SD) for parametric variables and as median (interquartile range [IQR]) for nonparametric variables. Data were visually examined for skew. Hypothesis testing of changes in DNA concentrations between the time periods were performed using the Friedman test, a nonparametric equivalent of repeated-measures analysis of variance (ANOVA). Differences between mtDNA and nDNA were compared at each time point using the Wilcoxon signed-rank test. DNA levels in the trauma cohort at different time points and healthy controls were compared using the Kruskal-Wallis test. Multiple comparisons were performed by using the Holm-Sidak approach.17 Differences between operative interventions were tested using two-way repeated-measures ANOVA. Correlation between continuous variables was calculated using Spearman’s correlation coefficient.
RESULTS
Study Population Demographics
Blood samples were obtained from 35 trauma patients (25 males, 10 females), with a median age of 38 years (IQR, 29–48) and a median Injury Severity Score (ISS) of 14 (IQR, 9–22). The 20 healthy controls’ (12 males, 8 females, p = 0.57 compared with trauma patients) median age was 38 years (IQR, 28–50) (p = 0.87 compared with trauma patients). All patients had experienced high-energy blunt trauma resulting in fractures that required surgical stabilization. Seventeen patients experienced polytrauma, and 18 experienced monotrauma. Median initial base deficit was −1 mEq/L (IQR, −3 to 0.9). The following interventions were performed: major pelvic surgery (n = 10), minor pelvic surgery (n = 11), femoral nailing (n = 7), tibial nailing (n = 7), and combined femoral and tibial nailing (n = 2). No patients had clinical signs of sepsis or microbiologically proven bacteremia during the perioperative period. Median time to surgery was 48 hours (IQR, 18–96) from injury. Thirteen patients received a blood product transfusion before surgery, and an additional nine received a transfusion in the postoperative period. Thirteen patients were admitted to the intensive care unit (median stay, 6 days; IQR, 3–11 days) (mean [SD] ventilator days, 3 [3]). Twelve patients developed clinical SIRS. Three patients developed MOF. All patients survived, and the median length of hospital stay was 18 days (IQR, 8–33).
Perioperative Changes in DNA Concentration
The median (IQR) plasma mtDNA concentration (ng/mL) (preoperative period, 108 [46–284]; immediate postoperative period, 96 [29–200]; 7 hours postoperatively, 88 [43–178]; 24 hours, 79 [36–172]; 3 days, 136 [65–263]; 5 days, 166 [101–434]) was elevated compared with that of the healthy controls (11 [5–19]) at all six perioperative time points (Kruskal-Wallis test, p < 0.0001) (Fig. 1A).
Figure 1.
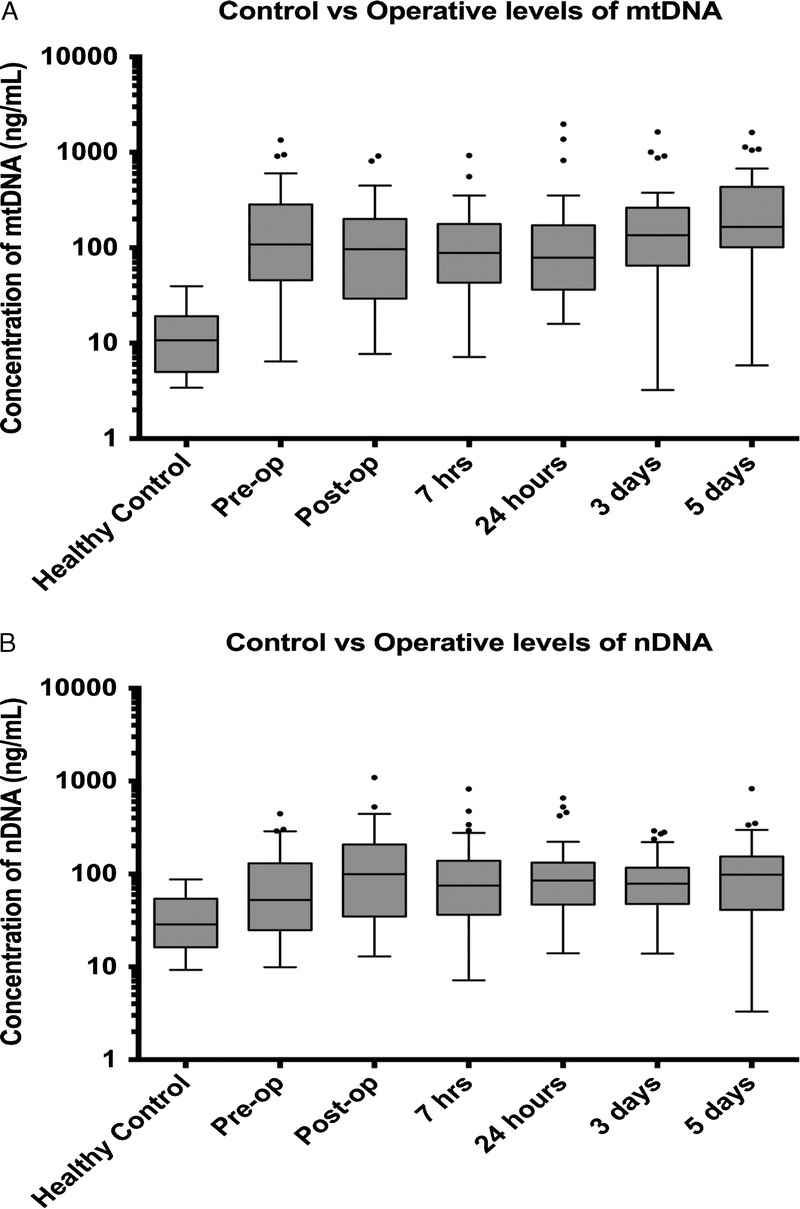
A, Free plasma mtDNA concentration of the trauma cohort was significantly higher than that of the healthy control subjects (Kruskal-Wallis test, p = <0.0001; Dunn post hoc test, p = <0.05) at all perioperative time points. There was no significant change in mtDNA concentration measured between any time points in trauma patients’ plasma. Spearman’s test was applied for nonparametric data comparing changes in mtDNA (omnibus p = 0.054). B, Free plasma nDNA concentration of the trauma cohort was significantly higher than that of healthy control subjects (Kruskal-Wallis test, p = 0.0069; Dunn post hoc test, p = <0.05) at all postoperative time points (preoperative comparison with healthy controls: Kruskal-Wallis test was nonsignificant). There was no statistically significant change in nDNA concentration measured between any time points in trauma patients’ plasma. Spearman’s test was applied for nonparametric data comparing changes in nDNA (omnibus p = 0.075).
The median (IQR) plasma nDNA concentration (ng/mL) (preoperative period, 52 [25–130]; immediate postoperative period, 100 [35–208]; 7 hours, 75 [36–139]; 24 hours, 85 [47–133]; 3 days, 79 [48–117]; 5 days, 99 [41–154]) was elevated compared with that of the healthy controls at all postoperative time points (29 [16–54]). (Kruskal-Wallis test, p = 0.0069) (Fig. 1B).
Plasma concentration of mtDNA was also found to be significantly elevated compared with nDNA levels in the study population at preoperative period (Wilcoxon test, p = 0.003), 3 days postoperatively (p = 0.003), and 5 days postoperatively (p = 0.0014) (Fig. 2).
Figure 2.
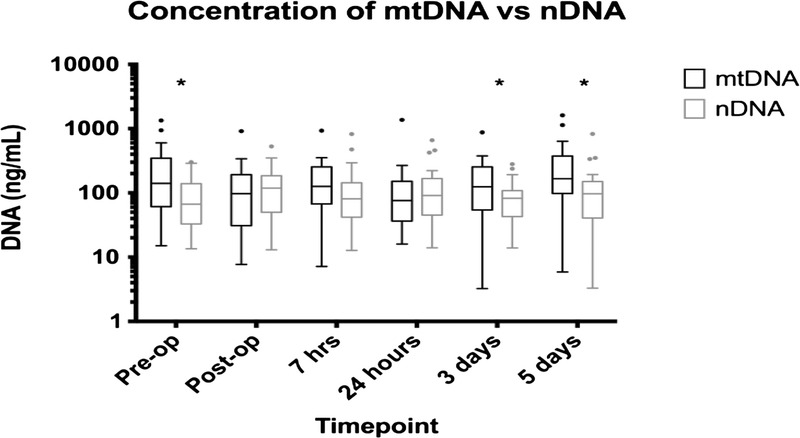
Statistically significant difference between free plasma mtDNA and nDNA levels at preoperative period (p = 0.003), 3 days postoperatively (p = 0.003), and 5 days postoperatively (p = 0.0014). Statistical comparison was made using the Wilcoxon matched-pair signed-rank test.
Those patient who had low preoperative serum mtDNA concentration had less pronounced postoperative mtDNA concentrations than those who had high concentrations (p = 0.0138, Wilcoxon matched-pairs rank-sum test). No correlation was found between the preoperative and postoperative nDNA concentrations.
There was no detectable bDNA in any patient included in the study cohort at any time point or in the healthy controls (data not presented).
Magnitude and Timing of Surgery
Major pelvic surgery was associated significantly higher mtDNA levels when compared with percutaneous, minimally invasive pelvic surgery, at day 5 postoperatively (two-way ANOVA and then Holm-Sidak test, p < 0.05) (Fig. 3).
Figure 3.
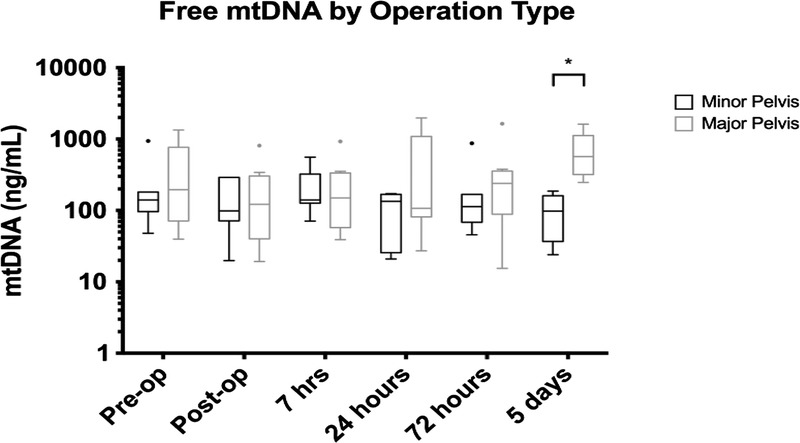
Statistically significant difference between free plasma mtDNA levels at 5 days postoperatively when comparing major pelvic versus minor pelvic surgery (p < 0.05). Statistical comparison was made using two-way ANOVA test.
There was a statistically significant correlation between the preoperative plasma mtDNA concentration and the time elapsed from injury. No significant correlation was found between time to surgery and preoperative nDNA levels (Fig. 4A and B).
Figure 4.
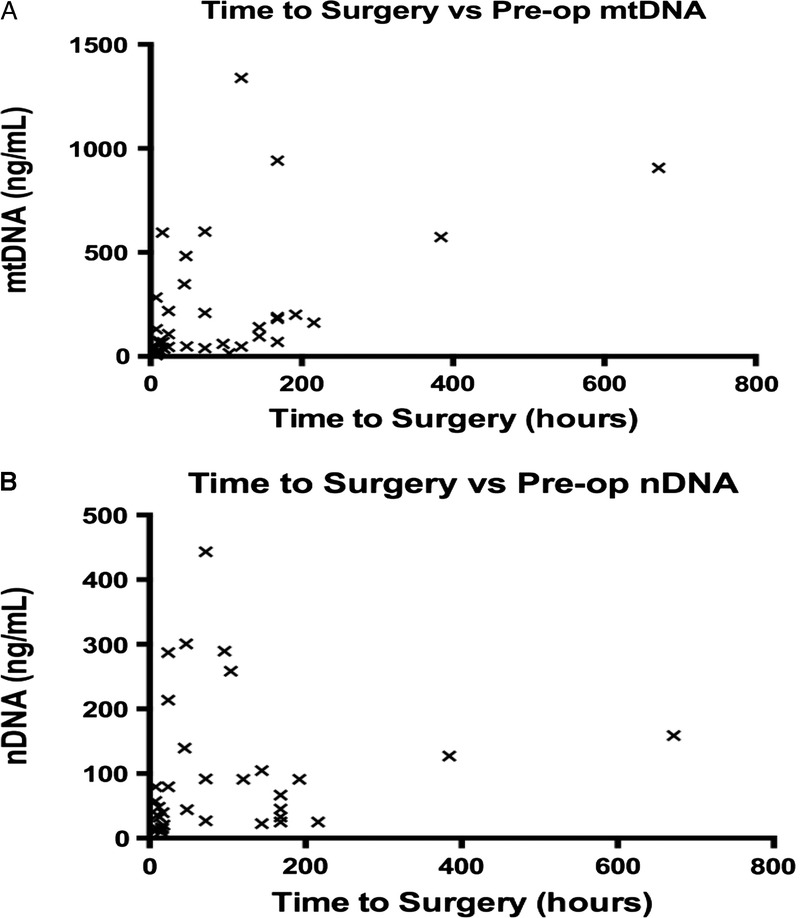
Statistically significant correlation between reduced time from injury to surgery and elevated preoperative levels of mtDNA (Spearman’s test, p = 0.0085). No significant correlation was found with preoperative nDNA levels (Spearman’s test, p = 0.055).
Tissue Necrosis, Fluid Resuscitation, and SIRS/MOF Association With mtDNA
No correlation was found between mtDNA levels and markers of cell necrosis, namely, creatine kinase (CK), lactate dehydrogenase (LDH), and aspartate aminotransferase (AST) (Fig. 5A–C). There was no correlation between CK and nDNA serum concentrations, but there was a significant correlation between AST and nDNA (72 hours postoperatively) and also between LDH and nDNA concentration (preoperative period, 24 hours postoperatively, and 72 hours postoperatively) (data not presented here). Immediate postoperative mtDNA levels were negatively correlated with intraoperative crystalloid fluid administration (p = 0.0017) (Fig. 6). No significant difference in plasma free mtDNA/nDNA was observed with SIRS (n = 12) versus no SIRS (n = 23) or MOF (n = 3) versus no MOF (n = 32).
Figure 5.
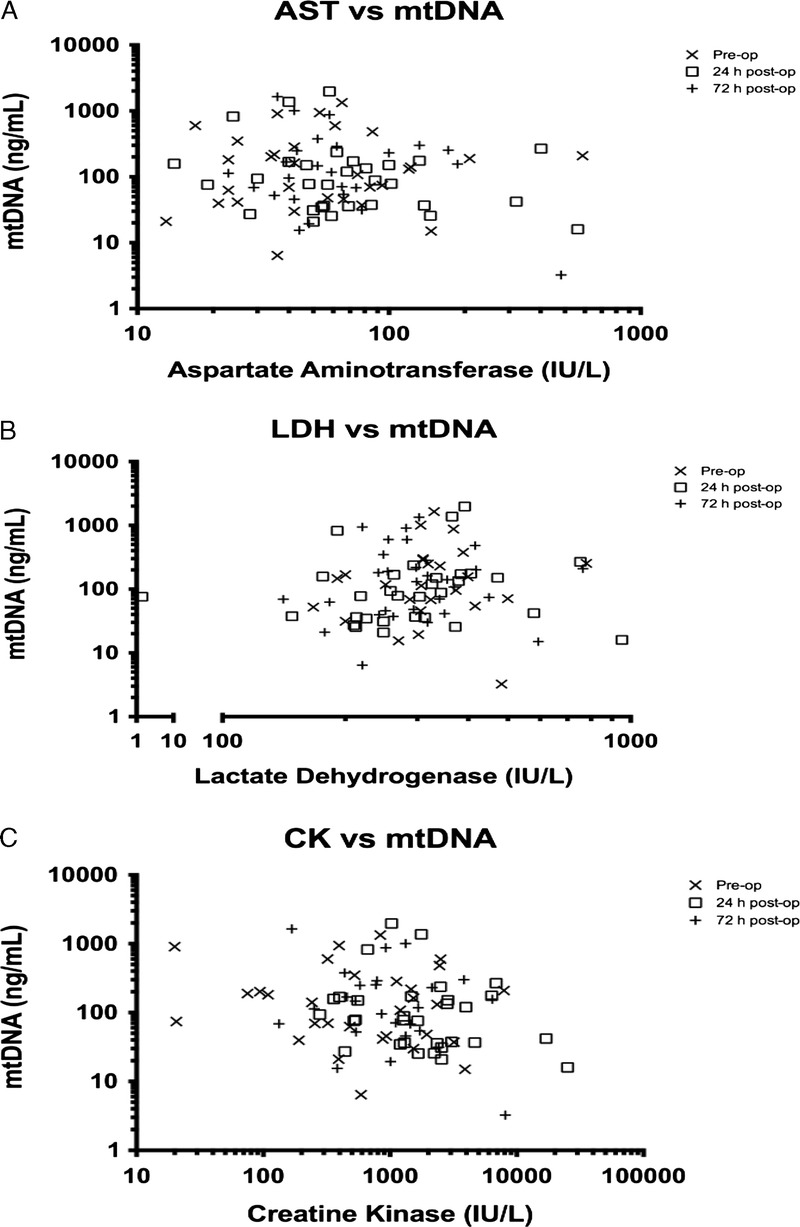
No statistically significant correlation to mtDNA levels (Spearman’s test) between AST (p = 0.47–0.84) (A), LDH (p = 0.13–0.62) (B), and CK (p = 0.39–0.48) (C).
Figure 6.
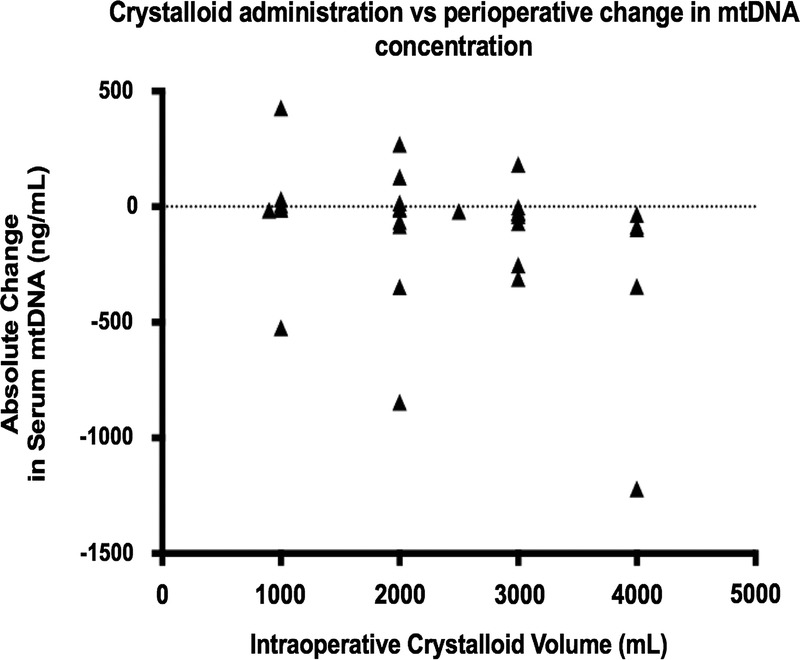
There was a statistically significant correlation (Spearman’s test) between volume of intraoperative crystalloid infused and postoperative mtDNA level (p = 0.0017).
DISCUSSION
We demonstrated that mtDNA release and subsequent elevated plasma titers are sustained for at least 5 days following major orthopedic interventions on trauma patients. Trauma patients have elevated mtDNA plasma levels preoperatively days after the initial traumatic tissue injury. The detected mtDNA concentration in the plasma is independent of tissue necrosis markers but negatively correlated with the magnitude of intraoperative fluid administration. Major pelvic surgery was associated with a higher elevation in plasma mtDNA titers compared with minor pelvic surgery toward the end of the observation period. Our cohort was not powered to demonstrate association between mtDNA or nDNA titers and outcomes. These findings are novel because while it has been demonstrated that mtDNA is released by injury,1 it has not previously been demonstrated in plasma days after surgery and the late increases in mtDNA levels rising significantly above nDNA levels were unknown so far.
The correlation between the preoperative mtDNA and time from injury to surgery indicates that mtDNA could be a valuable marker for optimal surgical timing. Independently from the elapsed time between injury and surgery, the preoperative mtDNA titers were significantly higher than those of the healthy controls. Most patients had comparable levels of plasma mtDNA between 3 days and 10 days after trauma with the previously published initial (within hours of injury) mtDNA concentrations.1 The magnitude of preoperative mtDNA concentrations was correlated with the postoperative increase of mtDNA concentrations (but not the nDNA), which could make mtDNA a valuable marker for optimal timing of major secondary surgical interventions.
Our data suggest that rather than increasing rapidly following surgery, absolute plasma levels of mtDNA actually declined in the immediate postoperative period (up to 7 hours postoperatively), when compared with preoperative titers. This could be explained by intraoperative hemodilution. Increased intraoperative fluid administration was correlated with decreased postoperative titers of mtDNA. This finding highlights the need to include fluid resuscitation among the reported variables in mtDNA research on trauma patients.
mtDNA concentration increased sequentially from 24 hours and 3 days postoperatively before peaking at Day 5. There was a strong trend (p = 0.054) in mtDNA concentration increase from 24 hours versus 3 days and 5 days. mtDNA was significantly elevated compared with nDNA concentration at 5 days postoperatively when comparing major and minor pelvic surgery. This indicates the potential link between the magnitude of surgery and postoperative DNA release. This could be caused by the fact that more invasive surgery can result in more severe inflammatory response. Unlike the initial trauma-related increase in mtDNA plasma concentration that occurs within hours of injury and correlates with CK concentrations,10 this sustained elevation of plasma mtDNA is independent of tissue necrosis markers, while nDNA shows correlation with AST and LDH concentrations. The mechanism of this mtDNA release is very likely to be other than direct tissue injury and subsequent cell necrosis.
The current literature suggests rapid increases followed by rapid decline in mtDNA titers following major trauma in humans1 and a rat model of traumatic hemorrhagic shock and subsequent surgery.2 There was a subgroup (eight patients) in a study performed in a polytrauma intensive care unit patient population where cell free DNA was measured over 10 days following admission where cell free DNA was noted to increase from Day 3 after injury, peaking at Day 7.18 The authors did not delineate the type of DNA measured (mtDNA vs. nDNA). The persistence of increased nDNA was confirmed in plasma of major trauma patients up to 28 days following injury.19 The same group noted increased mtDNA identified in the plasma of major trauma patients less than 4 hours after injury.20 This increased mtDNA titer was positively correlated with ISS, but the surgical interventions and potential sepsis were not accounted for.
Some studies18–20 did not specify their methodology to eliminate the cellular fraction in the plasma samples by including a second high-speed centrifugation step (12,000 rpm in approximately 10 minutes) before DNA extraction.17 Without this step, the white cell fraction may have been lysed during DNA extraction and could have contributed to the “free circulating DNA” measured.
There was also no correlation between AST or LDH and mtDNA. In sepsis, positive correlations had been shown between liver enzymes and plasma mtDNA levels, suggesting inflammatory end-organ damage and subsequent DNA release.21
With a lack of correlation between markers of tissue necrosis, that is, CK (skeletal muscle), AST, and LDH (liver) failing to correlate with mtDNA levels, alternative possible sources need to be considered. The default mode of cell death for neutrophils remains apoptosis, where little intracellular DNA, whether nDNA or mtDNA, escapes into the extracellular environment. Small amounts of DNA from apoptotic cells do escape degradation by scavenging macrophages22 and are released into the circulation. Under normal physiologic conditions, some 1 g to 10 g of DNA from nucleated leucocytes is degraded and cycled daily.23 Circulating DNA from dying cells is rapidly degraded by DNAse.24 The large titers of plasma DNA in trauma patients suggests deviation from normal physiologic process.
The possible source of free circulating DNA without proven cell necrosis could be that under certain conditions, neutrophils can extrude their DNA into the extracellular environment to form neutrophil extracellular traps (NETs).25 Our current data raise suspicion that an inflammatory mechanism (in contrast to necrosis) could be behind the sustained elevated plasma mtDNA titers in the postoperative period.
The significance of elevated mtDNA compared with nDNA concentration at several perioperative time points (preoperative period, 3 days postoperatively, and 5 days postoperatively) can be better understood by considering the size of the respective genomes. Human mitochondrial genome is 16,569 base pairs, and the human nuclear genome is 3.3 billion base pairs.8 This makes the mtDNA molecule 200 thousand times smaller than nDNA. There are variable numbers of mitochondria in different cell types and between 2 and 10 copies of the mitochondrial genome per mitochondria.8 Those cells with the greatest numbers of mitochondria such as striated muscle and liver would still have approximately six to seven times more nDNA if concentration was expressed as nanogram per milliliter as in this study. The potential release of mtDNA from viable nucleated cells without nDNA release has already been alluded to by studies on NET formation or NETosis.26,27
Most authors accept that the process of NETosis is a form of controlled cell death28,29 and nuclear chromatin is a major constituent of NETs.30,31 It has been postulated that some neutrophils remain viable while form NETs exclusively from mtDNA26 and eosinophil leukocytes have a mechanism for the “catapult-like release” of mitochondria.27 If such mechanisms for active mitochondrial product expulsion exist as a component of the innate immune response following major injury and subsequent surgery, perhaps, this may help explain why such high titers of mtDNA were measured in the plasma of patients in this study. Recently, we have demonstrated that trauma surgical interventions generate NETs and they primarily contain mtDNA in contrast to NETs produced in response to bacteria, which primarily consists of nDNA.30
That no significant bDNA was measurable in any patient at any time point confirms that the cellular processes occurred under essentially sterile conditions. This eliminates the possibility of sepsis-related inflammatory tissue damage driving the mtDNA or nDNA release.
The present study is limited by the lack of power to demonstrate statistical differences between groups such as SIRS versus no SIRS, which has been described before.31,32 Our study focused on the standard orthopedic trauma surgical interventions and mtDNA/nDNA release days after those; consequently, our study did not include a very high-risk, severely shocked, and major tissue injury cohort. Although the relatively standard surgical interventions were selected for inclusion, some heterogeneity with respect to the nature of surgery, surgical technique, and the period between injury and surgery are also potential confounding elements in this study.
In conclusion, our pilot study highlights the sustained presence of primarily mtDNA in trauma patients’ plasma following surgical interventions. That the timing and the magnitude of surgery correlates with mtDNA concentration makes it potentially attractive as a future marker for second hits and the development of postinjury complications.32,33 Our results and conclusions should be interpreted in the context of the heterogeneity of our population and the pilot nature of our study. Nevertheless, in future research related to postinjury DAMPs, we confidently recommend addressing routinely the timing and effect of surgical interventions and also fluid resuscitation to distinguish between trauma and treatment-associated responses.
Supplementary Material
AUTHORSHIP
A.E.W. and N.L. collected blood samples and patient demographics. D.J.M., M.B., and D.W.S. designed the experimental protocol. D.J.M. conducted the laboratory experiments. Initial data analysis was performed by D.J.M. Further statistical analysis was performed by B.M.H. with the generation of the figures used in the manuscript. The manuscript was written by D.J.M and Z.J.B. Critical appraisal of the manuscript was performed by D.W.S.
DISCLOSURE
This study was supported by a John Hunter Hospital Trauma Unit Research and Education Fund.
Footnotes
This study was presented at the 44th Annual Meeting of the Western Trauma Association, March 2–7, 2014, in Steamboat Springs, Colorado.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.jtrauma.com).
REFERENCES
- 1. Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser C. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010; 464 (7285): 104– 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang Q, Itagaki K, Hauser CJ. Mitochondrial DNA is released by shock and activates neutrophils via p38 MAP kinase. Shock. 2010; 34 (1): 55– 59. [DOI] [PubMed] [Google Scholar]
- 3. Hauser C, Sursal T, Rodriguez EK, Appleton PT, Zhang Q, Itagaki K. Mitochondrial DAMPs from femoral reamings activate neutrophils via formyl peptide receptors and P44/42 MAP kinase. J Orthop Trauma. 2010; 24 (9): 534– 538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Harris H, Raucci A. Alarmin(g) news about danger: workshop on innate danger signals and HMGB1. EMBO Rep. 2006; 7: 774– 778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sagan L. On the origin of mitosing cells. J Theor Biol. 1967; 14: 255– 274. [DOI] [PubMed] [Google Scholar]
- 6. Andersson S, Karlberg O, Canback B, Kurland C. On the origin of mitochondria: a genomics perspective. Phil Trans R Soc Lond B. 2003; 358: 165– 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, et al. A toll-like receptor recognizes bacterial DNA. Nature. 2000; 408: 740– 745. [DOI] [PubMed] [Google Scholar]
- 8. Hochhauser D. Relevance of mitochondrial DNA in cancer. Lancet. 2000; 356: 181– 182. [DOI] [PubMed] [Google Scholar]
- 9. Balogh ZJ, Reumann MK, Gruen RL, Mayer-Kuckuk P, Schuetz MA, Harris IA, Gabbe BJ. Advances and future directions for management of trauma patients with musculoskeletal injuries. Lancet. 2012; 380: 1109– 1119. [DOI] [PubMed] [Google Scholar]
- 10. Yamanouchi S, Kudo D, Yamada M, Miyagawa N, Furukawa H, Kushimoto S. Plasma mitochondrial DNA levels in patients with trauma and severe sepsis: time course and the association with clinical status. J Crit Care. 2013; 28: 1027– 1031. [DOI] [PubMed] [Google Scholar]
- 11. Lo YM, Rainer TH, Chan LY, Hjelm NM, Cocks RA. Plasma DNA as a prognostic marker in trauma patients. Clin Chem. 2000; 46: 319– 323. [PubMed] [Google Scholar]
- 12. Pape HC, Grimme K, Van Griensven M, Sott A, Giannoudis P, Morley J, Roise O, Ellingsen E, Hildebrand F, Wiese B, et al. Impact of intramedullary instrumentation versus damage control for femoral fractures on immunoinflammatory parameters: prospective randomized analysis by the EPOFF Study Group. J Trauma. 2003; 55 (1): 7– 13. [DOI] [PubMed] [Google Scholar]
- 13. Pape HC, van Griensven M, Rice J, Gänsslen A, Hildebrand F, Zech S, Winny M, Lichtinghagen R, Krettek C. Major secondary surgery in blunt trauma patients and perioperative cytokine liberation: determination of the clinical relevance of biochemical markers. J Trauma. 2001; 50 (6): 989– 1000. [DOI] [PubMed] [Google Scholar]
- 14. Muckart DJ, Bhagwanjee S. American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference definitions of the systemic inflammatory response syndrome and allied disorders in relation to critically injured patients. Crit Care Med. 1997; 25: 1789– 1795. [DOI] [PubMed] [Google Scholar]
- 15. Ciesla DJ, Moore EE, Johnson JL, Burch JM, Cothren CC, Sauaia A. A 12-year prospective study of postinjury multiple organ failure: has anything changed? Arch Surg. 2005; 140: 432– 438;discussion 438–440. [DOI] [PubMed] [Google Scholar]
- 16. Chiu R, Chan L, Lam N, Tsui N, Ng E, Rainer T, Lo YM. Quantitative analysis of circulating mitochondrial DNA in plasma. Clin Chem. 2003; 49 (5): 719– 726. [DOI] [PubMed] [Google Scholar]
- 17. Seaman MA, Levin JR, Serlin RC. New developments in pairwise multiple comparisons: some powerful and practicable procedures. Psychol Bull. 1991; 110: 577– 586. [Google Scholar]
- 18. Margraf S, Lögters T, Reipen J, Altrichter J, Scholz M, Windolf J. Neutrophil-derived circulating free DNA (cf-DNA/NETs): a potential prognostic marker for posttraumatic development of inflammatory second hit and sepsis. Shock. 2008; 30 (4): 352– 358. [DOI] [PubMed] [Google Scholar]
- 19. Lam N, Rainer T, Chiu R, Joynt G, Lo Y. Plasma mitochondrial DNA concentrations after trauma. Clin Chem. 2004; 50 (1): 213– 216. [DOI] [PubMed] [Google Scholar]
- 20. Lam N, Rainer T, Chan L, Joynt G, Lo Y. Time course of early and late changes in plasma DNA in trauma patients. Clin Chem. 2003; 49 (8): 1286– 1291. [DOI] [PubMed] [Google Scholar]
- 21. Sursal T, Stearns-Kurosawa D, Itagaki K, Oh SY, Sun S, Kurosawa S, Hauser C. Plasma bacterial and mitochondrial DNA distinguish bacterial sepsis from sterile systemic inflammatory response syndrome and quantify inflammatory tissue injury in nonhuman primates. Shock. 2013; 39 (1): 55– 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fleischhacker M, Schmidt B. Circulating nucleic acids (CNAs) and cancer—a survey. Biochim Biophys Acta. 2007; 1775: 181– 232. [DOI] [PubMed] [Google Scholar]
- 23. van der Vaart M, Pretorius PJ, Circulating DNA. Its origin and fluctuation. Ann N Y Acad Sci. 2008; 1137: 18– 26. [DOI] [PubMed] [Google Scholar]
- 24. Gaipl US, Sheriff A, Franz S, Munoz LE, Voll RE, Kalden JR, Herrmann M. Inefficient clearance of dying cells and autoreactivity. Curr Topic Microbiol Immunol. 2006; 305: 161– 176. [DOI] [PubMed] [Google Scholar]
- 25. Ballard JW, Whitlock MC. The incomplete natural history of mitochondria. Mol Ecol. 2004; 13: 729– 744. [DOI] [PubMed] [Google Scholar]
- 26. Yousefi S, Mihalache C, Kozlowski E, Schmid I, Simon H. Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death Differ. 2009; 16: 1438– 1444. [DOI] [PubMed] [Google Scholar]
- 27. Yousefi S, Gold J, Andina N, Lee J, Kelly A, Kozlowski E, Schmid I, Straumann A, Reichenbach J, Gleich G, et al. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat Med. 2007; 14 (9): 949– 953. [DOI] [PubMed] [Google Scholar]
- 28. Brinkmann V, Zychlinsky A. Neutrophil extracellular traps: is immunity the second function of chromatin? J Cell Biol. 2012; 198 (5): 773– 783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brinkmann V, Zychlinsky A. Beneficial suicide: why neutrophils die to make NETs. Nat Rev Microbiol. 2007; 5 (8): 577– 582. [DOI] [PubMed] [Google Scholar]
- 30. McIlroy DJ, Jarnicki AG, Au GG, Lott N, Smith DW, Hansbro PM, Balogh ZJ. Mitochondrial DNA neutrophil extracellular traps are formed after trauma and subsequent surgery. J Crit Care. 2014;29:1133.e1–5. [DOI] [PubMed]
- 31. Gu X, Yao Y, Wu G, Lv T, Luo L, Song Y, The plasma mitochondrial DNA is an independent predictor for post-traumatic systemic inflammatory response syndrome. PloS One. 8 (8): e72834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Simmons JD, Lee YL, Mulekar S, Kuck JL, Brevard SB, Gonzalez RP, Gillespie MN, Richards WO. Elevated levels of plasma mitochondrial DNA DAMPs are linked to clinical outcome in severely injured human subjects. Ann Surg. 2013; 258: 591– 596; discussion 596–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Balogh ZJ, McIlroy DJ, Smith DW, Hansbro PM. The origin and the role of mitochondrial DNA in post-injury inflammation. J Crit Care. 2013; 28: 1099– 1100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


