Abstract
With advances in care, increasing numbers of people with hemophilia (PWH) achieve near-normal life expectancies and present with typical age-related cardiovascular conditions. Evidence-based guidelines for medical or surgical management of cardiovascular conditions in individuals with hemophilia are limited. Published recommendations exist for the management of some common cardiovascular conditions (eg, ischemic heart disease, atrial fibrillation), but identifying optimal strategies for anticoagulant or antithrombotic therapy constitutes the primary challenge of managing nonoperative cardiovascular disease (CVD) in PWH. In general, as long as factor concentrates or other hemostatic therapies maintain adequate hemostasis, the recommended medical and surgical management of CVD in PWH parallels that in individuals without hemophilia. The presence of factor inhibitors complicates hemophilia management. Published outcomes of CVD treatment in PWH are similar to those in the general population. Specific knowledge about factor replacement, factor inhibitors, and disease-specific treatment distinguishes the cardiovascular care of PWH from similar care of individuals without this rare bleeding disorder. Furthermore, a multidisciplinary approach incorporating a hematologist with an onsite coagulation laboratory, ideally associated with a hemophilia treatment center, is integral to the management of CVD in PWH.
Keywords: hemophilia, cardiovascular diseases, cardiac surgery, atherosclerosis, atrial fibrillation
Hemophilia A and B (hereafter collectively referred to as “hemophilia”) encompass congenital deficiencies of the intrinsic pathway coagulation factors VIII (FVIII) and IX (FIX), respectively, with a variable risk for bleeding based on the type of hemophilia and the extent of factor deficiency. This risk for bleeding may complicate both medical and surgical management of congenital and acquired cardiovascular conditions in people with hemophilia (PWH), particularly those who are receiving anticoagulant or antithrombotic treatment or who require invasive measures for palliation or correction of a cardiovascular lesion.
PWH who receive repeated doses of factor as replacement may develop coagulation factor inhibitors that complicate the management of cardiovascular disease (CVD). Limited experience and a lack of evidence-based guidelines pose further challenges in the management of cardiovascular conditions in this population. As PWH are now achieving near-normal life expectancies due to advances in the management of their underlying disease, health care providers expect an increasing number of PWH presenting with typical cardiovascular conditions of the aging population.
This article examines the epidemiology and etiology of acquired CVD in PWH; summarizes the management of hemophilia, including hemostatic therapeutic options; and reviews the existing evidence and recommendations for managing various nonoperative and operative cardiovascular conditions in this unique population. Because of the specialized care required by these individuals, a multidisciplinary group of authors contributed to this work and provided a consensus set of recommendations for treating cardiovascular conditions in PWH. Other rare nonhemophilia congenital bleeding disorders lack data regarding CVD and are not considered in this consensus statement.
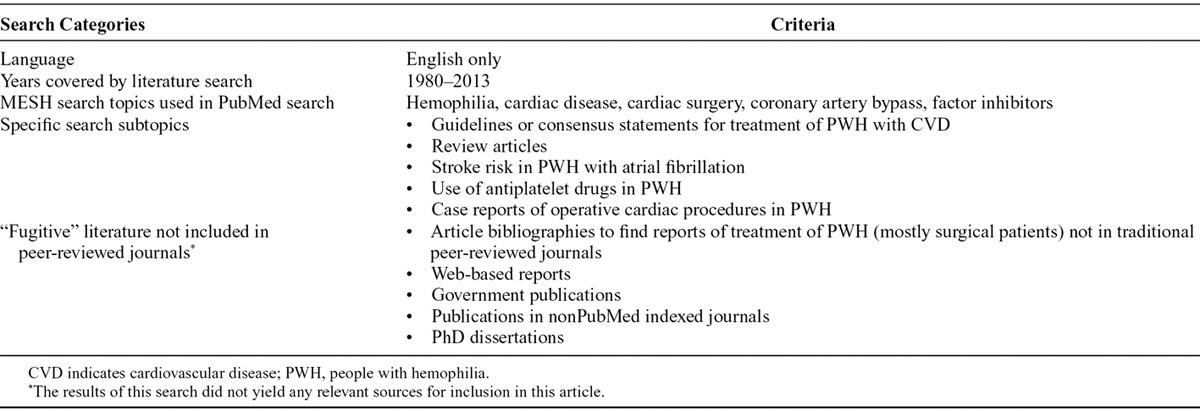
We performed a search of multiple sources to identify articles describing diagnoses and management of CVD in PWH. Table 1 lists the search categories and criteria used.
Table 1.
Search Categories and Criteria Used to Obtain Relevant Evidence About Cardiovascular Disease in People With Hemophilia
UNDERLYING DISEASE COURSE, LIFE EXPECTANCY, AND AGE-RELATED CARDIOVASCULAR DISEASE IN HEMOPHILIA
The clinical severity and bleeding risk in PWH depends on factor levels. Mild hemophilia is commonly defined by FVIII or FIX activity levels of greater than 5% (>0.05 IU/ml), constitutes 30–40% of hemophilia cases, and typically presents with bleeding episodes after hematologic stress (ie, surgery, vaginal delivery, or trauma).1 Moderate hemophilia is defined by FVIII or FIX activity levels between 1% and 5% (0.01–0.05 IU/ml), occurs in 10% of PWH, and presents with spontaneous bleeds or bleeding after operation or trauma.1 Severe hemophilia is characterized by FVIII or FIX activity levels of less than 1% (<0.01 IU/ml), occurs in 50% of PWH, and presents with spontaneous bleeding into joints and muscles and life-threatening (eg, intracranial) hemorrhage.1 Although the majority of cases of hemophilia A and B are inherited (X-linked recessive), about one third of PWH who are newly diagnosed have spontaneous mutations without any family history of bleeding.2
Complications may develop because of factor replacement therapy in PWH. Importantly, up to one third of individuals with severe hemophilia A develop alloantibody inhibitors to FVIII after replacement therapy.3 The incidence of FIX inhibitor development is much lower in individuals with hemophilia B (1–6%).3 In hemophilia A, formation of FVIII alloantibodies is highest in individuals with certain gene mutations, specifically intron 22 inversions, large deletions, and nonsense mutations.4 PWH transfused with plasma-derived factor replacement products risk development of transfusion-related viral infections, including human immunodeficiency virus (HIV) and hepatitis C virus (HCV). As of 2002, the first Multicenter Hemophilia Cohort Study found that concomitant HIV infection was present in more than half of HCV-seropositive PWH included in the study.5 However, adoption of viral inactivation methods beginning in the mid-1980s substantially reduced the risk of HIV and HCV transmission via plasma-derived FVIII and FIX concentrates.5
Due to advances in care, including comprehensive management in hemophilia treatment centers (HTCs), primary and secondary prophylaxis, and improvements in factor replacement therapies, PWH achieve near-normal life expectancies. Since the 1990s, life expectancies in high-income countries exceed 70 years in HIV-negative men with hemophilia.6,7 PWH with severe disease (very low FVIII or FIX levels), including those with HIV specifically, achieve lower life expectancies by approximately 10 years.6 PWH with factor inhibitors previously had a reduced life expectancy; however, an analysis from the 1990s showed that the presence of inhibitors did not increase mortality rates in individuals with severe hemophilia with or without HIV.8 An analysis of the Nationwide Inpatient Sample from 2007 found that the median age at death of hospitalized PWH in the United States (US) was 68 years, compared to 72 years in hospitalized individuals without hemophilia.9 Leading causes of death in PWH included typical age-related conditions like sepsis (38%), congestive heart failure (30%), respiratory failure (28%), and pneumonia (25%), rather than intracranial hemorrhage or HIV (16%).9 As the population of PWH ages, health care providers face increasing numbers of PWH presenting with typical age-related comorbidities, including ischemic heart disease (IHD), other atherosclerotic conditions, and degenerative valve disease.
The literature supporting any protective effect of hemophilia against IHD is conflicting. Some studies suggest that mortality from IHD is lower in PWH than in the general population,7,10,11 presumably due to a “hypocoagulable” state that prevents thrombus formation and coronary arterial occlusion after rupture of an atherosclerotic plaque.12,13 Others suggest that increased levels of various coagulation proteins, including FVIII and FIX, predispose affected individuals to atherogenesis14,15; however, reduced FVIII and FIX levels in PWH do not seem to ultimately protect against the development of atherosclerosis.13 In fact, current consensus warns that the prevalence of atherogenesis14–17 and endothelial dysfunction14 is similar between PWH and the general population. The extent of these abnormalities correlates with traditional cardiovascular risk factors rather than hemophilia severity.14,16–18 When compared with a cohort of hospitalized individuals without hemophilia, hospitalized PWH had similar rates of angina, myocardial infarction (MI), and cardiovascular risk factors such as hypertension, diabetes mellitus (DM), obesity, and hyperlipidemia.19
Although the risk of coronary artery thrombus formation may be low in PWH, a cautionary note exists. Other events such as plaque rupture with atheroemboli, coronary vasospasm, or intraplaque hemorrhage may lead to coronary events in PWH with coronary atherosclerosis.16 Isolated case reports suggest that supernormal FVIII infusions, like those given for factor replacement in PWH before an invasive procedure, can induce coronary thrombosis,20–22 as may other hemostatic therapies.21,23 The presence of other underlying cardiovascular risk factors may contribute to such events.20 PWH having procedures that typically employ anticoagulation such as cardiac operations or percutaneous coronary interventions (PCIs), require special considerations. Older patients with milder forms of hemophilia (mild or moderate factor level deficiencies) may be at particular thrombotic risk from factor replacement. These individuals require a delicate balance between anticoagulation or antiplatelet drugs and factor replacement.
PWH are susceptible to the same cardiovascular risk factors as individuals without hemophilia, and even more so to certain traditional risk factors such as hypertension15,24–26 and obesity related to limited mobility from arthropathy.18,27 In a cohort of more than 700 PWH from the Netherlands and United Kingdom, the predicted 10-year risk of fatal MI or stroke was significantly higher (8.9%) than in the general population (6.7%), based solely on typical factors determining cardiovascular risk (including age, blood pressure, total/high-density lipoprotein cholesterol, body mass index, and smoking or DM history).28 A recent 5-year cross-sectional study of PWH older than age 35 in the US found that PWH had twice the lifetime prevalence of coronary artery disease (CAD), stroke, and MI compared to nonHispanic white men overall.29 Nearly 40% of PWH in this study had 2 or more traditional risk factors for CVD.29 In a review of 36 cases of confirmed MI in PWH, 11 PWH had 1 or more acquired risk factors for CVD, including smoking, obesity, hypertension, and immobility.21 Systemic hemostatic therapies contributed to mortality in more than half (n = 22) of the PWH with MI.21 Other unique risk factors predisposing to IHD in PWH include type and severity of hemophilia (with hemophilia B and mild disease being more likely among PWH with IHD in 1 cohort),18 dyslipidemia, DM, and in PWH with HIV, hypertension from antiretroviral treatments.30
As PWH achieve longer life expectancies, their exposure to various cardiovascular risk factors increases. As a result, it is reasonable to expect greater numbers of PWH presenting with atherosclerotic disease in the future. Existing data already show a trend toward an increasing prevalence of IHD among PWH with increasing age, similar to the general population. Among more than 3000 PWH from the US in the late 1990s, the prevalence of IHD ranged from 0.05% in those younger than 30 years to 15.2% in those older than 60 years.18 Similarly, there will likely be an increase in PWH presenting with other atherosclerotic conditions such as carotid occlusive disease and peripheral arterial disease (PAD), and other age-related cardiovascular conditions. In addition, PWH are susceptible to other acquired cardiovascular conditions, regardless of age: for example, PWH with late-stage HIV infection may be susceptible to acquired dilated cardiomyopathy.31
STANDARD HEMOPHILIA MANAGEMENT
Familiarity with the general management of hemophilia, particularly the options for treatment or prevention of bleeding, is an essential starting point for understanding the particular aspects of treating PWH who also have CVD. Replacement of the deficient factor using specific factor concentrates is a mainstay of therapy, especially in PWH with active bleeding or in those requiring operative interventions. Treatment for bleeding episodes consists of both on-demand regimens (ie, at onset of discrete bleeding episodes) and prophylactic regimens. In the latter, replacement factor is given on a scheduled basis with the intent of preventing bleeding, especially hemarthroses, a particularly disabling bleeding-related complication in PWH.32 Administration of factor concentrates before, during, and after surgical or other invasive procedures limits bleeding, with the administered amounts of factor concentrates depending on hemophilia type and severity and the risk for bleeding associated with the procedure. Nonspecific blood products such as fresh frozen plasma (FFP) or cryoprecipitate contain relatively small amounts of hemophilia factors and are not viral inactivated.33 As a result, administration of these nonspecific products risks disease transmission and may prove insufficient to control or prevent bleeding in many PWH who have severe bleeding episodes.
Hemophilia Factor Replacement
Options for factor replacement include plasma-derived and recombinant products (Table 2).33,34 The screening of blood donors and harvested blood products along with adoption of viral inactivation methods has dramatically improved the safety of human plasma-derived factor concentrates.35 Specifically, there have been no reports of HIV or HCV transmission from clotting factor concentrates since 1986 and 1997, respectively.36 Still, concerns remain regarding the transmission of other pathogens, including nonenveloped viruses such as the parvoviruses and hepatitis A, or prions (variant Creutzfeldt-Jakob disease).35,37,38
Table 2.
Therapeutic Options for Treatment or Prevention of Bleeding in Hemophilia
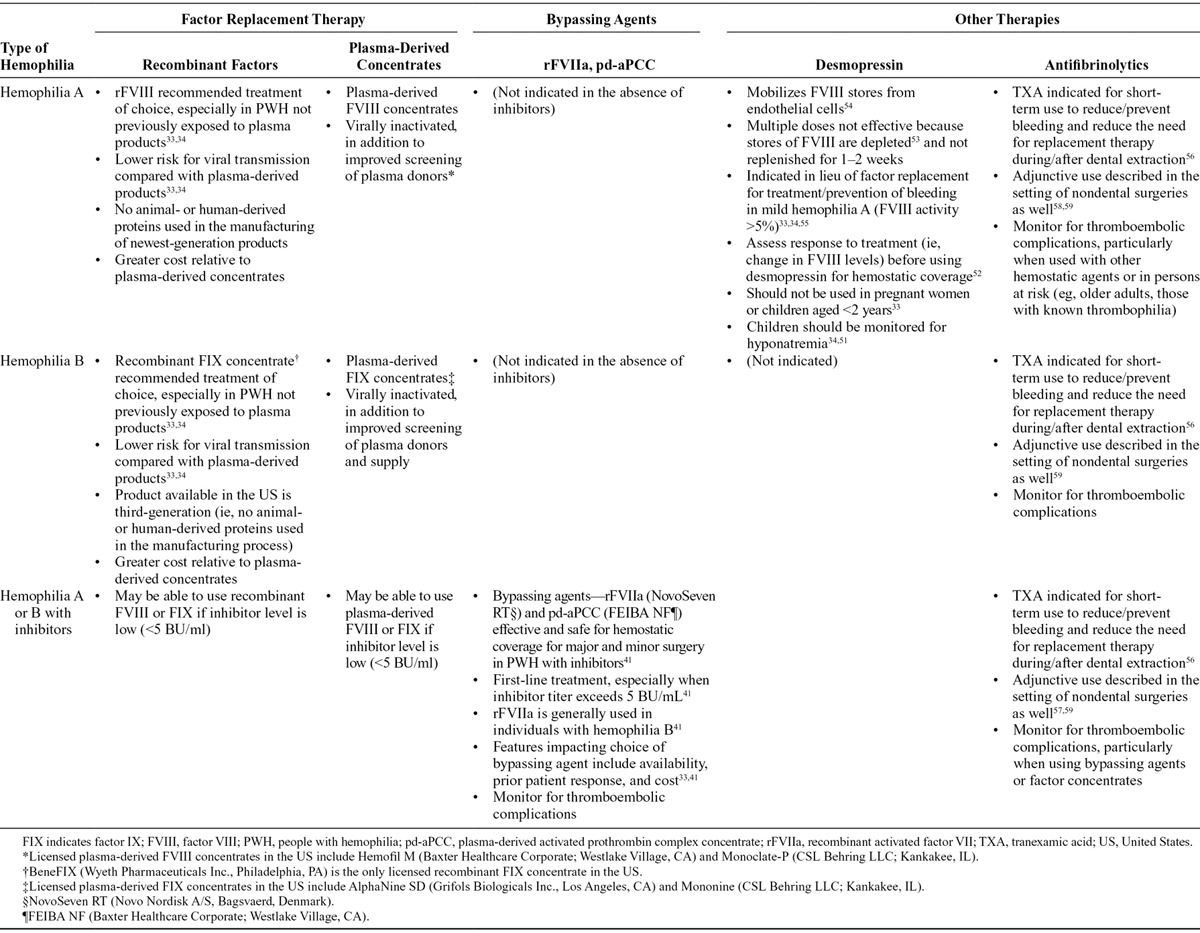
Recombinant products developed in the wake of the 1980s HIV epidemic significantly reduce the risk of transmitting blood-borne pathogens.33 The newest generation of recombinant products lack any human proteins in any of the production processes.33 Accordingly, the Medical Advisory and Scientific Council of the National Hemophilia Foundation recommends recombinant factor concentrates as the treatment of choice for bleeding control and for surgical coverage in individuals with hemophilia A or B.33 Consultation with a hematologist facilitates the choice of replacement product. Ultimately, the choice depends on several variables in addition to the potential for viral transmission, including availability, cost, and prior product exposure.35
Replacement factor dosing requires achievement of a prespecified percentage of normal factor activity that varies based on the specific indication (eg, hemostatic coverage for minor vs major surgery). For example, immediately before major operations, correction of factor activity level to 80–100% of normal is preferred to optimize hemostasis.39 Individuals with mild hemophilia (ie, with factor activity >5% of normal) require lesser amounts of factor concentrate than do those with severe hemophilia (ie, with factor activity level <1% of normal) and in some cases may not require any factor correction at all.
Complications of Factor Replacement
Currently, the most serious and costly complication of factor replacement therapy is the development of alloantibodies, or inhibitors, to the infused factor.40 Inhibitors develop in approximately 20–30% of patients with severe hemophilia A and in 1–6% of those with severe hemophilia B.3 Individuals with mild hemophilia typically have a low risk for developing inhibitors, but they are susceptible to developing inhibitors after receiving large amounts of factor concentrates for a surgical procedure, especially if they have had no previous exposure to exogenous factor replacement.6 The presence of inhibitors typically makes factor replacement ineffective, complicating the management of bleeding events and coverage for surgical or other invasive procedures.
In PWH who develop inhibitors, bypassing agents are generally the first-line systemic therapy for hemostatic coverage, particularly for high-titer (>5 Bethesda units/mL) inhibitors.41 These agents bypass the role of the inhibited factor in the coagulation cascade (Table 2). The bypassing agents consist of both recombinant activated factor VII {rFVIIa; NovoSeven RT [Coagulation Factor VIIa (Recombinant)], Novo Nordisk Inc., Bagsvaerd, Denmark} and a viral-inactivated, plasma-derived activated prothrombin complex concentrate (pd-aPCC) containing factors II, IX, and X in mostly nonactivated forms and factor VII mainly in the activated form [FEIBA NF (AntiInhibitor Coagulant Complex), Nanofiltered and Vapor Heated, Baxter Healthcare Corporation, Westlake Village, CA].
Concerns with the use of bypassing agents in the management of PWH with inhibitors include the lack of laboratory testing to predict efficacy42 and a risk of thrombotic events. Case reports describe MI and disseminated intravascular coagulation in individuals with concurrent liver disease, those of advanced age, and those receiving higher-than-recommended amounts of pd-aPCC.43–45 However, in a postmarketing survey of the use of FEIBA (pd-aPCC) in individuals with inhibitors, the incidence of thrombosis over 10 years was very low, with only 16 events occurring in the equivalent of 395,000 infusions.46 Likewise, the incidence of thromboembolic events that were possibly or probably attributable to rFVIIa in clinical studies within approved indications for PWH with inhibitors was 0.20%.47
Although not relevant to the hemophilia population discussed here, the off-label use of rFVIIa outside of the approved indications is associated with higher rates of thromboembolic events than in placebo controlled trials.48,49 Because pd-aPCC is used virtually exclusively in PWH with inhibitors,50 comparable data regarding the risk of thrombotic events with off-label use of pd-aPCC are not available. Ultimately, in PWH with inhibitors who require hemostatic coverage, the risk for thromboembolism must be weighed against the risk for bleeding on an individualized basis.
Nonfactor Replacement Therapies for People With Hemophilia
In some instances, administration of nonfactor replacement therapies33,34,51–59 occurs in lieu of, or in addition to, factor replacement products for surgical coverage or for management of perioperative bleeding (Table 2). Such therapies avoid the potential risks and costs associated with factor replacement.60 PWH may benefit from either of 2 types of nonfactor replacement therapy: desmopressin or antifibrinolytic agents. Desmopressin is effective in mild hemophilia A and usually allows for avoidance of FVIII concentrates.60 Desmopressin reduces blood loss61,62 and red blood cell transfusion requirements62 in select patients without bleeding disorders who experience bleeding after open-heart procedures. The antifibrinolytics, tranexamic acid and epsilon-aminocaproic acid, act as adjuncts in PWH who require operations. Both desmopressin60 and antifibrinolytics21 may predispose PWH to myocardial ischemia, so, like factor replacement therapies, they should be used with caution in PWH with potential IHD. This includes those individuals with traditional cardiovascular risk factors.54 Desmopressin may be particularly thrombogenic in individuals with underlying CAD as it induces release of both FVIII and von Willebrand factor from endothelial cell granules.54
GENERAL PRINCIPLES IN THE MANAGEMENT OF CARDIOVASCULAR DISEASE IN PEOPLE WITH HEMOPHILIA
The management of common cardiovascular conditions poses unique challenges in PWH, particularly in individuals requiring anticoagulant and antiplatelet therapy, or in those requiring invasive procedures. The presence of factor inhibitors further complicates management. Because of the lack of evidence-based guidelines,63 recommendations for the management of CVD in PWH are based on expert opinion, or on anecdotal experience from both cardiac and noncardiac procedures, mostly in patients without inhibitors. Given the relatively limited experience with managing CVD in this population, consultation with a hematologist who has experience in hemophilia management is paramount, particularly for assistance with navigating the fine balance between antithrombotic and hemostatic therapy required for many of these conditions. Furthermore, invasive cardiac procedures in PWH—particularly those with inhibitors—pose an extremely high-risk situation and require infrastructure and resources specifically aimed at treating PWH. The optimal setting for such procedures is a facility designated as an HTC, where all necessary ancillary services (eg, laboratory, blood bank, pharmacy), full-spectrum resources (eg, factor concentrates and other hemostatic therapies), and medical and surgical expertise are available.
NONOPERATIVE CARDIOVASCULAR CONDITIONS
Atrial Fibrillation and Other Cardiac Dysrhythmias
Nonvalvular atrial fibrillation (AF) is a common age-related cardiovascular condition that often requires antithrombotic therapy to prevent associated thromboembolic events, particularly stroke. The decision to initiate antithrombotic therapy is normally based on the projected risk for stroke as determined by a risk scoring system: either the CHADS2 score63 or the increasingly used CHA2DS2-VASc score.68,69 The acronyms reflect risk factors for thromboembolic complications (congestive heart failure, hypertension, age ≥75 years, DM, stroke, vascular disease history) and their relative weight in the calculation of the score (ie, 2 points given for prior stroke or transient ischemic attack).70 The CHA2DS2-VASc modification gives an additional point for female sex, 1 point for age of 65–74, and 2 points for age ≥75 and provides better stratification of risk. Neither scoring system has been studied extensively in PWH.
PWH who develop AF are not immune to thromboembolic complications; therefore, in high-risk cases, antithrombotic prophylaxis may be warranted, provided sufficient baseline factor levels are assured. There are no clinical trial data to support recommendations for antithrombotic therapy in PWH; therefore, therapy should be individualized, taking the comparative risks for bleeding versus thromboembolic complications into account. Whereas, in the general population, antithrombotic therapy is recommended for individuals at intermediate risk for stroke (CHADS2 or CHA2DS2-VASc score = 1),71 a higher threshold (CHADS2 ≥2) is proposed for initiating antithrombotic therapy in PWH.63 Mannucci et al provided an algorithm for managing antithrombotic therapy in PWH with AF (Fig. 1).63 However, several important changes have occurred in the options and recommendations for antithrombotic therapy for AF in the general population in the interim, including the recognition that aspirin alone does not sufficiently protect against thromboembolism in AF71 and the introduction of left atrial (LA) appendage occlusion devices.
Figure 1.
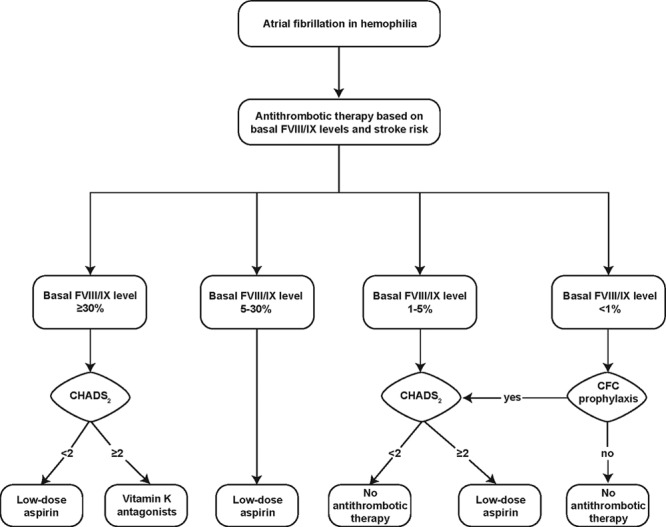
Recommended algorithm for management of atrial fibrillation in people with hemophilia without inhibitors.63 The primary determinant of management of AF in PWH is the baseline FVIII/FIX level (ie, hemophilia severity). People with severe hemophilia are considered candidates for thromboprophylaxis provided they are receiving factor concentrate prophylaxis. The decision to initiate antithrombotic therapy is further based on the individual projected risk for stroke, in this case determined by the CHADS2 scoring system. A score of ≥2 is considered indicative of a high risk for stroke. (See text for a description of the CHADS2 score calculation.) CFC indicates clotting factor concentrate; CHADS2, congestive heart failure, hypertension, age, diabetes mellitus, stroke; FVIII, factor VIII; FIX, factor IX; PWH, people with hemophilia. Republished with permission of Blood: Journal of the American Society of Hematology, from “How I treat age-related morbidities in elderly persons with hemophilia,” Mannucci, PM, et al.,114, 26 © 2009; permission conveyed through Copyright Clearance Center, Inc.
Pharmacologic options for antithrombotic therapy in PWH at lower risk for bleeding (ie, those with baseline factor levels >5% and those with severe hemophilia who are on clotting factor prophylaxis) include oral anticoagulants and antiplatelet agents (Table 3). Oral anticoagulant options include the vitamin K antagonist warfarin, which should be titrated to achieve an INR of 2.0–3.0, and the new oral anticoagulants, such as direct thrombin or activated factor X inhibitors.71 New oral anticoagulants offer the benefits of shorter half-lives and lower bleeding risk than warfarin but have no specific antidotes,72,73 and evidence of their use in PWH with AF is lacking. Oral anticoagulants offer better protection against stroke in AF than do antiplatelet agents, with a comparable risk for bleeding complications.71 In cases in which antiplatelet therapy is used, dual therapy incorporating both aspirin and clopidogrel is advised (Table 3), because the reduction in stroke risk imparted by aspirin alone is only moderate at best.71
Table 3.
Management Suggestions for Nonoperative Cardiovascular Disease in People With Hemophilia*
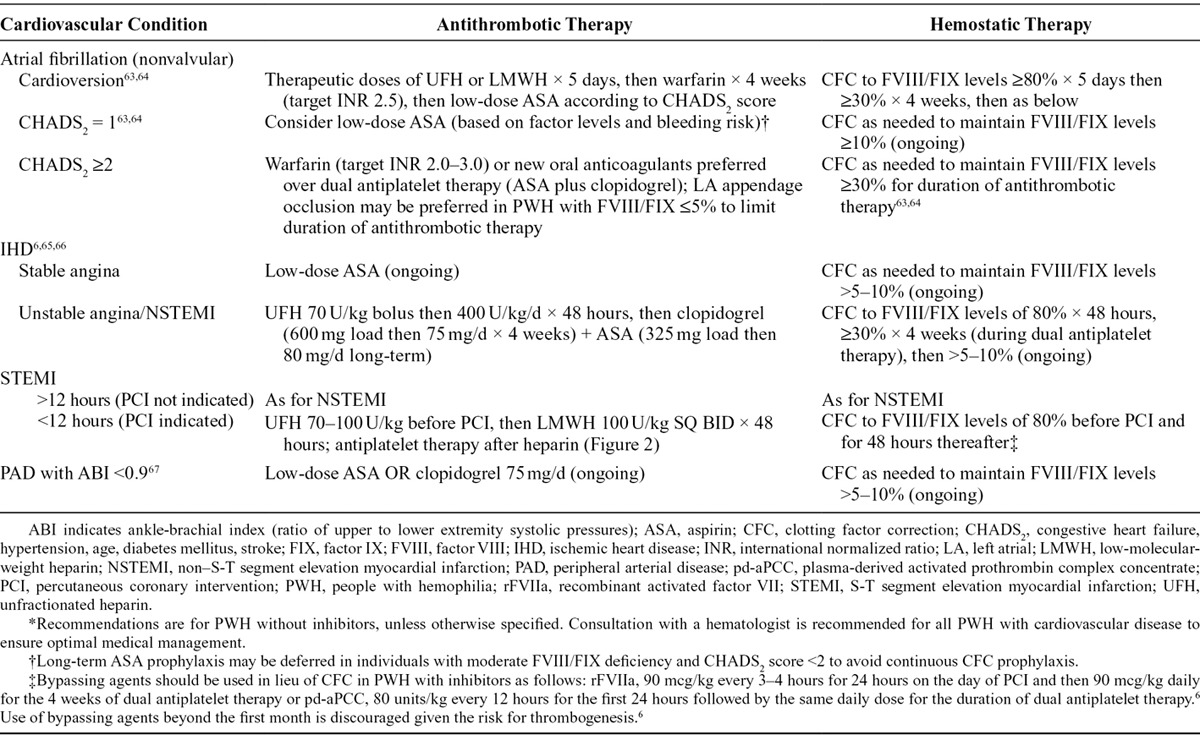
Mechanical interventions such as LA appendage occlusion or exclusion may alternatively be used to limit the duration of antithrombotic therapy in PWH with AF (Table 3). LA appendage occlusion devices reduce long-term risk of thromboembolism in AF, albeit with a trade-off of potential periprocedural complications. LA appendage occlusion may become the preferred strategy in PWH, especially those with factor levels ≤5%. This procedure has been described in a man with mild hemophilia A (FVIII level of 8%), allowing for a reduction of antithrombotic pharmacotherapy to a total of 6 weeks.74 Implantation of a LA appendage occlusion device should be done at an experienced center with a low complication rate. Antithrombotic prophylaxis is not recommended in PWH with inhibitors and AF given the heightened risk for bleeding and the difficulty in treating any bleeding episodes that might occur in this subpopulation of PWH.63
If cardioversion is elected, no antithrombotic therapy is needed before or after the procedure for AF of less than 48 hours duration.63 In PWH presenting with AF of longer duration, a transesophageal echocardiogram performed before cardioversion identifies periprocedural stroke risk related to the presence or absence of an LA thrombus. The absence of an LA thrombus permits avoidance of anticoagulation (and the attendant bleeding risk) in PWH before cardioversion.75 Cardioversion of AF requires anticoagulation with therapeutic doses of unfractionated heparin (UFH) or low-molecular-weight heparin (LMWH) during the procedure and for a period of 5 days thereafter (Table 3).63 PWH without inhibitors undergoing cardioversion simultaneously require factor replacement with either recombinant or plasma-derived FVIII or FIX, maintaining a trough factor activity level of around 80%.63 After discontinuation of periprocedural anticoagulation with UFH or LMWH, an additional 4 weeks of anticoagulation with a vitamin K antagonist is recommended, targeting an INR of 2.5.63 During this time, PWH should receive factor concentrates to maintain trough factor levels of 30%.63 Upon completion of this 4-week period of anticoagulation, PWH who remain in sinus rhythm convert to antithrombotic therapy based on basal factor level activity and stroke risk as outlined in Figure 1.63 No specific recommendations exist for the management of AF in PWH with inhibitors. Because of the use of anticoagulants and bypassing agents for bleeding prophylaxis, respectively, PWH with inhibitors who require anticoagulation for AF are at increased risk for both bleeding and thrombosis.
There is little in the literature pertaining to the evaluation or management of other dysrhythmias in PWH. Reports describe a child with severe hemophilia A who underwent cryoablation for atrioventricular nodal reentrant tachycardia76 and a 73-year-old man with severe hemophilia A who had uncomplicated diagnostic electrophysiologic studies.77 In these 2 patients, factor levels corrected to 80–100% of normal provided adequate hemostasis.
Coronary Artery and Ischemic Heart Disease
As in the general population, efforts in PWH focus on prevention of CAD when possible. Such efforts include screening adults with hemophilia for traditional cardiovascular risk factors after the age of 40 years,28 especially for hypertension and for obesity in PWH with arthropathy. Data suggest that screening and intervention for these traditional risk factors are currently suboptimal in PWH.26 Aggressive management of individual cardiovascular risk factors with appropriate medications and lifestyle modifications may allow for deferment of invasive interventions and antiplatelet medications in PWH with symptomatic CAD, especially those with inhibitors.78 Whether the benefits of low-dose aspirin prophylaxis for cardiovascular events outweigh the potential risk for bleeding in PWH is uncertain. Because of the bleeding risk, prophylactic low-dose aspirin therapy in PWH is currently not recommended as a rule.26 Chest pain syndromes may indicate myocardial ischemia in PWH, especially in those PWH with cardiovascular risk factors or when pain occurs after administration of factor replacement or bypassing agents.79
Evidence-based guidelines for the treatment of IHD in PWH are lacking, but published recommendations are available, including institutional guidelines from the Netherlands.65 In general, the recommended management of IHD in PWH parallels that in people without hemophilia and reflects the primary clinical manifestation of IHD [ie, stable angina vs an acute coronary syndrome (ACS)], with factor replacement as needed to maintain adequate factor levels (Fig. 2, Table 3).6,63,65,66 Specific recommendations for PWH with inhibitors do not exist, and published anecdotal experience with the management of IHD in this population is extremely limited.
Figure 2.
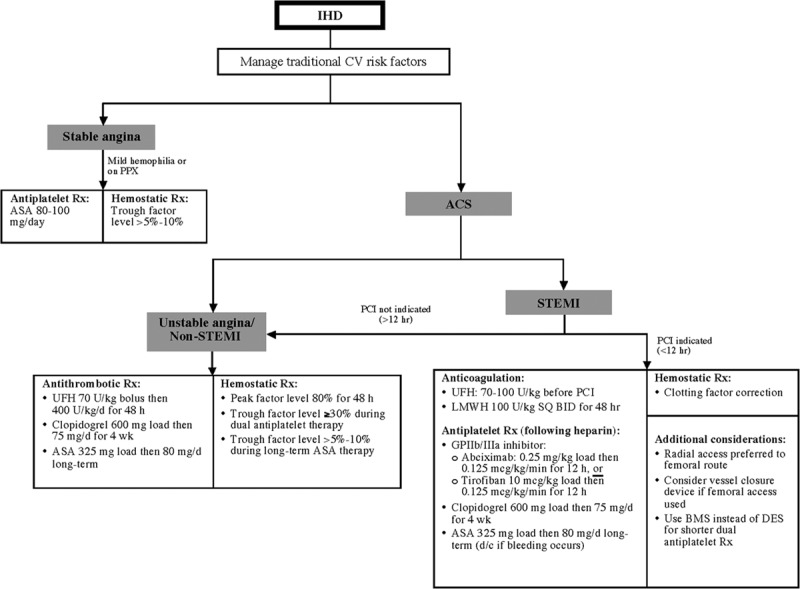
Suggested algorithm for management of ischemic heart disease in people with hemophilia without inhibitors.6,65,66 ACS indicates acute coronary syndrome; ASA, aspirin; BMS, bare metal stent; CV, cardiovascular; d/c, discontinue; DES, drug-eluting stent; GP, glycoprotein; IHD, ischemic heart disease; LMWH, low-molecular-weight heparin; PCI, percutaneous coronary intervention; PPX, prophylaxis; Rx, therapy; STEMI, S-T segment elevation myocardial infarction; UFH, unfractionated heparin.
In all forms of IHD, antiplatelet or antithrombotic pharmacotherapy is a mainstay of treatment. In PWH, the provision of antiplatelet or antithrombotic therapy requires concomitant maintenance of minimum factor levels to reduce bleeding risk (Fig. 2, Table 3). Some PWH (especially with mild hemophilia) achieve recommended trough factor levels on their own without ongoing factor correction.80 In PWH with moderate or severe disease who require long-term aspirin therapy, 1 source recommends concomitant factor replacement initially, followed by bolus or continuous factor infusion as needed for bleeding complications thereafter.81 Managing traditional cardiovascular risk factors is particularly important in PWH with severe disease who cannot receive antiplatelet agents,82 including those who are not maintained on factor prophylaxis.6,65
Recent articles summarize the “real-life” management and outcomes of ACS in PWH, including those managed with PCIs.80,83 PCI is primarily indicated in cases of S-T segment elevation myocardial infarction (STEMI) presenting within 12 hours (Fig. 2) and in certain high-risk non-STEMI patients.6,65 Prospective evaluation of the previously mentioned Dutch guidelines support the recommendations for PCI for STEMI,66 albeit in a small number of PWH, none of whom had severe hemophilia or inhibitors at the time of their procedures. There are several important considerations for performing PCI in PWH. Radial artery access is optimal in lieu of femoral access, especially in PWH with inhibitors.65,66,82,83 A recent study found that unselected individuals who underwent PCI for acute MI via the radial route had significantly lower bleeding rates, vascular complications, and mortality at 2 years compared with those who underwent PCI via the femoral route.84 However, many publications document catheterization via the femoral route in PWH with negligible complications.83,85 In cases where femoral access is used, a vessel closure device may reduce local bleeding complications.86
In PWH who require stenting, a bare metal stent (BMS) facilitates a shorter duration of dual antiplatelet therapy (typically 1 month) compared with a drug-eluting stent (DES, up to 12 months).63,65 A novel BMS under clinical development designed to enhance endothelialization by “recruitment” of endothelial progenitor cells [the Genous-R stent (OrbusNeich Medical Technologies, Fort Lauderdale, FL)] may allow an even shorter (ie, approximately 2 weeks) duration of dual antiplatelet therapy87 in PWH.88 Although the risk for restenosis in PWH is not known,65 expert consensus suggests that the risk of prolonged dual antiplatelet therapy outweighs any benefit that a DES offers in terms of a reduced risk for restenosis.63 Because of the limited ability of DESs to reduce mortality or reduce recurrent MI compared with BMSs, consensus recommendations suggest that only special circumstances like symptomatic restenosis or high risk for restenosis justify the use of DESs in PWH.83 PWH with mild disease (specifically, factor levels exceeding 25%) are good candidates for DES in ACSs, as they may not require factor replacement for the duration of dual antiplatelet therapy.66 Newer-generation DESs may permit shorter (ie, <6-month) periods of dual antiplatelet therapy66,85 but experience in PWH is nonexistent.
In general, the regimen for antithrombotic therapy during and after PCI mirrors that recommended for PWH presenting with other ACSs; however, PWH undergoing PCI should additionally receive glycoprotein IIb/IIIa inhibitors in the 12 hours after PCI (Fig. 2).6,65 The short-acting direct thrombin inhibitor bivalirudin was used in lieu of UFH during successful PCI in 4 PWH described in the literature; 3 of these individuals had severe hemophilia.83,89–91
Pretreatment with antiplatelet agents is recommended in the general population before PCI.92 In contrast, consensus suggests deferring antiplatelet therapy in PWH before possible PCI to limit bleeding events,93 especially in the minority who may require urgent operative coronary revascularization. This recommendation is somewhat controversial because withholding antiplatelet therapy in planned PCI risks the development of thrombi during PCI in PWH, especially when factor replacement is administered.93 To avoid the burden and costs related to ongoing clotting factor correction, the recommended duration of dual antiplatelet therapy in PWH is 1 month.66 This recommendation coincides with general guidelines for the minimal duration of dual antiplatelet therapy after placement of a BMS, the preferred stent for PWH with significant symptomatic coronary obstruction.66
After PCI in PWH, the recommendation is for near-complete [80%6 (Fig. 2) or 40–60%83] correction of clotting factor levels for at least 48 hours. This consensus includes factor correction to levels of at least 80% for the first 48 hours followed by daily dosing to a trough level of 30% for the duration of dual antiplatelet therapy.6 In PWH with inhibitors receiving prolonged antiplatelet therapy, continuous prophylaxis with bypassing agents beyond the first month after PCI risks thrombosis and is not recommended.6
Because of the extreme bleeding risk, the use of fibrinolytic therapy in lieu of PCI is contraindicated; however, a recent panel of experts from Europe concluded that fibrinolysis may be justified in PWH when primary PCI is unavailable, provided there is adequate factor correction (minimum of 50% and peak ≥80%) and the capacity for serial measurement of factor levels.94 When there is 3-vessel CAD or stenosis of the left main coronary artery, surgical coronary artery bypass grafting (CABG) is indicated.6 In some cases, multivessel PCI provides an alternative in PWH who are deemed too high risk for CABG.89
Other Atherosclerotic Conditions
PWH are susceptible to other atherosclerotic conditions in addition to IHD, although there is very little in the literature describing the presentation and management of additional conditions such as carotid occlusive disease or PAD. In one study, carotid intima media thickness increased significantly in PWH concurrent with the calculated 10-year cardiovascular risk, based on traditional cardiovascular risk factors.17 There are currently no descriptions of the medical management of carotid occlusive disease in PWH in the literature. Surgical management is discussed in the next section (“Cardiovascular Conditions Requiring Operations in PWH”).
PWH with cardiovascular risk factors are likely susceptible to PAD as well, encompassing atherosclerosis of the aorta and iliac and lower extremity arteries. A single study from 2000 found that, compared with age- and risk-matched controls, PWH had a significantly lower number of atherosclerotic plaques in the abdominal aorta and leg arteries as detected by color echo Doppler.95 Nevertheless, similar to IHD, the prevalence of PAD in PWH may be higher than previously believed, because risk factors for the development of PAD are similar to those for CAD. In addition, some evidence suggests that PAD is a marker of other atherosclerosis-related morbidities and mortality.67 PAD may go unrecognized in PWH for a number of reasons. PWH with severe hemophilic arthropathy may not be sufficiently mobile to manifest claudication, the classic presenting symptom of PAD.67 Claudication-related pain may also be erroneously attributed to arthropathy or may be masked by analgesics used to treat this condition.67 Therefore, PAD is a consideration in PWH with severe arthropathy and cardiovascular risk factors who present with atypical pain or worsening dysfunction of the lower extremities.67,96 In addition, PWH should undergo screening for PAD in a manner similar to the general population.96 Screening for PAD is particularly important in PWH over age 70 and in those under age 50 with a history of smoking or DM.
The ankle-brachial index (ABI), a ratio of upper to lower extremity systolic pressures, assesses the risk of PAD. In PWH with significant joint deformity precluding ABI measurement, a toe-brachial index or duplex ultrasonography are useful options for screening.67 In individuals with an ABI less than 0.9, measures to reduce overall cardiovascular risk should be implemented.67 To reduce the risk for peripheral ischemic events, antiplatelet therapy is typically employed, along with factor replacement as needed to maintain trough FVIII/FIX levels of more than 5% (Table 3).67 However, data supporting the use of antiplatelet therapy in PWH with PAD are limited.67 Individuals with mild hemophilia may tolerate low-dose aspirin without bleeding.67 In contrast, those with moderate or severe hemophilia may require factor correction to avoid bleeding with the use of antiplatelet therapy.67 The use of prophylactic antiplatelet therapy calls for an individualized management approach in consultation with a hematologist. Although pentoxifylline or cilostazol is generally used to manage claudication, the benefits and risk of bleeding attributable to these agents in PWH are unknown; therefore, their use should be discouraged until more information is available.67 Individuals with limb-threatening ischemia (ie, ischemic pain at rest, ischemic ulcers, or gangrene) or with claudication that interferes with quality of life despite pharmacologic treatment are candidates for surgical intervention.
CARDIOVASCULAR CONDITIONS REQUIRING OPERATIONS IN PEOPLE WITH HEMOPHILIA
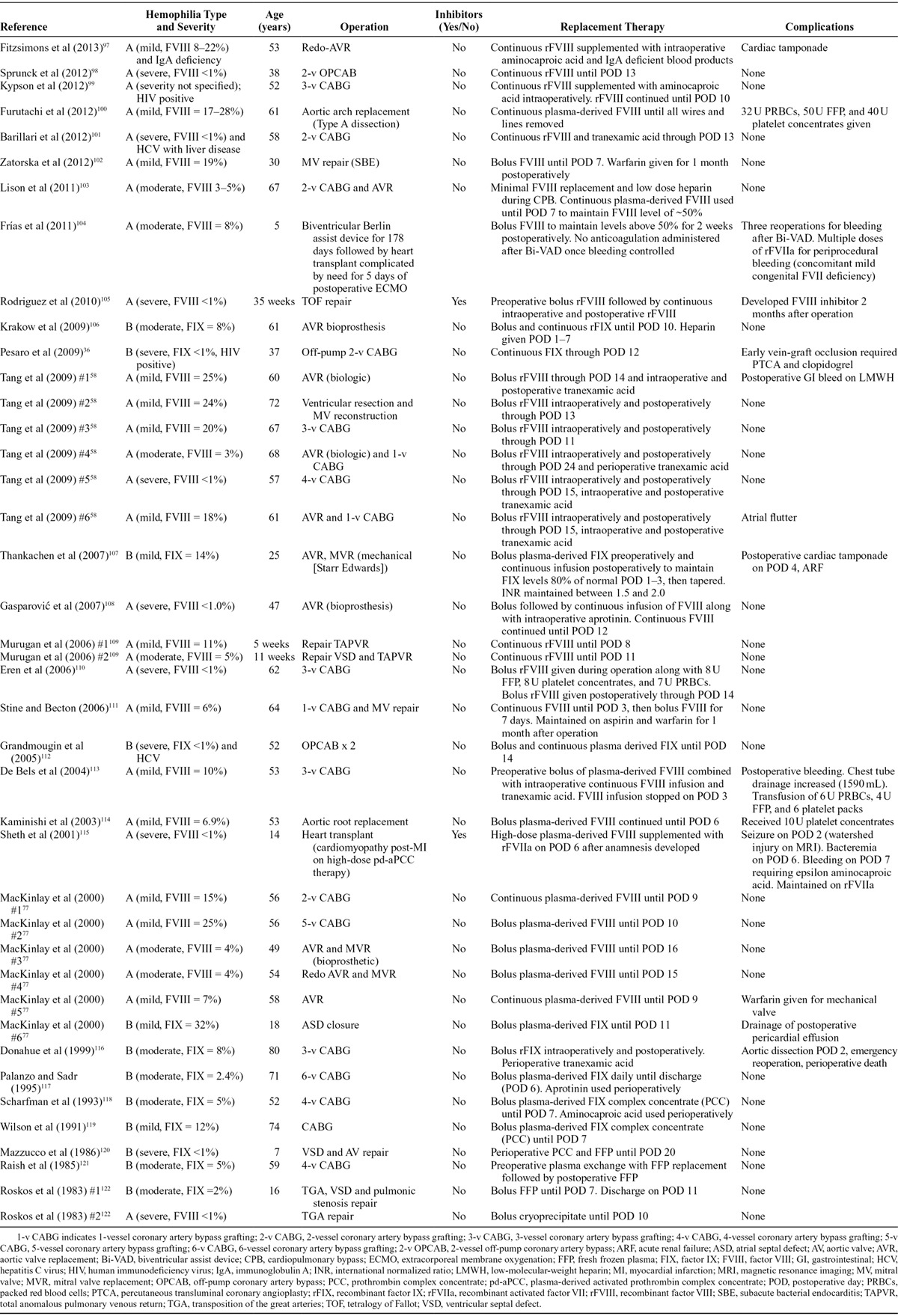
Numerous cardiovascular conditions, both acquired and congenital, require operations in PWH, including those with inhibitors. An extensive literature search revealed reports of cardiac operations in fewer than 50 PWH (Table 4). With such a small sample, assessment of clinical outcomes in this population is difficult. Results of cardiac procedures in PWH seem similar to those in people without hemophilia, but there is undoubtedly publication bias in these literature reports. A published summary of “best evidence” in this area exists,123 but evidence-based recommendations grounded on controlled trials or even on large observational studies do not exist for PWH undergoing cardiac procedures. Consequently, recommendations for the management of cardiac procedures in PWH come from expert consensus rather than large published series.
Table 4.
Reports of Cardiac Operations in People With Hemophilia: Survey of the English-Language Literature Between 1980 and 2013
General Perioperative Considerations
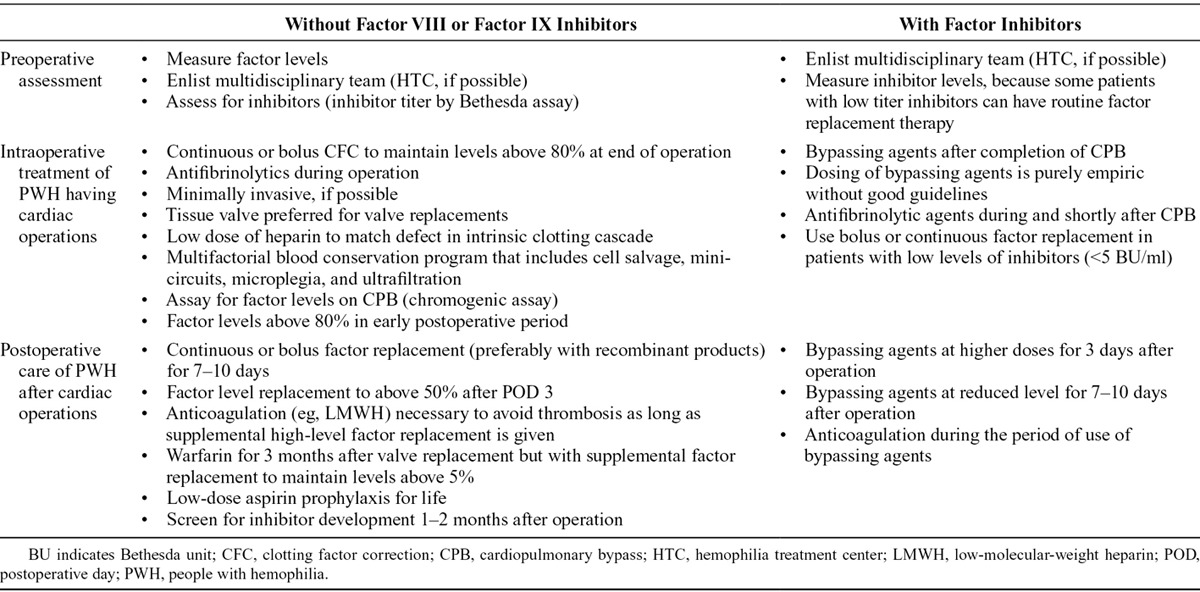
Table 5 summarizes general recommendations and considerations for surgical management of CVD in PWH. Given the challenges of ensuring hemostasis, particularly when systemic anticoagulation is required, operative intervention is a formidable undertaking in PWH, especially those with inhibitors. Accordingly, less invasive procedures—for example, PCI in lieu of CABG89 or transcatheter correction of cardiac lesions (eg, septal defects,124 patent ductus arteriosus125)—seem preferable whenever possible and appropriate. In PWH for whom elective surgery is the only or best option, preoperative coordination of all necessary personnel and resources is vital. Ample supplies of factor concentrates or bypassing agents and blood products are required. Consultation with a hematologist ideally affiliated with an HTC throughout the perioperative period is paramount.
Table 5.
Management Consensus for People With Hemophilia Requiring Surgical Treatment of Cardiovascular Disease
Cardiac transplantation poses a particular challenge when it comes to coordinating care, because the timing for this procedure cannot be planned in advance. Sufficient supplies of hemostatic agents are essential for this procedure, as is the ability to mobilize emergency laboratory, blood bank, and pharmacy support.115 Not surprisingly, relative to similar procedures in the general population, cardiac operations in PWH incur substantially increased costs (primarily due to hemostatic treatments) and resource utilization.115,123,126 Advance planning and involvement of a multidisciplinary team serves to optimize clinical outcomes while minimizing risk.
Numerous regimens exist for perioperative clotting factor correction. Most reports describe a factor activity level of 100% from immediately before operation extending to the conclusion of the procedure, using bolus dosing or continuous infusion. The latter avoids the fluctuation in factor levels that bolus dosing causes and potentially reduces the total amounts of factor ultimately consumed.81 Consensus suggests that maintaining factor levels in the range of 80–100% of normal activity through the early postoperative period, at least to postoperative day 3, is optimal. Thereafter, standard recommendations endorse maintenance of trough factor levels of 50% until wound healing is nearly complete (postoperative day 10–14).102 In PWH with inhibitors, unless the inhibitor titer is low (≤5 Bethesda units/mL) or there is an opportunity to eradicate the inhibitor before operation, bypassing agents substitute for clotting factor replacement (Table 2).41 In addition to factor replacement therapies, adjunctive use of tranexamic acid for postoperative hemostatic coverage in PWH is an option.58,125 In individuals with refractory bleeding during or after operation, acquired hemostatic perturbations, such as consumptive or dilutional coagulopathies from cardiopulmonary bypass (CPB) or deep hypothermia, are possibilities.127 Cases complicated by acquired coagulopathy may require additional hemostatic products (eg, FFP, platelets) independent of hemophilia factor replacement.
Procedures requiring CPB in PWH constitute high-risk undertakings, given the need for systemic anticoagulation. Consequently, off-pump or minimally invasive variations of procedures that typically require extracorporeal circulatory support, such as CABG,36,98,112 confer less risk to PWH. Anecdotal evidence suggests that minimally invasive procedures reduce the risk for bleeding and coagulopathy in PWH.36,98,112 Published experience with management of anticoagulation and hemostasis during CPB in PWH is limited and entirely anecdotal. Ultimately, this procedure requires a highly individualized approach. In many PWH described in the literature, procedures using CPB incorporated standard heparinization protocols,58,77,105,108,114,116,128 after 100% correction of factor levels by bolus or continuous administration of factor concentrates.77,105,108–111,113,116,118 For 1 patient, the bypass circuit was primed with FFP in lieu of saline to minimize hemodilution.115 However, use of this large amount of FFP (as much as 1500 mL) confers additional risk of transfusion-related complications without providing much evidence of benefit.
The monitoring of anticoagulant activity during CPB in PWH is problematic. Activated clotting time (ACT) is most often used to monitor heparin effect. Importantly, the accuracy of ACT monitoring is uncertain unless there are near-normal factor levels.126 In addition, the utility and accuracy of ACT monitoring during CPB when using hemostatic coverage with bypassing agents is uncertain. For monitoring hemostatic therapy during cardiac surgery (with or without CPB), the measurement of PTT or factor levels is a viable option. For this purpose, it is necessary to have reliable venous access at a site separate from where factor concentrates will be administered, preferably placed in advance of the operation.109 High heparin concentrations during CPB preclude accurate determination of FVIII levels.105,113 To determine factor levels in this setting, a chromogenic assay with heparin neutralization should be used instead of a coagulation-based assay.109 Point-of-care tests such as thromboelastography monitor perioperative coagulation in settings where hemostatic perturbations are likely (including cardiac operations). However, experience with thromboelastography for directing perioperative hemostatic management specifically in PWH is extremely limited,129 especially in the setting of cardiac surgery. In PWH with factor inhibitors who require bypassing agents for hemostatic coverage, there is no reliable biochemical means of monitoring hemostatic response, other than by clinical parameters.
Valve Replacement
There are several important considerations specific to PWH undergoing valve replacement, including the need for postoperative anticoagulation. In the majority of PWH undergoing valve replacement, a bioprosthetic valve is preferred to avoid the need for prolonged anticoagulation.58,103,128 Published reports suggest that anticoagulation strategies in the immediate postoperative period vary and include LMWH for a period of up to approximately 10 days,58,106,107 warfarin,77 or no therapy.77,128 Specific institutional recommendations for anticoagulation from centers in Italy and the Netherlands advocate the use of LMWH (5000–7000 U twice daily) for 10 days after valve replacement, in conjunction with factor replacement.6 Subsequently, in those who receive bioprosthetic valves, warfarin derivatives targeting an INR of 2.5–3.5 are recommended for a period of 3 months, during which time trough factor levels should be maintained at ≥5%.6 In certain cases, based on age or hemodynamics,107 PWH may receive a mechanical prosthesis because of hypothetical superior longevity. Use of a mechanical valve mandates indefinite anticoagulation and maintenance of factor levels above 30% by continuous prophylaxis.63 One case report, however, described the initial deferment of anticoagulation after mechanical valve replacement in a man with moderate hemophilia A who developed FVIII inhibitors.130 Instead, measurement of D-dimer levels and monitoring of echocardiography eventually pointed to evidence of thrombosis and prompted warfarin therapy.130 Experience with annuloplasty in this population is very limited, with only 2 case reports identified in the literature.102,111 Individuals without hemophilia who undergo annuloplasty typically receive anticoagulation for 3 months after the procedure, similar to those undergoing bioprosthetic valve replacement.102 Whether PWH require similar anticoagulation after annuloplasty is uncertain.
Lower Extremity Peripheral Arterial Disease
To date, there is only a single description of surgical intervention for PAD in the setting of hemophilia: a 61-year-old man with mild hemophilia A who underwent femoral-popliteal bypass using an autogenous saphenous vein graft.131 The patient received hemostatic coverage with intermittent boluses of plasma-derived FVIII. Other details of the procedure are lacking. Stenting is a possible option in PWH who have aortoiliac disease.132 However, the benefits of this approach in PWH are uncertain, especially in view of the need for ongoing antiplatelet or antithrombotic therapy.67
Acquired Aortic Conditions
A few cases of elective repair of abdominal aortic aneurysm in PWH appear in the literature. These aneurysms had the gross and histologic appearance of typical atherosclerotic aneurysms.133,134 Two patients with mild133 and moderate134 hemophilia A had surgical graft replacement; 1 had a collagen-coated Dacron bifurcation graft placed to avoid the need for preclotting.133 More recently, a man with severe hemophilia B had endovascular aneurysm repair (EVAR).135 All individuals met the criteria for elective repair, based on aneurysm size or on a rapid increase in diameter.136 In all cases, factor levels were corrected to ≥100% of normal for the operation using corresponding factor concentrates, followed by maintenance of factor levels at 80–100% for approximately 1 week after the procedure.133–135 Intraoperative heparinization was employed in 2 cases.134,135 In individuals without bleeding disorders, blood loss and transfusion requirements are significantly reduced for EVAR compared with open procedures.135 Whereas logic supports the use of minimally invasive procedures such as EVAR in PWH,135 given the existence of only a single reported case of EVAR in PWH, it is difficult to generalize this recommendation to the entire population.
Literature reports describe the surgical management of aortic dissection in PWH.100,116,127,137 Because emergent intervention is necessary, the management of acute aortic dissection poses challenges beyond those of elective cardiovascular procedures in PWH. Surgical and other considerations are summarized in a recently published first-ever case report describing management of an acute type A dissection in an individual with hemophilia A.127
Carotid Occlusive Disease
Two reports document carotid endarterectomy after cerebrovascular events in a total of 3 PWH: 1 with mild hemophilia B,138 1 with moderate hemophilia B,139 and 1 with mild hemophilia A.138 Two of these individuals had substantial cardiovascular risk factors, and 1 had prior CABG. These reports did not provide extensive operative details. Management of hemophilia factor replacement involved continuous intraoperative infusion of recombinant FIX in both individuals with hemophilia B,138,139 in 1 case continuing for 48 hours postprocedure.139 In the other case, postoperative FIX replacement was achieved by bolus dosing continued for 7 days after operation.138 Ironically, both individuals with hemophilia B developed carotid artery pseudotumors within 8 weeks of endarterectomy, and both underwent successful excision of their pseudotumors under FIX cover.138,139 The individual with hemophilia A received bolus dosing of FVIII during endarterectomy and for 10 days thereafter.138 A Dacron patch was used for carotid closure in 1 of the men with hemophilia B.139
CONCLUSIONS
As the population of PWH ages, cardiovascular health care providers will encounter increasing numbers of PWH presenting with typical age-related cardiovascular conditions, in addition to other acquired or congenital conditions spanning all ages. The management of cardiovascular conditions in PWH may prove especially challenging when antithrombotic therapy or surgical intervention is indicated, particularly in the presence of inhibitors. Further complicating the challenges of ensuring hemostasis in PWH is the lack of evidence-based guidelines upon which to base therapeutic decisions. Accordingly, current recommendations for the medical and surgical management of common cardiovascular conditions in PWH derive from anecdotal experience and expert opinion. Most recommendations reflect guidelines and common practices for people without hemophilia. Ultimately, the rigorous, systematic investigation of management strategies for many cardiovascular conditions is unobtainable, given the relative rarity of hemophilia and even smaller numbers of PWH with any given cardiovascular condition. The utilization of data from existing global registries or from newly created registries may provide useful information regarding approaches to antithrombotic and hemostatic therapy in PWH. In the meantime, the best options include individualization of treatment protocols, with coordinated input from a multidisciplinary team. To optimize resource utilization and clinical outcome and to minimize bleeding risk and complications, close consultation with a hematologist, ideally in association with an HTC, is essential.
Acknowledgments
Writing assistance was provided by Lara Primak, MD, of ETHOS Health Communications in Newtown, Pennsylvania, with financial support from Novo Nordisk Inc., in compliance with international Good Publication Practice guidelines.
Footnotes
Disclosure: Victor A. Ferraris has been a paid consultant for Baxter Healthcare (CME activity), Haemonetics (advisory board), and AstraZeneca (advisory board). Leonard I. Boral has previously been a paid consultant for Alexion Pharmaceuticals. Alice J. Cohen and Susan S. Smyth declare no competing interests. Gilbert C. White is a paid consultant for Bayer (grant review); Baxter (Data Safety Monitoring Board); and CSL Behring, Novo Nordisk, and Pfizer (advisory boards); is a cofounder of and scientific advisory board member for Entegrion; and is on the scientific advisory board for Asklepios.
REFERENCES
- 1.Boggio LN, Kessler CM. Hemophilia A and B. In: Kitchens CS, Alving BM, Kessler CM, editors. In: Consultative Hemostasis and Thrombosis. 2nd ed. Philadelphia: Elsevier Health Sciences; 2007. pp. 45–59. [Google Scholar]
- 2.Kulkarni R, Soucie JM. Pediatric hemophilia: a review. Semin Thromb Hemost. 2011;37:737–744. doi: 10.1055/s-0031-1297164. [DOI] [PubMed] [Google Scholar]
- 3.DiMichele DM. Inhibitors in Hemophilia: A Primer. 4th ed. Montreal: World Federation of Hemophilia; 2008. [Google Scholar]
- 4.Bolton-Maggs PH, Pasi KJ. Haemophilias A and B. Lancet. 2003;361:1801–1809. doi: 10.1016/S0140-6736(03)13405-8. [DOI] [PubMed] [Google Scholar]
- 5.Goedert JJ, Eyster ME, Lederman MM, et al. End-stage liver disease in persons with hemophilia and transfusion-associated infections. Blood. 2002;100:1584–1589. [PubMed] [Google Scholar]
- 6.Mannucci PM, Mauser-Bunschoten EP. Cardiovascular disease in haemophilia patients: a contemporary issue. Haemophilia. 2010;16(suppl 3):58–66. doi: 10.1111/j.1365-2516.2010.02262.x. [DOI] [PubMed] [Google Scholar]
- 7.Plug I, Van Der Bom JG, Peters M, et al. Mortality and causes of death in patients with hemophilia, 1992–2001: a prospective cohort study. J Thromb Haemost. 2006;4:510–516. doi: 10.1111/j.1538-7836.2006.01808.x. [DOI] [PubMed] [Google Scholar]
- 8.Darby SC, Keeling DM, Spooner RJ, et al. The incidence of factor VIII and factor IX inhibitors in the hemophilia population of the UK and their effect on subsequent mortality, 1977–99. J Thromb Haemost. 2004;2:1047–1054. doi: 10.1046/j.1538-7836.2004.00710.x. [DOI] [PubMed] [Google Scholar]
- 9.Goel R, Krishnamurti L. Mortality, health care utilization and associated diagnoses in hospitalized patients with haemophilia in the United States: first reported nationwide estimates. Haemophilia. 2012;18:688–692. doi: 10.1111/j.1365-2516.2012.02774.x. [DOI] [PubMed] [Google Scholar]
- 10.Darby SC, Kan SW, Spooner RJ, et al. Mortality rates, life expectancy, and causes of death in people with hemophilia A or B in the United Kingdom who were not infected with HIV. Blood. 2007;110:815–825. doi: 10.1182/blood-2006-10-050435. [DOI] [PubMed] [Google Scholar]
- 11.Triemstra M, Rosendaal FR, Smit C, et al. Mortality in patients with hemophilia. Changes in a Dutch population from 1986 to 1992 and 1973 to 1986. Ann Intern Med. 1995;123:823–827. doi: 10.7326/0003-4819-123-11-199512010-00002. [DOI] [PubMed] [Google Scholar]
- 12.Ragni MV. Aging in haemophilia: getting to the heart of the matter. Thromb Haemost. 2011;105:207–208. doi: 10.1160/TH10-12-0818. [DOI] [PubMed] [Google Scholar]
- 13.Srámek A, Reiber JH, Gerrits WB, et al. Decreased coagulability has no clinically relevant effect on atherogenesis: observations in individuals with a hereditary bleeding tendency. Circulation. 2001;104:762–767. doi: 10.1161/hc3501.094232. [DOI] [PubMed] [Google Scholar]
- 14.Biere-Rafi S, Tuinenburg A, Haak BW, et al. Factor VIII deficiency does not protect against atherosclerosis. J Thromb Haemost. 2012;10:30–37. doi: 10.1111/j.1538-7836.2011.04499.x. [DOI] [PubMed] [Google Scholar]
- 15.Tuinenburg A, Rutten A, Kavousi M, et al. Coronary artery calcification in hemophilia A: no evidence for a protective effect of factor VIII deficiency on atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:799–804. doi: 10.1161/ATVBAHA.111.238162. [DOI] [PubMed] [Google Scholar]
- 16.Foley CJ, Nichols L, Jeong K, et al. Coronary atherosclerosis and cardiovascular mortality in hemophilia. J Thromb Haemost. 2010;8:208–211. doi: 10.1111/j.1538-7836.2009.03669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zwiers M, Lefrandt JD, Mulder DJ, et al. Coronary artery calcification score and carotid intima–media thickness in patients with hemophilia. J Thromb Haemost. 2012;10:23–29. doi: 10.1111/j.1538-7836.2011.04514.x. [DOI] [PubMed] [Google Scholar]
- 18.Kulkarni R, Soucie JM, Evatt BL Hemophilia Surveillance System Project Investigators. Prevalence and risk factors for heart disease among males with hemophilia. Am J Hematol. 2005;79:36–42. doi: 10.1002/ajh.20339. [DOI] [PubMed] [Google Scholar]
- 19.Ragni MV, Moore CG. Atherosclerotic heart disease: prevalence and risk factors in hospitalized men with haemophilia A. Haemophilia. 2011;17:867–871. doi: 10.1111/j.1365-2516.2011.02501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alsolaiman MM, Chang K, Arjomand H, et al. Acute left anterior descending artery occlusion in a hemophiliac A patient during recombinant factor VIII infusion: treatment with coronary angioplasty. Catheter Cardiovasc Interv. 2000;50:468–472. doi: 10.1002/1522-726x(200008)50:4<468::aid-ccd22>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 21.Girolami A, Ruzzon E, Fabris F, et al. Myocardial infarction and other arterial occlusions in hemophilia a patients. A cardiological evaluation of all 42 cases reported in the literature. Acta Haematol. 2006;116:120–125. doi: 10.1159/000093642. [DOI] [PubMed] [Google Scholar]
- 22.Maresca L, Giallauria F, D’Agostino MA, et al. A severe haemophiliac patient with acute coronary syndrome admitted to cardiac rehabilitation. Haemophilia. 2012;18:e45–e47. doi: 10.1111/j.1365-2516.2011.02746.x. [DOI] [PubMed] [Google Scholar]
- 23.Bovenzi F, De Luca L, Signore N, et al. Abciximab for the treatment of an acute thrombotic coronary occlusion during stent implantation in a patient with severe hemophilia B. Ital Heart J. 2003;4:728–730. [PubMed] [Google Scholar]
- 24.Biere-Rafi S, Baarslag MA, Peters M, et al. Cardiovascular risk assessment in haemophilia patients. Thromb Haemost. 2011;105:274–278. doi: 10.1160/TH10-07-0460. [DOI] [PubMed] [Google Scholar]
- 25.Fransen van de Putte DE, Fischer K, Makris M, et al. Increased prevalence of hypertension in haemophilia patients. Thromb Haemost. 2012;108:750–755. doi: 10.1160/TH12-05-0313. [DOI] [PubMed] [Google Scholar]
- 26.Lim MY, Pruthi RK. Cardiovascular disease risk factors: prevalence and management in adult hemophilia patients. Blood Coagul Fibrinolysis. 2011;22:402–406. doi: 10.1097/MBC.0b013e328345f582. [DOI] [PubMed] [Google Scholar]
- 27.Konkle BA, Kessler C, Aledort L, et al. Emerging clinical concerns in the ageing haemophilia patient. Haemophilia. 2009;15:1197–1209. doi: 10.1111/j.1365-2516.2009.02066.x. [DOI] [PubMed] [Google Scholar]
- 28.Fransen van de Putte DE, Fischer K, Makris M, et al. Unfavourable cardiovascular disease risk profiles in a cohort of Dutch and British haemophilia patients. Thromb Haemost. 2013;109:16–23. doi: 10.1160/TH12-05-0332. [DOI] [PubMed] [Google Scholar]
- 29.Sharathkumar AA, Soucie JM, Trawinski B, et al. Prevalence and risk factors of cardiovascular disease (CVD) events among patients with haemophilia: experience of a single haemophilia treatment centre in the United States (US). Haemophilia. 2011;17:597–604. doi: 10.1111/j.1365-2516.2010.02463.x. [DOI] [PubMed] [Google Scholar]
- 30.Friis-Møller N, Reiss P, Sabin CA, et al. DAD Study Group. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356:1723–1735. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- 31.Jacob AJ, Sutherland GR, Boon NA, et al. Dilated cardiomyopathy in haemophiliacs infected with the human immunodeficiency virus. Scott Med J. 1993;38:112–113. doi: 10.1177/003693309303800406. [DOI] [PubMed] [Google Scholar]
- 32.Manco-Johnson MJ, Abshire TC, Shapiro AD, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357:535–544. doi: 10.1056/NEJMoa067659. [DOI] [PubMed] [Google Scholar]
- 33.MASAC Recommendations Concerning Products Licensed for the Treatment of Hemophilia and Other Bleeding Disorders. National Hemophilia Foundationwebsite; Available at: http://www.hemophilia.org/NHFWeb/MainPgs/MainNHF.aspx?menuid=57&contentid=693. Updated October 2013. Accessed October 5, 2013. [Google Scholar]
- 34.Keeling D, Tait C, Makris M. Guideline on the selection and use of therapeutic products to treat haemophilia and other hereditary bleeding disorders. A United Kingdom Haemophilia Center Doctors’ Organisation (UKHCDO) guideline approved by the British Committee for Standards in Haematology. Haemophilia. 2008;14:671–684. doi: 10.1111/j.1365-2516.2008.01695.x. [DOI] [PubMed] [Google Scholar]
- 35.Franchini M. The modern treatment of haemophilia: a narrative review. Blood Transfus. 2013;11:178–182. doi: 10.2450/2012.0166-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pesaro AE, Gaz MV, Karbstein R, et al. Coronary artery bypass surgery, angioplasty and long term anti-platelet treatment in a type B hemophilia patient. Clinics (Sao Paulo) 2009;64:822–823. doi: 10.1590/S1807-59322009000800019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hermans C, Brackmann HH, Schinco P, et al. The case for wider use of recombinant factor VIII concentrates. Crit Rev Oncol Hematol. 2012;83:11–20. doi: 10.1016/j.critrevonc.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 38.Ludlam CA, Powderly WG, Bozzette S, et al. Clinical perspectives of emerging pathogens in bleeding disorders. Lancet. 2006;367:252–261. doi: 10.1016/S0140-6736(06)68036-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim DK, Kim DI, Kim MS, et al. Successful percutaneous coronary intervention for acute coronary syndrome in a patient with severe hemophilia A. Korean Circ J. 2010;40:527–529. doi: 10.4070/kcj.2010.40.10.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kempton CL, White GC., 2nd How we treat a hemophilia A patient with a factor VIII inhibitor. Blood. 2009;113:11–17. doi: 10.1182/blood-2008-06-160432. [DOI] [PubMed] [Google Scholar]
- 41.Kulkarni R. Comprehensive care of the patient with haemophilia and inhibitors undergoing surgery: practical aspects. Haemophilia. 2013;19:2–10. doi: 10.1111/j.1365-2516.2012.02922.x. [DOI] [PubMed] [Google Scholar]
- 42.Hoffman M, Dargaud Y. Mechanisms and monitoring of bypassing agent therapy. J Thromb Haemost. 2012;10:1478–1485. doi: 10.1111/j.1538-7836.2012.04793.x. [DOI] [PubMed] [Google Scholar]
- 43.Chavin SI, Siegel DM, Rocco TA, Jr, et al. Acute myocardial infarction during treatment with an activated prothrombin complex concentrate in a patient with factor VIII deficiency and a factor VIII inhibitor. Am J Med. 1988;85:245–249. doi: 10.1016/s0002-9343(88)80353-x. [DOI] [PubMed] [Google Scholar]
- 44.Fukui H, Fujimura Y, Takahashi Y, et al. Laboratory evidence of DIC under FEIBA treatment of a hemophilic patient with intracranial bleeding and high titre factor VIII inhibitor. Thromb Res. 1981;22:177–184. doi: 10.1016/0049-3848(81)90319-4. [DOI] [PubMed] [Google Scholar]
- 45.Schimpf K, Zeltsch C, Zeltsch P. Myocardial infarction complicating activated prothrombin complex concentrate substitution in patient with hemophilia A. Lancet. 1982;2:1043. doi: 10.1016/s0140-6736(82)90071-x. [DOI] [PubMed] [Google Scholar]
- 46.Ehrlich HJ, Henzl MJ, Gomperts ED. Safety of factor VIII inhibitor bypass activity (FEIBA): 10-year compilation of thrombotic adverse events. Haemophilia. 2002;8:83–90. doi: 10.1046/j.1365-2516.2002.00532.x. [DOI] [PubMed] [Google Scholar]
- 47.NovoSeven RT (Coagulation Factor VIIa [Recombinant], Room Temperature Stable) [prescribing information] Bagsvaerd, Denmark: Novo Nordisk A/S; 2014. [Google Scholar]
- 48.Hardy JF, Bélisle S, Van der Linden P. Efficacy and safety of recombinant activated factor VII to control bleeding in nonhemophiliac patients: a review of 17 randomized controlled trials. Ann Thorac Surg. 2008;86:1038–1048. doi: 10.1016/j.athoracsur.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 49.Hsia CC, Chin-Yee IH, McAlister VC. Use of recombinant activated factor VII in patients without hemophilia: a meta-analysis of randomized control trials. Ann Surg. 2008;248:61–68. doi: 10.1097/SLA.0b013e318176c4ec. [DOI] [PubMed] [Google Scholar]
- 50.Aledort LM. Factor VIII inhibitor bypassing activity (FEIBA)—addressing safety issues. Haemophilia. 2008;14:39–43. doi: 10.1111/j.1365-2516.2007.01594.x. [DOI] [PubMed] [Google Scholar]
- 51.Dunn AL, Cox Gill J. Adenotonsillectomy in patients with desmopressin responsive mild bleeding disorders: a review of the literature. Haemophilia. 2010;16:711–716. doi: 10.1111/j.1365-2516.2009.02145.x. [DOI] [PubMed] [Google Scholar]
- 52.Horrow JC. Desmopressin and antifibrinolytics. Int Anesthesiol Clin. 1990;28:230–236. doi: 10.1097/00004311-199002840-00009. [DOI] [PubMed] [Google Scholar]
- 53.Mannucci PM, Bettega D, Cattaneo M. Patterns of development of tachyphylaxis in patients with haemophilia and von Willebrand disease after repeated doses of desmopressin (DDAVP). Br J Haematol. 1992;82:87–93. doi: 10.1111/j.1365-2141.1992.tb04598.x. [DOI] [PubMed] [Google Scholar]
- 54.Virtanen R, Kauppila M, Itälä M. Percutaneous coronary intervention with stenting in a patient with haemophilia A and an acute myocardial infarction following a single dose of desmopressin. Thromb Haemost. 2004;92:1154–1156. [PubMed] [Google Scholar]
- 55.Desmopressin Acetate Injection, USP [prescribing information] Lake Forest, Illinois: Hospira, Inc.; 2007. [Google Scholar]
- 56.Cyclokapron® (tranexamic acid injection) [prescribing information] New York, NY: Pfizer, Inc.; 2013. [Google Scholar]
- 57.Giangrande PL, Wilde JT, Madan B, et al. Consensus protocol for the use of recombinant activated factor VII [eptacog alfa (activated); NovoSeven] in elective orthopaedic surgery in haemophilic patients with inhibitors. Haemophilia. 2009;15:501–508. doi: 10.1111/j.1365-2516.2008.01952.x. [DOI] [PubMed] [Google Scholar]
- 58.Tang M, Wierup P, Terp K, et al. Cardiac surgery in patients with haemophilia. Haemophilia. 2009;15:101–107. doi: 10.1111/j.1365-2516.2008.01895.x. [DOI] [PubMed] [Google Scholar]
- 59.Tengborn L. Fibrinolytic Inhibitors in the Management of Bleeding Disorders. 2nd ed. Montreal: World Federation of Hemophilia; 2012. [Google Scholar]
- 60.Cattaneo M. The use of desmopressin in open-heart surgery. Haemophilia. 2008;14(suppl 1):40–47. doi: 10.1111/j.1365-2516.2007.01608.x. [DOI] [PubMed] [Google Scholar]
- 61.Cattaneo M, Harris AS, Strömberg U, et al. The effect of desmopressin on reducing blood loss in cardiac surgery—a meta-analysis of double-blind, placebo-controlled trials. Thromb Haemost. 1995;74:1064–1070. [PubMed] [Google Scholar]
- 62.Despotis GJ, Levine V, Saleem R, et al. Use of point-of-care test in identification of patients who can benefit from desmopressin during cardiac surgery: a randomised controlled trial. Lancet. 1999;354:106–110. doi: 10.1016/S0140-6736(98)12494-7. [DOI] [PubMed] [Google Scholar]
- 63.Mannucci PM, Schutgens RE, Santagostino E, et al. How I treat age-related morbidities in elderly persons with hemophilia. Blood. 2009;114:5256–5263. doi: 10.1182/blood-2009-07-215665. [DOI] [PubMed] [Google Scholar]
- 64.Mannucci PM. Management of antithrombotic therapy for acute coronary syndromes and atrial fibrillation in patients with hemophilia. Expert Opin Pharmacother. 2012;13:505–510. doi: 10.1517/14656566.2012.656591. [DOI] [PubMed] [Google Scholar]
- 65.Schutgens RE, Tuinenburg A, Roosendaal G, et al. Treatment of ischaemic heart disease in haemophilia patients: an institutional guideline. Haemophilia. 2009;15:952–958. doi: 10.1111/j.1365-2516.2009.02020.x. [DOI] [PubMed] [Google Scholar]
- 66.Tuinenburg A, Damen SA, Ypma PF, et al. Cardiac catheterization and intervention in haemophilia patients: prospective evaluation of the 2009 institutional guideline. Haemophilia. 2013;19:370–377. doi: 10.1111/hae.12109. [DOI] [PubMed] [Google Scholar]
- 67.Fogarty PF, Olin JW, Kessler CM, et al. An algorithmic approach to peripheral artery disease in hemophilia: extrapolation of management principles from noncoagulopathic patients. Blood Coagul Fibrinolysis. 2012;23:23–29. doi: 10.1097/MBC.0b013e32834cb372. [DOI] [PubMed] [Google Scholar]
- 68.Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 69.Olesen JB, Lip GY, Hansen ML, et al. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ. 2011;342:d124. doi: 10.1136/bmj.d124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gage BF, Waterman AD, Shannon W, et al. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 71.You JJ, Singer DE, Howard PA, et al. American College of Chest Physicians. Antithrombotic therapy for atrial fibrillation: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 suppl):e531S–e575S. doi: 10.1378/chest.11-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kalus JS. Pharmacologic interventions for reversing the effects of oral anticoagulants. Am J Health Syst Pharm. 2013;70(10 suppl 1):S12–S21. doi: 10.2146/ajhp130041. [DOI] [PubMed] [Google Scholar]
- 73.Tripodi A, Palareti G. New anticoagulant drugs for treatment of venous thromboembolism and stroke prevention in atrial fibrillation. J Intern Med. 2012;271:554–565. doi: 10.1111/j.1365-2796.2012.02541.x. [DOI] [PubMed] [Google Scholar]
- 74.Cheung VT, Hunter RJ, Ginks MR, et al. Management of thromboembolic risk in persons with haemophilia and atrial fibrillation: is left atrial appendage occlusion the answer for those at high risk? Haemophilia. 2013;19:e84–e86. doi: 10.1111/hae.12055. [DOI] [PubMed] [Google Scholar]
- 75.Cid AR, Zorio E, Haya S, et al. Treatment in a haemophiliac A patient with paroxysmal atrial fibrillation and ischemic heart disease. Haemophilia. 2007;13:760–762. doi: 10.1111/j.1365-2516.2007.01443.x. [DOI] [PubMed] [Google Scholar]
- 76.DeWitt ES, DiMichele DM, Larsen K, et al. Successful cryoablation of atrioventricular nodal reentrant tachycardia in a child with hemophilia A. J Pediatr Hematol Oncol. 2010;32:404–406. doi: 10.1097/MPH.0b013e3181e0040d. [DOI] [PubMed] [Google Scholar]
- 77.MacKinlay N, Taper J, Renisson F, et al. Cardiac surgery and catheterization in patients with haemophilia. Haemophilia. 2000;6:84–88. doi: 10.1046/j.1365-2516.2000.00384.x. [DOI] [PubMed] [Google Scholar]
- 78.Lim MY, Pruthi RK. Impact of lifestyle modification on symptomatic coronary artery disease in a haemophilia patient with inhibitors. Haemophilia. 2011;17:e1006–e1007. doi: 10.1111/j.1365-2516.2011.02543.x. [DOI] [PubMed] [Google Scholar]
- 79.Guo Y, Zhang X, Huang D, et al. Acute myocardial infarction in a Chinese patient with haemophilia A and risk factors for coronary artery disease: a case report with autopsy. Haemophilia. 2010;16:691–693. doi: 10.1111/j.1365-2516.2010.02212.x. [DOI] [PubMed] [Google Scholar]
- 80.Lim MY, Pruthi RK. Outcomes of management of acute coronary syndrome in patients with congenital bleeding disorders: a single center experience and review of the literature. Thromb Res. 2012;130:316–322. doi: 10.1016/j.thromres.2012.02.050. [DOI] [PubMed] [Google Scholar]
- 81.Ferrario C, Renders F, Cairoli A, et al. Management of an acute coronary syndrome in a patient with severe haemophilia A. Haemophilia. 2007;13:763–765. doi: 10.1111/j.1365-2516.2007.01530.x. [DOI] [PubMed] [Google Scholar]
- 82.Coppola A, Tagliaferri A, Franchini M. The management of cardiovascular diseases in patients with hemophilia. Semin Thromb Hemost. 2010;36:91–102. doi: 10.1055/s-0030-1248728. [DOI] [PubMed] [Google Scholar]
- 83.Fefer P, Gannot S, Lubetsky A, et al. Percutaneous coronary intervention in patients with haemophilia presenting with acute coronary syndrome: an interventional dilemma: case series, review of the literature, and tips for management. J Thromb Thrombolysis. 2013;35:271–278. doi: 10.1007/s11239-012-0802-y. [DOI] [PubMed] [Google Scholar]
- 84.Valgimigli M, Saia F, Guastaroba P, et al. REAL Registry Investigators. Transradial versus transfemoral intervention for acute myocardial infarction: a propensity score-adjusted and -matched analysis from the REAL (REgistro regionale AngiopLastiche dell’Emilia-Romagna) multicenter registry. JACC Cardiovasc Interv. 2012;5:23–35. doi: 10.1016/j.jcin.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 85.Coppola A, De Simone C, Di Capua M, et al. Acute coronary syndrome and severe haemophilia: an unusual association with challenging treatment. Thromb Haemost. 2010;103:1270–1272. doi: 10.1160/TH09-11-0766. [DOI] [PubMed] [Google Scholar]
- 86.Smolka G, Kulach A, Dabek J, et al. Percutaneous coronary intervention with stent implantation in haemophilic A patient with unstable angina. Haemophilia. 2007;13:428–431. doi: 10.1111/j.1365-2516.2007.01436.x. [DOI] [PubMed] [Google Scholar]
- 87.Piscione F, Cassese S, Galasso G, et al. A new approach to percutaneous coronary revascularization in patients requiring undeferrable non-cardiac surgery. Int J Cardiol. 2011;146:399–403. doi: 10.1016/j.ijcard.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 88.Petrillo G, Cirillo P, Leosco D, et al. Percutaneous coronary intervention in a patient with acute non-ST-elevation myocardial infarction and haemophilia A: a ‘genous’ experience. Haemophilia. 2011;17:e245–e246. doi: 10.1111/j.1365-2516.2010.02355.x. [DOI] [PubMed] [Google Scholar]
- 89.Arora UK, Dhir M, Cintron G, et al. Successful multi-vessel percutaneous coronary intervention with bivalirudin in a patient with severe hemophilia A: a case report and review of literature. J Invasive Cardiol. 2004;16:330–332. [PubMed] [Google Scholar]
- 90.Krolick MA. Successful percutaneous coronary intervention in a patient with severe haemophilia A using bivalirudin as the sole procedural anticoagulant. Haemophilia. 2005;11:415–417. doi: 10.1111/j.1365-2516.2005.01103.x. [DOI] [PubMed] [Google Scholar]
- 91.Quintero D, Biria M, Meyers DG. Percutaneous coronary intervention in a patient with acute ST-elevation myocardial infarction and hemophilia A. J Invasive Cardiol. 2008;20:240–241. [PubMed] [Google Scholar]
- 92.King SB, 3rd, Smith SC, Jr, Hirshfeld JW, Jr, et al. ACC/AHA/SCAI. 2007 focused update of the ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice guidelines. J Am Coll Cardiol. 2008;51:172–209. doi: 10.1016/j.jacc.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 93.Mafrici A, Baudo F. Hemophilia and percutaneous coronary interventions. Ital Heart J. 2003;4:731–733. [PubMed] [Google Scholar]
- 94.Staritz P, de Moerloose P, Schutgens R, et al. ADVANCE Working Group. Applicability of the European Society of Cardiology guidelines on management of acute coronary syndromes to people with haemophilia—an assessment by the ADVANCE Working Group. Haemophilia. 2013;19:833–840. doi: 10.1111/hae.12189. [DOI] [PubMed] [Google Scholar]
- 95.Bilora F, Boccioletti V, Zanon E, et al. Hemophilia A, von Willebrand disease, and atherosclerosis of abdominal aorta and leg arteries: factor VIII and von Willebrand factor defects appear to protect abdominal aorta and leg arteries from atherosclerosis. Clin Appl Thromb Hemost. 2001;7:311–313. doi: 10.1177/107602960100700411. [DOI] [PubMed] [Google Scholar]
- 96.Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease). Circulation. 2006;11:e463–e654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 97.Fitzsimons MG, Walton K, Makar R, et al. Redo aortic valve replacement in a patient with immunoglobulin A deficiency and hemophilia A. Ann Thorac Surg. 2013;96:311–313. doi: 10.1016/j.athoracsur.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 98.Sprunck A, Maechel AL, Levy F, et al. Off-pump myocardial revascularization in a patient with haemophilia A: a case report and operative strategies. Haemophilia. 2012;18:e20–e22. doi: 10.1111/j.1365-2516.2011.02660.x. [DOI] [PubMed] [Google Scholar]
- 99.Kypson AP, Rodriguez E, Anderson CA. Coronary surgery in a hemophiliac with continuous factor VIII replacement. Asian Cardiovasc Thorac Ann. 2012;20:191–192. doi: 10.1177/0218492311421579. [DOI] [PubMed] [Google Scholar]
- 100.Furutachi A, Furukawa K, Oda Y, et al. Total arch replacement for acute aortic dissection (Stanford A) in a patient with hemophilia A. Ann Thorac Surg. 2012;93:e61–e62. doi: 10.1016/j.athoracsur.2011.10.028. [DOI] [PubMed] [Google Scholar]
- 101.Barillari G, Pasca S, Erice F, et al. Successful double bypass in a patient with severe hemophilia A: a case report. J Thromb Thrombolysis. 2012;33:193–196. doi: 10.1007/s11239-011-0664-8. [DOI] [PubMed] [Google Scholar]
- 102.Zatorska K, Orłowska-Baranowska E, Abramczuk E, et al. Short and long-term management of haemophilia A patient requiring heart valve surgery. Haemophilia. 2012;18:e352–e354. doi: 10.1111/j.1365-2516.2012.02812.x. [DOI] [PubMed] [Google Scholar]
- 103.Lison S, Spannagl M, Dietrich W Working Group of Perioperative Hemostasis. Hemophilia A in cardiac operations: a model of reduced thrombin generation. Ann Thorac Surg. 2011;91:1606–1608. doi: 10.1016/j.athoracsur.2010.10.077. [DOI] [PubMed] [Google Scholar]
- 104.Frías MÁ, Jaraba S, Ibarra I, et al. Ventricular assist device as a bridge to transplant, and extracorporeal membrane oxygenation for primary graft failure in a child with hemophilia A. Pediatr Crit Care Med. 2011;12:e432–e435. doi: 10.1097/PCC.0b013e31822f1b63. [DOI] [PubMed] [Google Scholar]
- 105.Rodriguez V, Burkhart HM, Schmidt KA, et al. Hemostatic management of an infant with severe hemophilia A and tetralogy of Fallot for cardiac bypass surgery. Pediatr Blood Cancer. 2010;55:1399–1401. doi: 10.1002/pbc.22646. [DOI] [PubMed] [Google Scholar]
- 106.Krakow EF, Walker I, Lamy A, et al. Cardiac surgery in patients with haemophilia B: a case report and review of the literature. Haemophilia. 2009;15:108–113. doi: 10.1111/j.1365-2516.2008.01918.x. [DOI] [PubMed] [Google Scholar]
- 107.Thankachen R, George B, Shukla V, et al. Aortic and mitral valve replacement in a patient with hemophilia B. Asian Cardiovasc Thorac Ann. 2007;15:526–527. doi: 10.1177/021849230701500618. [DOI] [PubMed] [Google Scholar]
- 108.Gasparović H, Zupancic-Salek S, Brida V, et al. Aortic valve replacement in a patient with severe hemophilia. Coll Antropol. 2007;31:355–357. [PubMed] [Google Scholar]
- 109.Murugan SJ, Viswanathan S, Thomson J, et al. Heart surgery in infants with hemophilia. Ann Thorac Surg. 2006;81:336–339. doi: 10.1016/j.athoracsur.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 110.Eren A, Friedl R, Hannekum A, et al. Cardiac surgery in a patient with haemophilia A. Thorac Cardiovasc Surg. 2006;54:212–214. doi: 10.1055/s-2005-872859. [DOI] [PubMed] [Google Scholar]
- 111.Stine KC, Becton DL. Use of factor VIII replacement during open heart surgery in a patient with haemophilia A. Haemophilia. 2006;12:435–436. doi: 10.1111/j.1365-2516.2006.01283.x. [DOI] [PubMed] [Google Scholar]
- 112.Grandmougin D, Delolme MC, Reynaud J, et al. Off-pump myocardial revascularization in a diabetic patient with severe hemophilia B and impaired left ventricular function: hematological and operative strategies. J Card Surg. 2005;20:366–369. doi: 10.1111/j.1540-8191.2005.2004108.x. [DOI] [PubMed] [Google Scholar]
- 113.De Bels D, Demeere JL, Dugauquier J, et al. Continuous infusion of factor VIIIc during heart surgery in a patient with haemophilia A. Eur J Anaesthesiol. 2004;21:984–986. doi: 10.1017/s026502150423037x. [DOI] [PubMed] [Google Scholar]
- 114.Kaminishi Y, Aizawa K, Saito T, et al. Modified Bentall operation in a patient with hemophilia A. Jpn J Thorac Cardiovasc Surg. 2003;51:68–70. doi: 10.1007/BF02719171. [DOI] [PubMed] [Google Scholar]
- 115.Sheth S, Dimichele D, Lee M, et al. Heart transplant in a factor VIII-deficient patient with a high-titre inhibitor: perioperative management using high-dose continuous infusion factor VIII and recombinant factor VIIa. Haemophilia. 2001;7:227–232. doi: 10.1046/j.1365-2516.2001.00483.x. [DOI] [PubMed] [Google Scholar]
- 116.Donahue BS, Emerson CW, Slaughter TF. Case 1–1999. Elective and emergency cardiac surgery on a patient with hemophilia B. J Cardiothorac Vasc Anesth. 1999;13:92–97. doi: 10.1016/s1053-0770(99)90181-7. [DOI] [PubMed] [Google Scholar]
- 117.Palanzo DA, Sadr FS. Coronary artery bypass grafting in a patient with haemophilia B. Perfusion. 1995;10:265–270. doi: 10.1177/026765919501000410. [DOI] [PubMed] [Google Scholar]
- 118.Scharfman WB, Rauch AE, Ferraris V, et al. Treatment of a patient with factor IX deficiency (hemophilia B) with coronary bypass surgery. J Thorac Cardiovasc Surg. 1993;105:765–766. [PubMed] [Google Scholar]
- 119.Wilson CJ, Frankville D, Robinson B, et al. Perioperative management of coronary artery bypass surgery in a patient with factor IX deficiency. J Cardiothorac Vasc Anesth. 1991;5:160–162. doi: 10.1016/1053-0770(91)90332-n. [DOI] [PubMed] [Google Scholar]
- 120.Mazzucco A, Stellin G, Cantele P, et al. Repair of ventricular septal defect and aortic regurgitation associated with severe hemophilia B. Ann Thorac Surg. 1986;42:97–99. doi: 10.1016/s0003-4975(10)61846-2. [DOI] [PubMed] [Google Scholar]
- 121.Raish RJ, Witte DL, Goldsmith JC. Successful cardiac surgery following plasmapheresis in a patient with hemophilia B. Transfusion. 1985;25:128–130. doi: 10.1046/j.1537-2995.1985.25285169203.x. [DOI] [PubMed] [Google Scholar]
- 122.Roskos RR, Gilchrist GS, Kazmier FJ, et al. Management of hemophilia A and B during surgical correction of transposition of the great arteries. Mayo Clin Proc. 1983;58:182–186. [PubMed] [Google Scholar]
- 123.Rossi M, Jayaram R, Sayeed R. Do patients with haemophilia undergoing cardiac surgery have good surgical outcomes? Interact Cardiovasc Thorac Surg. 2011;13:320–331. doi: 10.1510/icvts.2011.272401. [DOI] [PubMed] [Google Scholar]
- 124.Fabricius AM, Krueger M, Falk V, et al. Floating thrombus on an ASD occluder device in a patient with hemophilia A. Thorac Cardiovasc Surg. 2001;49:312–313. doi: 10.1055/s-2001-17800. [DOI] [PubMed] [Google Scholar]
- 125.Saxena A, Jindal RC, Juneja R, et al. Transcatheter closure of patent ductus arteriosus in a patient with haemophilia-A. Indian Heart J. 2000;52:339–340. [PubMed] [Google Scholar]
- 126.Thiagarajan RR, Roth SJ, Margossian S, et al. Extracorporeal membrane oxygenation as a bridge to cardiac transplantation in a patient with cardiomyopathy and hemophilia A. Intensive Care Med. 2003;29:985–988. doi: 10.1007/s00134-003-1748-5. [DOI] [PubMed] [Google Scholar]
- 127.Diplaris KT, Karfis EA, Ampatzidou F, et al. Acute type-a dissection in a patient with severe hemophilia A. J Cardiothorac Vasc Anesth. 2012;26:660–663. doi: 10.1053/j.jvca.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 128.Meagher PD, Rickard KA, Richards JG, et al. Aortic and mitral valve replacement in a patient with severe haemophilia A. Aust N Z J Med. 1981;11:76–79. doi: 10.1111/j.1445-5994.1981.tb03743.x. [DOI] [PubMed] [Google Scholar]
- 129.Pivalizza EG. Perioperative use of the Thrombelastograph in patients with inherited bleeding disorders. J Clin Anesth. 2003;15:366–370. doi: 10.1016/s0952-8180(03)00022-9. [DOI] [PubMed] [Google Scholar]
- 130.Ghosh K, Shetty S. Anticoagulation in haemophilia patients with prosthetic valve replacement. Haemophilia. 2004;10:743. doi: 10.1111/j.1365-2516.2004.01029.x. [DOI] [PubMed] [Google Scholar]
- 131.Gerhardt A, Grotemeyer D, Sandmann W, et al. A hemophilia patient with C1 domain Arg2150His mutation developed a high titer inhibitor not inhibiting autologous factor VIII after switching to third generation recombinant product. Blood (ASH Annual Meeting Abstracts) 2005;106:4060. [Google Scholar]
- 132.Olin JW, Sealove BA. Peripheral artery disease: current insight into the disease and its diagnosis and management. Mayo Clin Proc. 2010;85:678–692. doi: 10.4065/mcp.2010.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bergqvist D, Thimberg L, Bergentz SE, et al. Abdominal aortic aneurysm surgery in a hemophiliac. Vasa. 1985;14:394–397. [PubMed] [Google Scholar]
- 134.Kobayashi M, Matsushita M, Nishikimi N, et al. Treatment for abdominal aortic aneurysm in a patient with hemophilia A: a case report and review of the literature. J Vasc Surg. 1997;25:945–948. doi: 10.1016/s0741-5214(97)70228-3. [DOI] [PubMed] [Google Scholar]
- 135.Marrocco-Trischitta MM, Melissano G, Castellano R, et al. Endovascular abdominal aortic aneurysm repair in a patient with severe hemophilia B. J Endovasc Ther. 2009;16:120–123. doi: 10.1583/08-2584.1. [DOI] [PubMed] [Google Scholar]
- 136.Brewster DC, Cronenwett JL, Hallett JW, Jr, et al. Joint Council of the American Association for Vascular Surgery and Society for Vascular Surgery. Guidelines for the treatment of abdominal aortic aneurysms. Report of a subcommittee of the Joint Council of the American Association for Vascular Surgery and Society for Vascular Surgery. J Vasc Surg. 2003;37:1106–1117. doi: 10.1067/mva.2003.363. [DOI] [PubMed] [Google Scholar]
- 137.Chun PK, Flannery EP, Bowen TE. Open-heart surgery in patients with hematologic disorders. Am Heart J. 1983;105:835–842. doi: 10.1016/0002-8703(83)90249-1. [DOI] [PubMed] [Google Scholar]
- 138.Bowles L, Yee TT. Carotid endarterectomy in 2 patients with hemophilia. Haemophilia. 2012;18:4. [Google Scholar]
- 139.Malam Y, Tsui J, Sheikh SE, et al. Journal rubric. Haemophilic pseudotumour of the carotid artery. Vasc Med. 2012;17:193–194. doi: 10.1177/1358863X11429998. [DOI] [PubMed] [Google Scholar]


