Abstract
Gastric arrhythmias occur in gastroparesis, however, their meaning and significance has been debated. Recently, an improved understanding of dysrhythmia has begun to emerge, informed by cellular, physiological, and engineering progress. This review discusses these advances and considers clinical applications. An important foundation has been defining histopathological abnormalities in gastroparesis. The finding that interstitial cells of Cajal are depleted and injured provides mechanisms for arrhythmogenesis in gastroparesis, and has reinvigorated clinical interest in electrical diagnostics. Electrogastrography (EGG) has been the dominant method to date, demonstrating consistent associations between arrhythmias and gastroparesis, however, is fundamentally limited by its summative nature, low signal quality, and incomplete sensitivity and specificity. Recently, high-resolution (HR; multi-electrode) mapping has emerged, providing superior spatial data on arrhythmic patterns and mechanisms, and enabling new insights and classifications. However, HR mapping is currently limited by invasiveness, and low-resolution approaches are being assessed as bridging techniques until this limitation is overcome.
Keywords: Electrogastrography, interstitial cells of Cajal, tachygastria, slow wave
Introduction
Gastroparesis remains a complex clinical and research challenge, with few effective therapies.1,2 Among several underlying factors, considerable interest over recent decades has focused on a putative role for gastric electrical arrhythmia.3 While the nature and significance of gastric arrhythmia has been a source of considerable controversy, an enhanced understanding is now emerging that could contribute diagnostic and therapeutic value to the problem of gastroparesis.
This review discusses current knowledge of gastric arrhythmia in the context of gastroparesis, and evaluates potential clinical implications in diagnostic testing. Future diagnostic and therapeutic directions for this rapidly evolving field are also considered.
The Human ‘Gastric Conduction System’
Recent advances in normal human gastric electrophysiology have been significant, such that most textbooks are outdated and a brief overview here is warranted. Although several details still await clarification, current progress now represents a useful foundation for performing and interpreting tests of gastric electrical function.
Gastric contractions are coordinated by bioelectrical slow waves, which are generated and propagated by networks of interstitial cells of Cajal (ICC). Slow waves are transmitted from ICC to the electrically-coupled smooth muscle cells, which generate secondary responses that are integrated with other modulatory factors to effect contractions.4 In humans, ICC anastomose throughout abundant networks within the gastric myenteric plexus (ICC-MP), as well as running parallel to smooth muscle fibres in the longitudinal and circular muscular layers (intramuscular ICC; ICC-IM).5 Propagation within these layers is also facilitated by a further ‘septal’ subclass of ICC that encase and connect muscle bundles (ICC-SEP). Animal studies have shown that a break in ICC continuity occurs at the pylorus, serving as an isolating electrical barrier from the distinct slow wave patterns of the duodenum.6
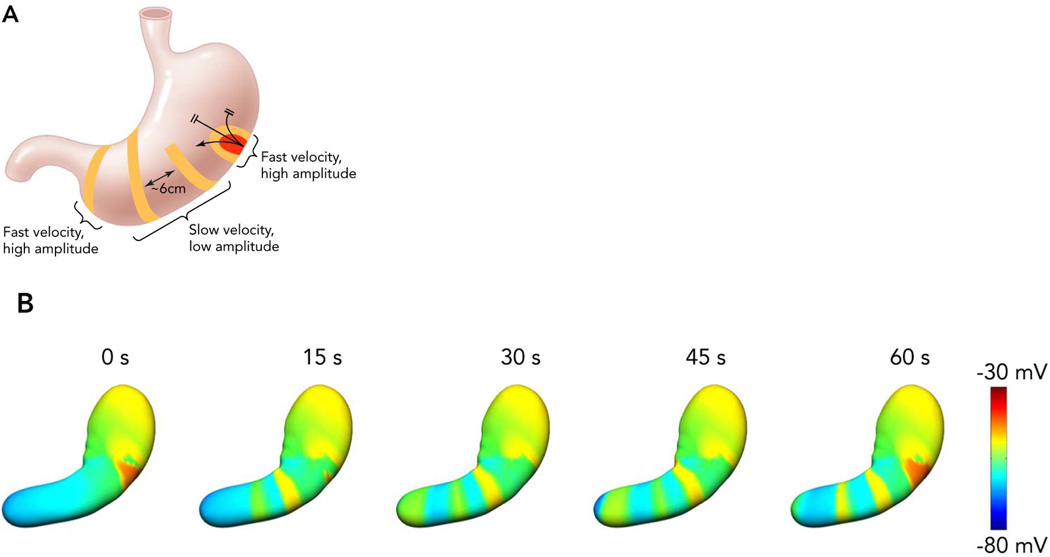
In health, gastric slow waves arise in a defined pacemaker region that is located at the mid to upper corpus of the greater curvature (Figure 1).7 Antegrade propagation from this site is facilitated by an underlying frequency gradient within ICC, with cells in the pacemaker region dominating because they have the highest intrinsic frequency.8 Slow waves propagate toward the pylorus at intervals of around 20 s (i.e. 3 ‘cycles per minute’ or cpm), and at a constant speed of ~3 mm/s, such that successive wavefronts become evenly spaced at intervals of around 6 cm. Within the antrum, there is an acceleration in velocity and the wave spacing then becomes greater (Figure 1B).7 Slow waves do not normally excite the fundus in-vivo.
Figure 1.
The normal human gastric slow wave conduction pattern.7,13 A: Slow waves arise from a pacemaker region in the corpus at the greater curvature. Slow waves initially propagate in all directions, and rapidly in the circumferential axis, forming complete ring wavefronts in the mid corpus. Multiple wavefronts accrue, spaced ~6 cm apart, before a transition to rapid activation within the antrum. Higher amplitude signals are observed whenever conduction velocity is rapid.13 B: Simulations demonstrating this activation pattern over time13,30. Multiple wavefronts propagate as successive rings around the lumen, with each cycle taking 1 min to reach the pylorus. From Cheng LK, Du P, O’Grady G. Mapping and modeling gastrointestinal bioelectricity: from engineering bench to bedside. Physiology. 2013;28:310–317; with permission.
Considerable discussion has focused on what slow wave frequency ranges should be considered normal in humans, with a commonly cited reference range being 2.5 – 3.6 cpm, such that ‘bradygastric’ or ‘tachygastric’ frequencies are defined outside this range.9 While these classifications remain useful, particularly for electrogastrography (EGG) interpretation, their importance in humans may have been overstated in the past because it now appears that much abnormal activity in humans occurs within this normal range.10
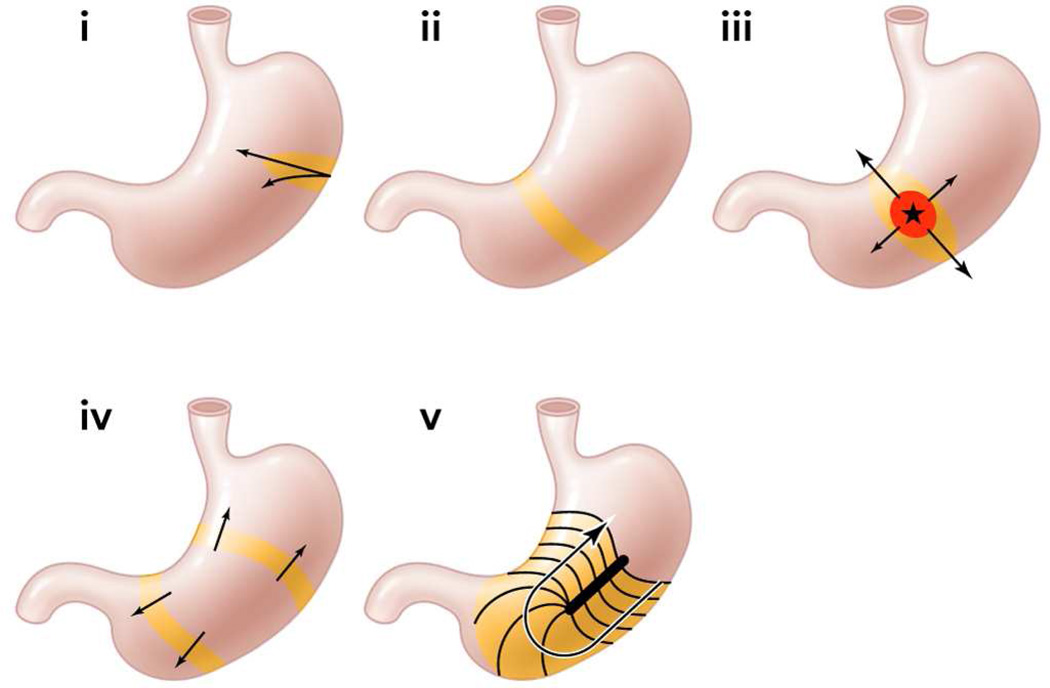
An important recent finding for arrhythmia analysis has been to clarify the role of velocity anisotropy in normal human gastric conduction. Studies in several species initially documented the presence of rapid conduction in the vicinity of the normal pacemaker, compared with the surrounding corpus (Figure 1),7,11,12 and it is now known that this occurs because conduction is ~2.5× more rapid in the transverse direction of the human stomach than in the longitudinal (organoaxial) direction.10 Wavefronts spread out rapidly and circumferentially from the pacemaker, however, by the mid-corpus, complete rings of activation have formed such that the rapid circumferential conduction ceases, and the rings thereafter move slowly and longitudinally down the stomach (Figure 1B).11,13 Importantly, during gastric arrhythmias, rapid circumferential propagation re-emerges to become a major determinant of conduction patterns, as a consequence of either ectopic pacemaking or disruption to the normal ring wavefronts by conduction blocks (Figure 2).10,13
Figure 2.
Illustration of velocity anisotropy during normal and arrhythmic gastric conduction.10,13 In human stomach, conduction is 2.5× more rapid in the circumferential gastric axis. i. At the normal pacemaker site, conduction occurs rapidly in the circumferential axis. ii. Complete ring wavefronts rapidly form by the mid corpus, such that circumferential conduction ceases and slow longitudinal conduction dominates. Iii. Rapid circumferential propagation accompanies ectopic pacemaking, inducing elliptical wavefront patterns. iv: Organized ring wavefronts may arise from ectopic sources that propagate in both antegrade and retrograde directions. V. In conduction blocks, rapid circumferential propagation occurs beneath the site of block. From Cheng LK, Du P, O’Grady G. Mapping and modeling gastrointestinal bioelectricity: from engineering bench to bedside. Physiology. 2013;28:310–317; with permission.
It is probable that there two complimentary gastric conduction pathways are active to explain these anisotropic velocity properties. For example, ICC-MP and ICC-IM might form a bidirectionally-coupled network, such that the leading network switches between ICC-MP (dominant during longitudinal conduction) and circular ICC-IM (dominant during circumferential conduction), depending on the presence or absence of complete gastric ring wavefronts.13 However, this hypothesis awaits experimental validation, and the role of other ICC populations must be clarified.
The Pathological Basis for Arrhythmias in Gastroparesis
Research by the U.S. Gastroparesis Clinical Research Consortium (GpCRC) has recently clarified the cellular pathologies underlying idiopathic and diabetic gastroparesis. A reduced density of ICC was found to be the most prominent cellular abnormality in both aetiologies, with remaining ICC also showing injury.14,15 Other abnormalities were also routinely documented, including a decreased density nerve fibres, inflammatory infiltrate, and a marked stromal fibrosis.14,15 However, the ICC loss may be of particular functional significance to gastroparesis because it has been correlated with delayed gastric emptying in humans,16 as well as in experimental models.17
Given the central role played by ICC in generating and propagating slow waves, these pathological abnormalities now provide a rational basis for the genesis of arrhythmias in gastroparesis. This concept is also supported by a study showing that the severity of ICC loss correlates with the presence of arrhythmias when assessed by EGG.18 While the exact chain of events responsible for these relationships still requires investigation, recent work discussed below indicates several plausible mechanisms may be contributing.
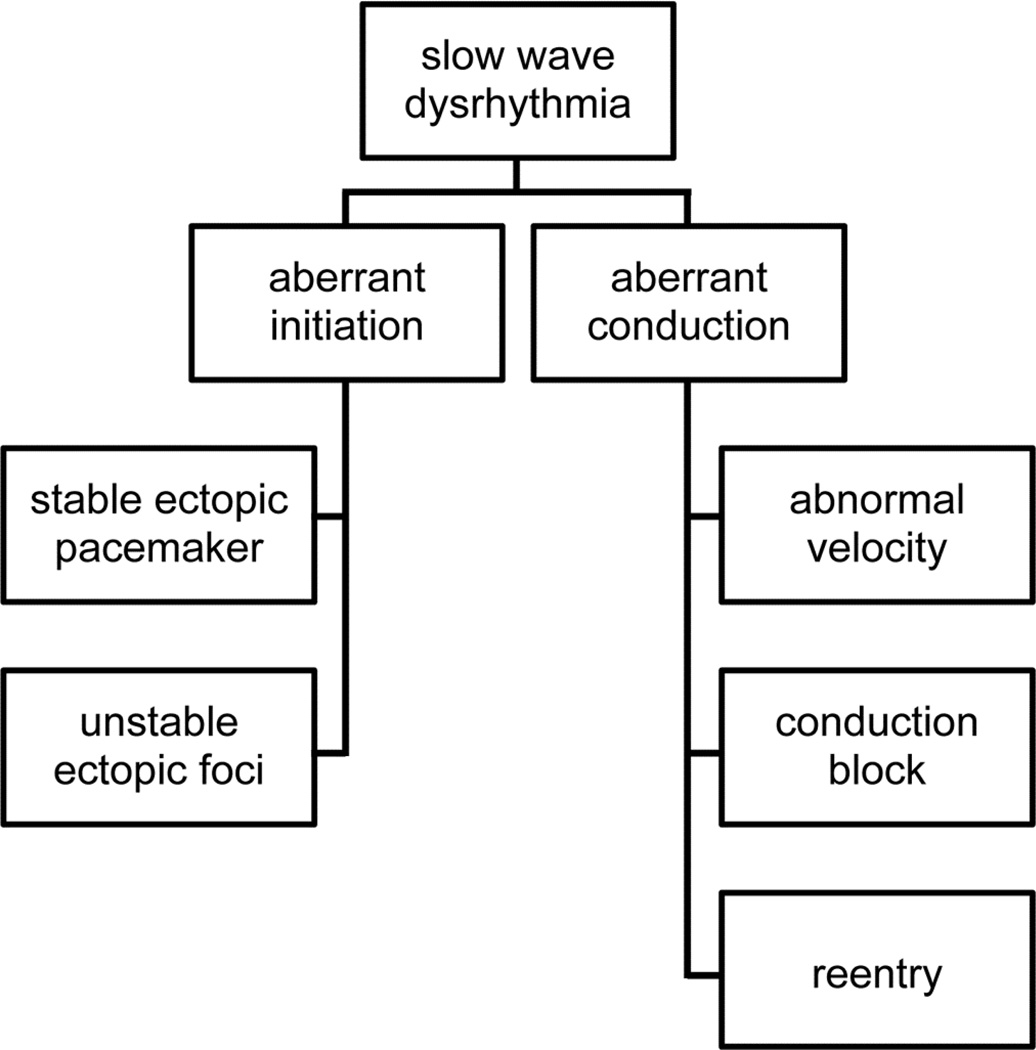
One helpful classification for understanding gastric arrhythmias, adapted from cardiac rhythm analysis, is to consider them as either ‘disorders or initiation’ or ‘disorders of conduction’ (Figure 3).10,19 Under this schema, it can be surmised that disorders of slow wave initiation result from abnormalities to intrinsic ICC frequencies, whereas disorders of conduction result from disruption to slow wave entrainment through ICC networks.20
Figure 3.
An arrhythmia classification system based on spatial analyses of abnormal patterns.
In view of the above histopathological findings recently elucidated in gastroparesis, distinct mechanisms may be proposed to contribute to ICC initiation and conduction abnormalities. In terms of initiation disorders, it is known that non-lethal stressors can promote aberrant frequency responses within ICC, including in humans, adversely affecting intrinsic frequency gradients.21,22 Cholinergic regulation is also known to modulate ICC-IM frequencies, potentially inducing arrhythmias,23 and a role for autonomic neuropathy in aberrant slow wave initiation therefore deserves further investigation. In terms of conduction abnormalities, biophysically-based modeling studies have shown that slowed haphazard conduction occurs in states of ICC loss,24 potentially promoting arrhythmias. In addition, conduction blocks must result when ICC loss falls below a critical threshold.10 The presence of fibrosis might also be significant, because in cardiac electrophysiology fibrosis is well known to slow electrical conduction and to play an important role in arrhythmogenesis.25
Of clinical interest, another patient group where ICC pathologies may be relevant are those with chronic unexplained nausea and vomiting (CUNV). It was recently shown that this poorly characterised disease state, in which gastric emptying is normal, shows substantial clinical overlap with gastroparesis, being equivalent in terms of demographics, symptoms, disease duration, health care utilisation, and quality of life.26 Emerging work now further suggests that CUNV might even be considered within the same disease category as gastroparesis, because these patients also appear to display reduced ICC and electrical arrhythmias that are similar to those described in gastroparesis.27 Accumulating evidence points to a threshold of ICC loss at which failure of gastric emptying becomes likely, at around ~3 cell bodies per high-powered field within circular muscle, which may be reached in gastroparesis but not in CUNV.27,28
Clinical Methods for Gastric Electrical Testing
Electrogastrography
Electrogastrography (EGG) has long been the most widely researched and practiced technique for assessing gastric electrical activity and arrhythmias, involving the placement of cutaneous electrodes over the surface of the epigastrium.29 A detailed explanation of how to conduct and interpret EGG is available elsewhere.9,29
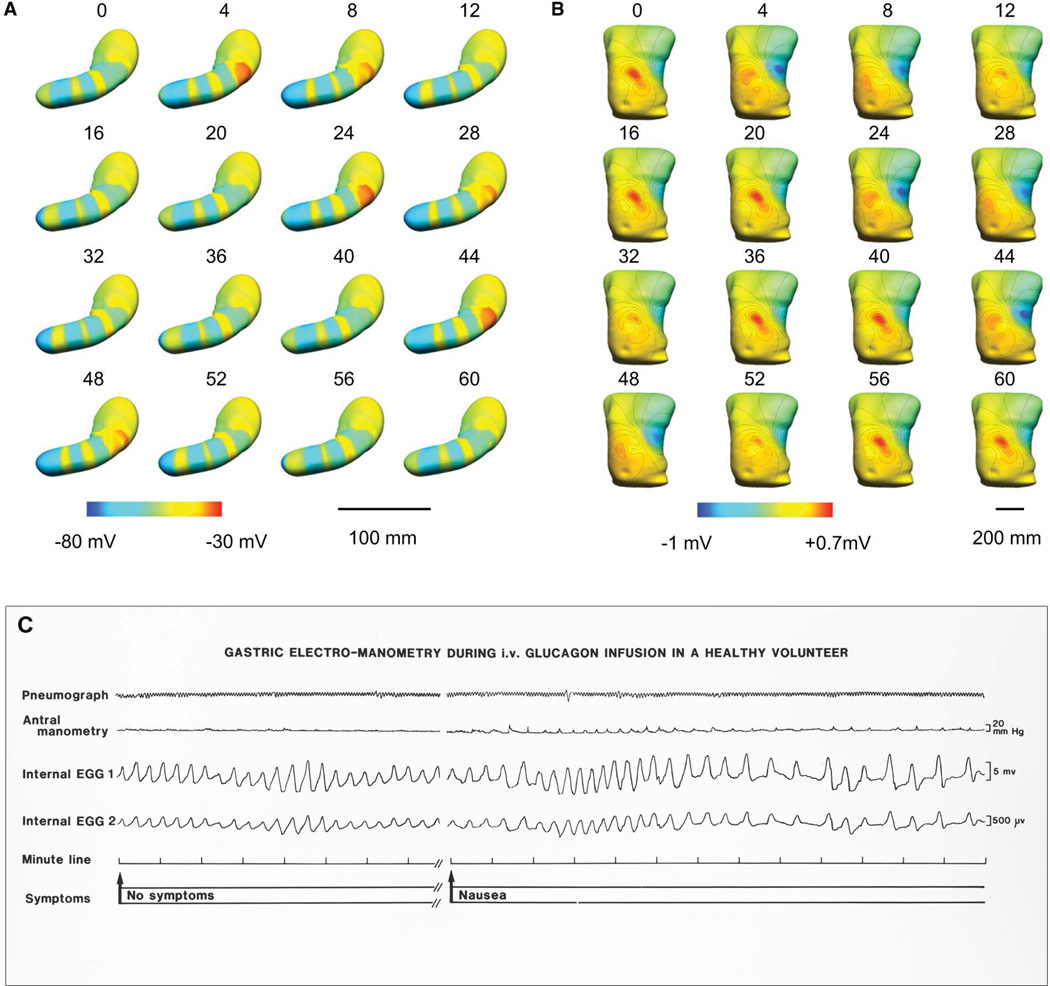
EGG is clinically attractive due to its low invasiveness, however, this lack of invasiveness also underlies its several limitations. Because cutaneous signals are recorded at distance from their gastric sources, they offer only a fundamentally limited, highly summative, representation of gastric activation, and it is currently difficult to relate the meaning of these highly integrated signals back to the underlying slow wave events.30 This problem is further compounded by the fact that three wavefronts may be simultaneously active in the human stomach at any one time, and possibly more during arrhythmia, which are then summated within a single EGG waveform (Figure 4).7
Figure 4.
A.B. The relationship between slow waves and EGG analyzed by a virtual model. Multiple slow waves propagate in the human stomach at any one time, however, EGG achieves only a summative view. A. Gastric pattern over 60 s of propagation. Red represents depolarized activity, blue the resting membrane potentials. B. Simulated resultant body surface EGG potentials of this same activity. Red represents positive potentials and the blue negative potentials in the torso field. The contour lines represent 0.1-mV increments. From Du P, O’Grady G, Cheng LK, Pullan AJ. A multi-scale model of the electrophysiological basis of the human electrogastrogram. Biophys J. 2010;99:2784–2792; with permission. C. Example slow wave traces showing arrhythmic frequencies associated with nausea, following glucagon infusion in a healthy volunteer. While reflective of frequency, the summative detail achieved by current EGG methods is in contrast to the rich spatial detail achieved in HR mapping (Figures 5–7).
Despite these considerable limitations, EGG can reliably reflect the underlying dominant slow wave frequency,31 and for this reason, the primary focus of EGG analyses to date have been in the frequency domain, include analyzing the stability of frequency.9,29 While such data is useful, the recent evidence that human gastric arrhythmias routinely occur at normal frequencies means that frequency-focused approaches may generally underestimate arrhythmia occurrence.10 Nevertheless, a large number of successful EGG studies have accrued in the literature in recent decades that do consistently demonstrate clear associations between arrhythmic EGG parameters and gastroparesis (e.g.32,33).
Despite its diagnostic promise, clinical adoption of EGG has remained weak, with practice limited to a small number of specialist referral and research centres. In addition to the limitations above, other factors have contributed to this lack of general clinical interest. The signals are of low amplitude and susceptible to artifacts, such that analyses can be difficult in non-specialist hands.34 The test is also perceived as having an incomplete sensitivity and specificity, inconsistent associations with symptoms, and validated protocols defining the role of EGG in clinical algorithms are lacking.9 The positive predictive value of EGG for normal gastric emptying ranges from 65–100% in published studies, vs 50–80% for predicting abnormal gastric emptying;9 although, it should be noted that EGG and gastric emptying measure different aspects of motility physiology.
Nevertheless, EGG still retains an active and useful role, particularly in research studies, as well as clinically in the screening and diagnosis for motility disorders.9,29 The promise and limitations of EGG also continue to motivate the development of improved foundations and clinical methods of conducting the test, for example, by introducing multichannel approaches.30,35 However, more accurate and invasive tests of gastric electrical function are much needed to further progress this field.
Extracellular Recordings
Extracellular recordings taken directly from the gastric surface provide the most useful method of analysing gastric electrical activity in clinical practice. Extracellular recordings may be undertaken at the mucosal or serosal surfaces, classically by using a small number of electrodes placed at sparse intervals,3,36 or more recently, by applying dense arrays of electrodes to achieve multi-electrode (high-resolution; HR) mapping.
The biophysical basis of extracellular recordings was recently addressed, demonstrating robust knowledge of how this technique relates to the underlying electrical activation.37 Extracellular recordings reflect the localized conduction of slow waves through a defined area of tissue beneath and around the electrode. The potentials approximate the second-derivative of the intracellular slow wave time-course, typically adopting a biphasic or triphasic morphology, with the steep negative descent in these signals corresponding to the time of arrival of a wavefront beneath the electrode.37
As with EGG, several studies using sparse electrodes have clearly linked arrhythmia with gastroparesis.36,38 However, the limitations of such studies must again be appreciated. Single point or linear reconstructions from sparse electrodes give no spatial detail about gastric slow wave patterns, meaning that these analyses have again been largely limited to frequency and rhythm domains, which are not always reliable predictors of arrhythmic onset.10 Spatial aliasing of spares data can also lead to misinterpretations.7,13
Mucosal recordings and ‘low resolution mapping’
Clinically-relevant extracellular techniques have evolved over the last several decades, and particularly approaches to measure mucosal electrical activity. Different systems to secure mucosal electrodes have been trialled, including one early system utilizing external rare-earth magnets that enabled a comparison of internal (mucosal) and external (cutaneous) recordings simultaneously, providing validity for these approaches.39
Some mucosal recording techniques have utilized more than one electrode thus allowing low-resolution reconstructions of propagation to be estimated. More recently, with the advent of temporary gastric electrical stimulation, mucosal recordings, both single point and multiple sites, have been obtained at the time of surgery as part of routine clinical practice.40,41 In addition the same low resolution approaches can be used serosally, often at the time of gastric stimulator insertion, and with the delivery of energy through proximal to distal electrodes.
High-resolution Electrical Mapping
In recent years, the limitations with the sparse electrode techniques led Lammers et al to introduce multi-electrode (high-resolution; HR) mapping of slow wave activity.42 HR mapping involves the placement of dense arrays of many electrodes to track the propagation of slow wave propagation patterns with fine spatiotemporal accuracy.43 These techniques are well established in the clinical management of modern cardiac arrhythmias, and are now showing similar potential in the GI field.44
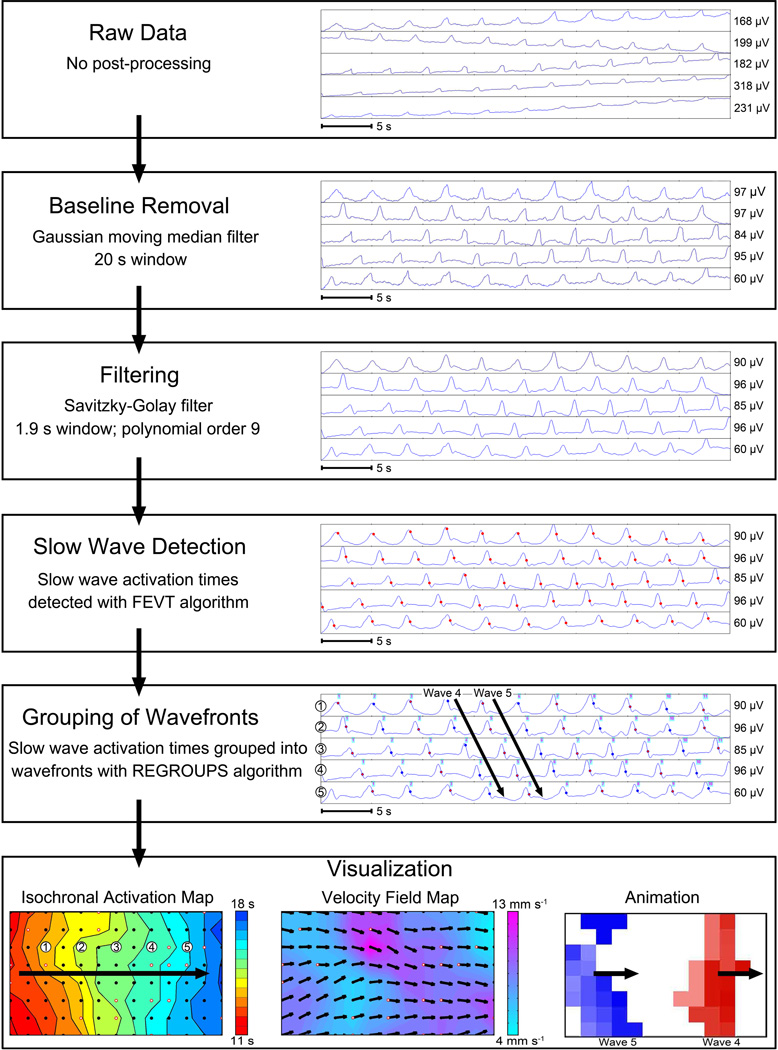
Progress in GI HR mapping is currently accelerating due to engineering achievements. These advances include mass-producing HR arrays suitable for human applications,43 and automated methods for analyzing and visualizing the vast volumes of retrieved data.45 The steps involved in HR mapping analyses are summarized in Figure 5.
Figure 5.
An example of HR mapping analysis, based on intestinal multi-electrode recordings. First, raw data is filtered to remove baseline wander and extraneous noise.58 Slow-wave ‘activation times’ are then marked at the point of steepest negative descent (red dots), before ‘grouping’ of individual events into their propagation cycles. Algorithms and software are now available to automate these time-intensive tasks,59,60 but careful manual review and correction are still currently required for accurate results in humans. Three forms of visualization are illustrated. Isochronal activation maps (bottom left), which quantify propagation patterns in spatiotemporal detail. Each dot represents an electrode, and each color band shows the ‘isochronal interval’ (area propagated per unit time; here 0.5 s).60 Velocity field maps (bottom center) display speed using a color gradient and propagation direction using arrows at each electrode.61 Propagation patterns may also be animated (bottom right),45 with multiple waves passing through this mapped area concurrently. From O’Grady G, Angeli T, Du P et al. Abnormal initiation and conduction of slow wave activity in gastroparesis, defined by high-resolution electrical mapping. Gastroenterology. 2012;143:589–598; with permission.
The outputs of HR mapping are proving a critical advance in this field, initiating a new era in understanding of the events underlying arrhythmic initiation and maintenance.44 A surprising complexity to gastric arrhythmias is being revealed, necessitating new terms and classification schemes adapted from cardiology, including focal events and re-entry as mechanisms of tachygastria, and conduction block and retrograde escape rhythms as mechanism of bradygastria.19,46 It has also been possible to assess the dynamic evolution of arrhythmic patterns through time, including defining wavefront interactions and collisions.
Despite its clear advantages, the major limitation of HR mapping is its invasiveness. All studies to date have been performed in fasted anesthetized subjects subjected to surgery. To achieve clinical translation, major technical advances are still required to enable effective endoscopic approaches that can be routinely employed in clinical practice for accurate arrhythmia analysis.
High-Resolution Mapping in Gastroparesis
Recently, clinical translation of HR mapping was achieved in patients with gastroparesis, who were mapped at the time of gastric stimulator implantation.10 This work enabled several novel clinically-relevant insights into the nature and mechanisms of arrhythmias, some of which have been discussed above, and which can be summarized as follows:
Gastric arrhythmias may be usefully classified as disorders of initiation and disorders of conduction (Figure 3). Both of these classes appear to be prevalent in gastroparesis, however, most past EGG and sparse electrode analyses had focused disproportionately on disorders of initiation.
In humans, gastric arrhythmias routinely occur at normal slow wave frequencies. Therefore, many arrhythmic events may be missed by techniques focused mainly on frequency-based analyses, including EGG and sparse electrode studies. This issue party explains inconsistencies seen in past arrhythmia investigations.
Rapid circumferential conduction of slow wave activity (i.e. 2.5× velocity of longitudinal conduction) is a determining feature of arrhythmic activation patterns, always emerging at sites of ectopic pacemaking or when normal gastric ring wavefronts are disrupted (Figure 6).
This rapid circumferential conduction may serve to quickly restore normal antegrade propagation during arrhythmia, but may also lead to organized retrograde propagations that interfere with proximal wavefronts (Figure 6c). In addition, rapid circumferential conduction is always accompanied by a large increase in the amplitude of the extracellular potentials during arrhythmia (Figure6c).13
In general, however, the amplitude of extracellular slow wave recordings appears to be reduced in gastroparesis compared to healthy controls, possibly reflecting a reduced bulk of excited cells, consistent with the underlying pathology of the disease.
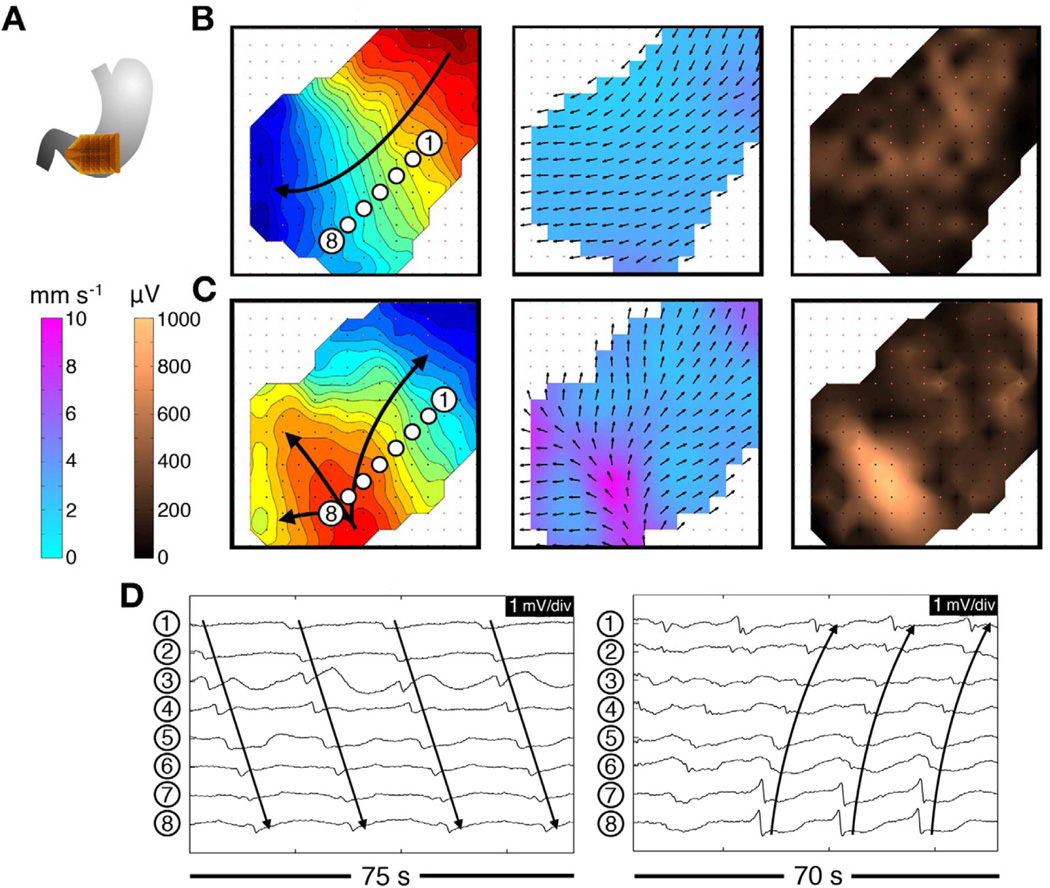
Figure 6.
An example of regular tachygastria mapping at HR in a patient with diabetic gastroparesis. Isochronal intervals = 1 second. A. Array position. B. Normal activity was initially mapped for 280 seconds (3.3 cpm); isochronal, velocity, and amplitude maps are shown. C. Regular tachygastria followed due to an ectopic pacemaker (4.0 cpm). Isochronal maps show organized retrograde propagation, with velocity and amplitude maps demonstrating the emergence of rapid circumferential propagation and associated high-amplitude potentials near the ectopic focus. D. Representative electrograms from these normal (left) and arrhythmic activities (right). From O’Grady G, Angeli T, Du P et al. Abnormal initiation and conduction of slow wave activity in gastroparesis, defined by high-resolution electrical mapping. Gastroenterology. 2012;143:589–598; with permission.
Representative examples of tachygastric patterns mapped in gastroparesis using high-resolution methods are shown in Figures 6 and 7.
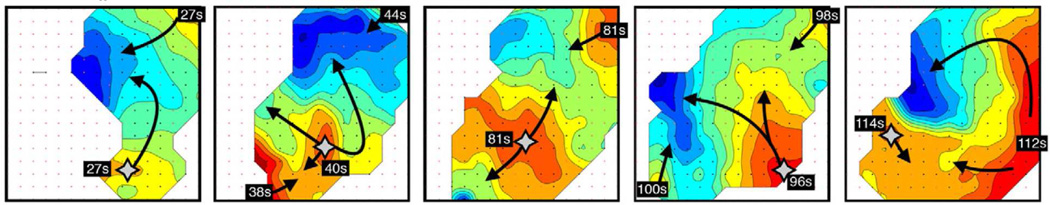
Figure 7.
An example of an irregular tachygastria defined by HR mapping, due to unstable ectopic events arising at multiple locations (stars; duration >200 seconds). The activity was mapped from the same patient and in the same location as that shown in Figure 6. Isochronal maps of 5 representative cycles (isochronal intervals = 1 s) showing chaotic tissue activation and wave collisions, resulting in a range or frequencies across the mapped field (1.4 –5.7 cpm). From O’Grady G, Angeli T, Du P et al. Abnormal initiation and conduction of slow wave activity in gastroparesis, defined by high-resolution electrical mapping. Gastroenterology. 2012;143:589–598; with permission.
Do Gastric Arrhythmias Cause Gastroparesis Symptoms?
One of the areas of controversy regarding gastric arrhythmias has been concerning their role in symptom generation. Previous studies have demonstrated somewhat inconsistent associations with symptoms, possibly reflecting the limitations of past research techniques, and leading some to consider arrhythmias a mere epiphenomenon.
The strongest links between arrhythmias and symptoms are concerning nausea.47 In general, arrhythmias are measurable whenever nausea occurs, including in chronic disease states such as gastroparesis, as well as in more acute states such as motion sickness, following chemotherapy, and nausea of pregnancy.48,49 Arrhythmias are therefore a useful objective biomarker for nausea, and can be considered an important potential therapeutic target for anti-nausea therapies.47
Supporting a true role for arrhythmias in nausea genesis is the observation that during motion sickness simulations, rhythm disturbances clearly arise and peak prior to symptoms, with their severity correlating with nausea intensity.50,51 In addition, gastric pacing appears to have potential to both revert arrhythmia and reduce nausea, and in patients with gastroparesis, improvements in EGG parameters have been linked with the resolution of nausea and vomiting.32,52 Further research is still needed to confirm these findings, and correlations between arrhythmias and symptoms other than nausea are less certain.
Future Directions
Knowledge of gastric arrhythmias is now evolving rapidly, underpinned by the recent technical advances describe above. The crucial challenge must be to now translate this knowledge into improved clinical methods of testing gastric electrical activity.
The essential step must be to develop an endoscopic approach to HR mapping. This goal constitutes a major engineering challenge, due to the difficulties of safely instrumenting a complex device via the oesophagus, and the problem of resolving satisfactory signals at the high-impedance mucosal interface. Nevertheless, published approaches have shown proof-of-principle, suggesting it is feasible.53
In the meantime, low-resolution methods may serve as an effective bridging technique, such as by using serial placements of sparse electrodes, with the analyses guided by current HR mapping methods. For example, the knowledge that slow wave amplitudes increase ~2.5× near arrhythmic foci, as a consequence of resultant rapid circumferential conduction, could become a useful biomarker of arrhythmic onset in sparse electrodes.10,13 A number of strategies such as laparoscopic and wireless telemetry mapping systems are now being developed to exploit such information in clinical trials within coming years.54,55
Future work must also address therapies; at present there have been few studies designed to reverse arrhythmias and/or their symptoms. Both gastric pacing and high-frequency (low-energy) electrical stimulation approaches have been attempted, but require further investigation using HR techniques before their potential can be fully established.56 There are also few trials of pharmacological agents to treat arrhythmias, though target pathways of potential therapeutic value have been identified.20
Much work is needed before these advances translate to improved care. However, a preliminary clinical protocol has been proposed and implemented for arrhythmia management, serving as a potential template for future advances (Figure 8).
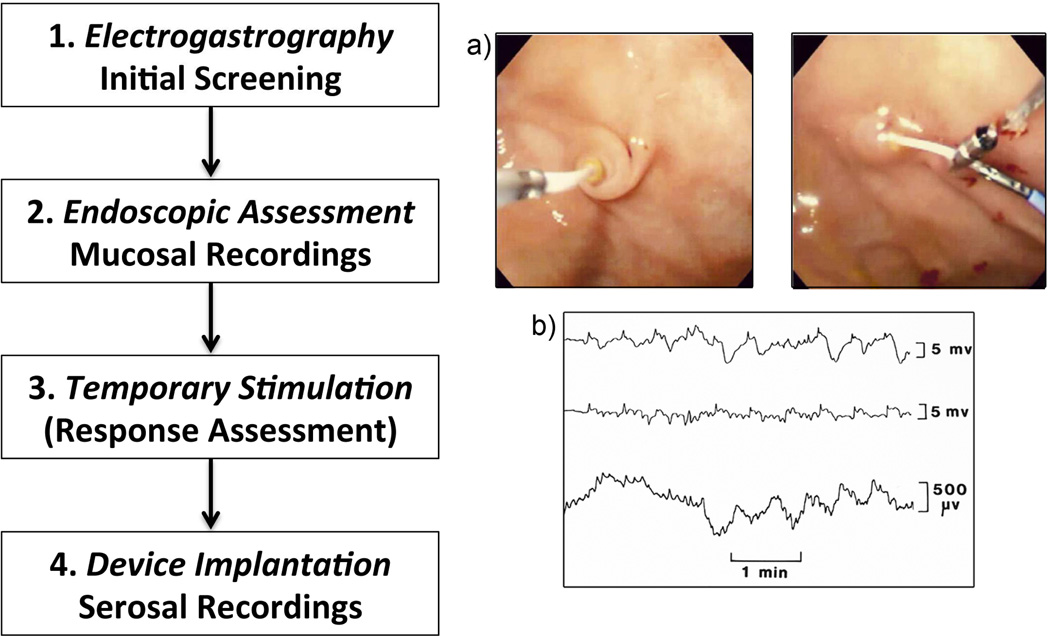
Figure 8.
A preliminary clinical protocol being implemented for arrhythmia management. There is a current need to advance methods for each of these components (1–4), including applying mapping-based strategies. a) Examples of mucosal slow wave recording (left) and temporary stimulator placement (right); b) Example electrograms from mucosal recordings (top two rows) and simultaneous EGG trace (bottom row). From Ayinala S, Batista O, Goyal A, et al. Temporary gastric electrical stimulation with orally or PEG-placed electrodes in patients with drug refractory gastroparesis. Gastrointest Endosc. 2005 Mar;61(3):455–61; with permission.
In summary, there has been substantial recent progress in gastric arrhythmia due to complementary pathological, engineering, physiological and clinical advances. As a result, the opportunity now exists to definitively define the role and value of new electrical diagnostic tests in clinical practice. However, it still remains to be seen whether arrhythmias can be meaningfully treated in a way that improves organ function and quality of life in patients.
Key Points.
The discovery that interstitial cells of Cajal (ICC) are depleted in gastroparesis provides mechanistic insights into arrhythmogenesis, and reinvigorates clinical interest in gastric electrical testing.
Gastric arrhythmias may play a role in pathophysiology and symptom generation in gastroparesis, and in particular, may contribute to chronic nausea.
Electrogastrography (EGG) has been the dominant clinical method to date, and has demonstrated an association between arrhythmias and gastroparesis; however, EGG is critically limited by its summative nature and incomplete sensitivity.
The advent of high-resolution (HR; multi-electrode) mapping has been a key advance, and is enabling new insights and classifications.
HR mapping is currently limited by its invasiveness and low resolution recordings are therefore being assessed as a bridging method until endoscopic high-resolution recording systems become available for clinical use.
Acknowledgments
Funding
This work was principally supported by the NIH (R01 DK64775) and the New Zealand Health Research Council.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
TLA is a licensor, consultant, and investigator for Medtronic, Inc.
References
- 1.Hasler WL. Gastroparesis: pathogenesis, diagnosis and management. Nat Rev Gastroenterol Hepatol. 2011;8:438–453. doi: 10.1038/nrgastro.2011.116. [DOI] [PubMed] [Google Scholar]
- 2.Camilleri M, Parkman HP, Shafi MA, Abell TL, Gerson L. Clinical guideline: management of gastroparesis. Am J Gastroenterol. 2013;108:18–37. doi: 10.1038/ajg.2012.373. quiz 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.You CH, Lee KY, Chey WY, Menguy R. Electrogastrographic study of patients with unexplained nausea, bloating, and vomiting. Gastroenterology. 1980;79:311–314. [PubMed] [Google Scholar]
- 4.Huizinga JD, Lammers WJEP. Gut peristalsis is coordinated by a multitude of cooperating mechanisms. Am J Physiol Gastrointest Liver Physiol. 2009;296:1–8. doi: 10.1152/ajpgi.90380.2008. [DOI] [PubMed] [Google Scholar]
- 5.Torihashi S, Horisawa M, Watanabe Y. c-Kit immunoreactive interstitial cells in the human gastrointestinal tract. J Auton Nerv Syst. 1999;75:38–50. doi: 10.1016/s0165-1838(98)00174-x. [DOI] [PubMed] [Google Scholar]
- 6.Wang XY, Lammers WJ, Bercik P, Huizinga JD. Lack of pyloric interstitial cells of Cajal explains distinct peristaltic motor patterns in stomach and small intestine. Am J Physiol Gastrointest Liver Physiol. 2005;289:G539–G549. doi: 10.1152/ajpgi.00046.2005. [DOI] [PubMed] [Google Scholar]
- 7.O’Grady G, Du P, Cheng LK, et al. The origin and propagation of human gastric slow wave activity defined by high-resolution mapping. Am J Physiol Gastrointest Liver Physiol. 2010;299:585–592. doi: 10.1152/ajpgi.00125.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hinder RA, Kelly KA. Human gastric pacesetter potential. Site of origin, spread, and response to gastric transection and proximal gastric vagotomy. Am J Surg. 1977;133:29–33. doi: 10.1016/0002-9610(77)90187-8. [DOI] [PubMed] [Google Scholar]
- 9.Parkman HP, Hasler WL, Barnett JL, Eaker EY. Electrogastrography: a document prepared by the gastric section of the American Motility Society Clinical GI Motility Testing Task Force. Neurogastroenterol Motil. 2003;2003:89–102. doi: 10.1046/j.1365-2982.2003.00396.x. [DOI] [PubMed] [Google Scholar]
- 10.O’Grady G, Angeli T, Du P, et al. Abnormal initiation and conduction of slow wave activity in gastroparesis, defined by high-resolution electrical mapping. Gastroenterology. 2012;143:589–598. doi: 10.1053/j.gastro.2012.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lammers WJ, Ver Donck L, Stephen B, Smets D, Schuurkes JA. Origin and propagation of the slow wave in the canine stomach: the outlines of a gastric conduction system. Am J Physiol Gastrointest Liver Physiol. 2009;296:1200–1210. doi: 10.1152/ajpgi.90581.2008. [DOI] [PubMed] [Google Scholar]
- 12.Egbuji JU, O’Grady G, Du P, et al. Origin, propagation and regional characteristics of porcine gastric slow wave activity determined by high-resolution mapping. Neurogastroenterol Motil. 2010;22:e292–e300. doi: 10.1111/j.1365-2982.2010.01538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Grady G, Du P, Paskaranandavadivel N, et al. Rapid high-amplitude circumferential slow wave conduction during normal gastric pacemaking and dysrhythmia. Neurogastroenterol Motil. 2012;24:e299–e312. doi: 10.1111/j.1365-2982.2012.01932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grover M, Farrugia G, Lurken MS, et al. Cellular changes in diabetic and idiopathic gastroparesis. Gastroenterology. 2011;140:1575.e8–1585.e8. doi: 10.1053/j.gastro.2011.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faussone-Pellegrini MS, Grover M, Pasricha PJ, et al. Ultrastructural differences between diabetic and idiopathic gastroparesis. J Cell Mol Med. 2012;16:1573–1581. doi: 10.1111/j.1582-4934.2011.01451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grover M, Bernard CE, Pasricha PJ, et al. Clinical-histological associations in gastroparesis: results from the Gastroparesis Clinical Research Consortium. Neurogastroenterol Motil. 2012;24:531–539. e249. doi: 10.1111/j.1365-2982.2012.01894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi KM, Gibbons SJ, Nguyen TV, et al. Heme oxygenase-1 protects interstitial cells of Cajal from oxidative stress and reverses diabetic gastroparesis. Gastroenterology. 2008;135:2055–2064. 2064.e1. doi: 10.1053/j.gastro.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin Z, Sarosiek I, Forster J, Damjanov I, Hou Q, McCallum RW. Association of the status of interstitial cells of Cajal and electrogastrogram parameters, gastric emptying and symptoms in patients with gastroparesis. Neurogastroenterol Motil. 2010;22:56–61. e10. doi: 10.1111/j.1365-2982.2009.01365.x. [DOI] [PubMed] [Google Scholar]
- 19.O’Grady G, Egbuji JU, Du P, et al. High-resolution spatial analysis of slow wave initiation and conduction in porcine gastric dysrhythmia. Neurogastroenterol Motil. 2011;23:e345–e355. doi: 10.1111/j.1365-2982.2011.01739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Grady G, Wang TH, Du P, Angeli T, Lammers WJ, Cheng LK. Recent progress in gastric dysrhythmia: pathophysiology, clinical significance and future horizons. Clin Exp Pharmacol Physiol. 2013 doi: 10.1111/1440-1681.12288. Online Ahead of Press [doi to follow] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xue S, Valdez DT, Tremblay L, Collman PI, Diamant NE. Electrical slow wave activity of the cat stomach: its frequency gradient and the effect of indomethacin. Neurogastroenterol Motil. 1995;7:157–167. doi: 10.1111/j.1365-2982.1995.tb00221.x. [DOI] [PubMed] [Google Scholar]
- 22.O’Grady G, Pullan AJ, Cheng LK. The analysis of human gastric pacemaker activity. J Physiol. 2012;590:1299–1300. doi: 10.1113/jphysiol.2011.224014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forrest AS, Ordog T, Sanders KM. Neural regulation of slow-wave frequency in the murine gastric antrum. Am J Physiol Gastrointest Liver Physiol. 2006;290:G486–G495. doi: 10.1152/ajpgi.00349.2005. [DOI] [PubMed] [Google Scholar]
- 24.Du P, O’Grady G, Gibbons SJ, et al. Tissue-specific mathematical models of slow wave entrainment in wild-type and 5-HT2B knockout mice with altered interstitial cell of Cajal networks. Biophys J. 2010;98:1772–1781. doi: 10.1016/j.bpj.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munoz V, Zlochiver S, Jalife J. Fibrosis and Fibroblast Infiltration: An Active Structural Substrate for Altered Propagation and Spontaneous Tachyarrhythmias. In: Zipes DP, Jalife J, editors. Cardiac Electrophysiology, From Cell to Bedside. Philadelphia: Saunders Elsevier; 2009. pp. 215–221. [Google Scholar]
- 26.Pasricha PJ, Colvin R, Yates K, et al. Characteristics of patients with chronic unexplained nausea and vomiting and normal gastric emptying. Clin Gastroenterol Hepatol. 2011;9:567–576. doi: 10.1016/j.cgh.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Angeli TR, Cheng LK, Du P, et al. High-resolution mapping reveals gastric slow wave dysrhythmias in chronic nausea and vomiting. Neurogastroenterol Motil. 25:S1–S12. [Google Scholar]
- 28.Gomez-Pinilla PJ, Gibbons SJ, Sarr MG, et al. Changes in interstitial cells of cajal with age in the human stomach and colon. Neurogastroenterol Motil. 2011;23:36–44. doi: 10.1111/j.1365-2982.2010.01590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen JD, Lin Z, Yin Y. Electrogastrography. In: Parkman HP, McCallum RW, Rao SC, editors. GI Motility Testing: A Laboratory and Office Handbook. Thorofare, NJ: SLACK Incorporated; 2011. pp. 81–92. [Google Scholar]
- 30.Du P, O’Grady G, Cheng LK, Pullan AJ. A multi-scale model of the electrophysiological basis of the human electrogastrogram. Biophys J. 2010;99:2784–2792. doi: 10.1016/j.bpj.2010.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smout AJPM, Van der schee EJ, Grashuis JL. What is measured in electrogastrography? Dig Dis Sci. 1980;25:179–187. doi: 10.1007/BF01308136. [DOI] [PubMed] [Google Scholar]
- 32.Koch KL, Stern RM, Stewart WR, Vasey MW. Gastric emptying and gastric myoelectrical activity in patients with diabetic gastroparesis: effect of long-term domperidone treatment. Am J Gastroenterol. 1989;84:1069–1075. [PubMed] [Google Scholar]
- 33.Chen JD, Lin Z, Pan J, McCallum RW. Abnormal gastric myoelectrical activity and delayed gastric emptying in patients with symptoms suggestive of gastroparesis. Dig Dis Sci. 1996;41:1538–1545. doi: 10.1007/BF02087897. [DOI] [PubMed] [Google Scholar]
- 34.Verhagen MAMT, van Schelven LJ, Samsom M, Smout AJPM. Pitfalls in the analysis of electrogastrographic recordings. Gastroenterology. 1999;177:453–460. doi: 10.1053/gast.1999.0029900453. [DOI] [PubMed] [Google Scholar]
- 35.Simonian HP, Panganamamula K, Chen JZ, Fisher RS, Parkman HP. Multichannel electrogastrography (EGG) in symptomatic patients: a single center study. Am J Gastroenterol. 2004;99:478–485. doi: 10.1111/j.1572-0241.2004.04103.x. [DOI] [PubMed] [Google Scholar]
- 36.Bortolotti M, Sarti P, Barara L, Brunelli F. Gastric myoelectrical activity in patients with chronic idiopathic gastroparesis. J Gastroint Motil. 1990;2:104–108. [Google Scholar]
- 37.Angeli TR, Du P, Paskaranandavadivel N, et al. The bioelectrical basis and validity of gastrointestinal extracellular slow wave recordings. J Physiol. 2013;591:4567–4579. doi: 10.1113/jphysiol.2013.254292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin ZY, McCallum RW, Schirmer BD, Chen JD. Effects of pacing parameters on entrainment of gastric slow waves in patients with gastroparesis. Am J Physiol. 1998;274:G186–G191. doi: 10.1152/ajpgi.1998.274.1.G186. [DOI] [PubMed] [Google Scholar]
- 39.Abell TL, Malagelada JR. Glucagon-evoked gastric dysrhythmias in humans shown by an improved electrogastrographic technique. Gastroenterology. 1985;88:1932–1940. doi: 10.1016/0016-5085(85)90022-8. [DOI] [PubMed] [Google Scholar]
- 40.Daram SR, Tang SJ, Abell TL. Video: temporary gastric electrical stimulation for gastroparesis: endoscopic placement of electrodes (ENDOstim) Surg Endosc. 2011;25:3444–3445. doi: 10.1007/s00464-011-1710-5. [DOI] [PubMed] [Google Scholar]
- 41.Abell TL, Johnson WD, Kedar A, et al. A double-masked, randomized, placebo-controlled trial of temporary endoscopic mucosal gastric electrical stimulation for gastroparesis. Gastrointest Endosc. 2011;74:496.e3–503.e3. doi: 10.1016/j.gie.2011.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lammers WJ, al-Kais A, Singh S, Arafat K, el-Sharkawy TY. Multielectrode mapping of slow-wave activity in the isolated rabbit duodenum. J Appl Physiol. 1993;74:1454–1461. doi: 10.1152/jappl.1993.74.3.1454. [DOI] [PubMed] [Google Scholar]
- 43.Du P, O’Grady G, Egbuji JU, et al. High-resolution mapping of in vivo gastrointestinal slow wave activity using flexible printed circuit board electrodes: methodology and validation. Ann Biomed Eng. 2009;37:839–846. doi: 10.1007/s10439-009-9654-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lammers WJ. Arrhythmias in the gut. Neurogastroenterol Motil. 2013;25:353–357. doi: 10.1111/nmo.12116. [DOI] [PubMed] [Google Scholar]
- 45.Yassi R, O’Grady G, Paskaranandavadivel N, et al. The Gastrointestinal Electrical Mapping Suite (GEMS): Software for analysing and visualising gastrointestinal multi-electrode recordings. BMC Gastroenterol. 2012;12:60. doi: 10.1186/1471-230X-12-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lammers WJEP, Ver Donck L, Stephen B, Smets D, Schuurkes JAJ. Focal activities and re-entrant propagations as mechanisms of gastric tachyarrhythmias. Gastroenterology. 2008;135:1601–1611. doi: 10.1053/j.gastro.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 47.Koch KL. Gastric dysrhythmias: a potential objective measure of nausea. Exp Brain Res. 2014 doi: 10.1007/s00221-014-4007-9. In Press [doi to follow] [DOI] [PubMed] [Google Scholar]
- 48.O’Grady G, Wang T-H, Du P, Angeli T, Lammers WJEP, Cheng LK. Recent progress in gastric dysrhythmia: pathophysiology, clinical significance and future horizons. Clin Exp Pharmacol Physiol. 2014 doi: 10.1111/1440-1681.12288. In Press: doi to follow. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koch KL, Stern RM. Handbook of electrogastrography. Oxford: Oxford University Press; 2004. [Google Scholar]
- 50.Owyang C, Hasler WL. Physiology and pathophysiology of the interstitial cells of Cajal: from bench to bedside. VI. Pathogenesis and therapeutic approaches to human gastric dysrhythmias. Am J Physiol Gastrointest Liver Physiol. 2002;283:G8–G15. doi: 10.1152/ajpgi.00095.2002. [DOI] [PubMed] [Google Scholar]
- 51.Hasler WL, Kim MS, Chey WD, Stevenson V, Stein B, Owyang C. Central cholinergic and alpha-adrenergic mediation of gastric slow wave dysrhythmias evoked during motion sickness. Am J Physiol. 1995;268:G539–G547. doi: 10.1152/ajpgi.1995.268.4.G539. [DOI] [PubMed] [Google Scholar]
- 52.McCallum RW, Chen JD, Lin Z, Schirmer BD, Williams RD, Ross RA. Gastric pacing improves emptying and symptoms in patients with gastroparesis. Gastroenterology. 1998;114:456–461. doi: 10.1016/s0016-5085(98)70528-1. [DOI] [PubMed] [Google Scholar]
- 53.Coleski R, Hasler WL. Coupling and propagation of normal and dysrhythmic gastric slow waves during acute hyperglycaemia in healthy humans. Neurogastroenterol Motil. 2009;21:492–499. e1. doi: 10.1111/j.1365-2982.2008.01235.x. [DOI] [PubMed] [Google Scholar]
- 54.O’Grady G, Du P, Egbuji JU, et al. A novel laparoscopic device for measuring gastrointestinal slow-wave activity. Surg Endosc. 2009;23:2842–2848. doi: 10.1007/s00464-009-0515-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Farajidavar A, O’Grady G, Rao SM, Cheng LK, Abell T, Chiao JC. A miniature bidirectional telemetry system for in vivo gastric slow wave recordings. Physiol Meas. 2012;33:N29–N37. doi: 10.1088/0967-3334/33/6/N29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.O’Grady G, Du P, Lammers WJ, et al. High-resolution entrainment mapping for gastric pacing: a new analytic tool. Am J Physiol Gastrointest Liver Physiol. 2010;298:314–321. doi: 10.1152/ajpgi.00389.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cheng LK, Du P, O’Grady G. Mapping and modeling gastrointestinal bioelectricity: from engineering bench to bedside. Physiology. 2013;28:310–317. doi: 10.1152/physiol.00022.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Paskaranandavadivel N, O’Grady G, Du P, Cheng LK. Comparison of filtering methods for extracellular gastric slow wave recordings. Neurogastroenterol Motil. 2013;25:79–83. doi: 10.1111/nmo.12012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Erickson JC, O’Grady G, Du P, et al. Falling-edge, variable threshold (FEVT) method for the automated detection of gastric slow wave events in serosal high-resolution electrical recordings. Ann Biomed Eng. 2010;38:1511–1529. doi: 10.1007/s10439-009-9870-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Erickson JC, O’Grady G, Du P, Egbuji JU, Pullan AJ, Cheng LK. Automated cycle partitioning and visualization of high-resolution activation time maps of gastric slow wave recordings: the Region Growing Using Polynomial Surface-estimate stabilization (REGROUPS) Algorithm. Ann Biomed Eng. 2011;39:469–483. doi: 10.1007/s10439-010-0170-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Paskaranandavadivel N, O’Grady G, Du P, Pullan AJ, Cheng LK. An improved method for the estimation and visualization of velocity fields from gastric high-resolution electrical mapping. IEEE Trans Biomed Eng. 2012;59:882–889. doi: 10.1109/TBME.2011.2181845. [DOI] [PMC free article] [PubMed] [Google Scholar]