Abstract
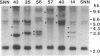
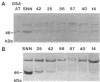
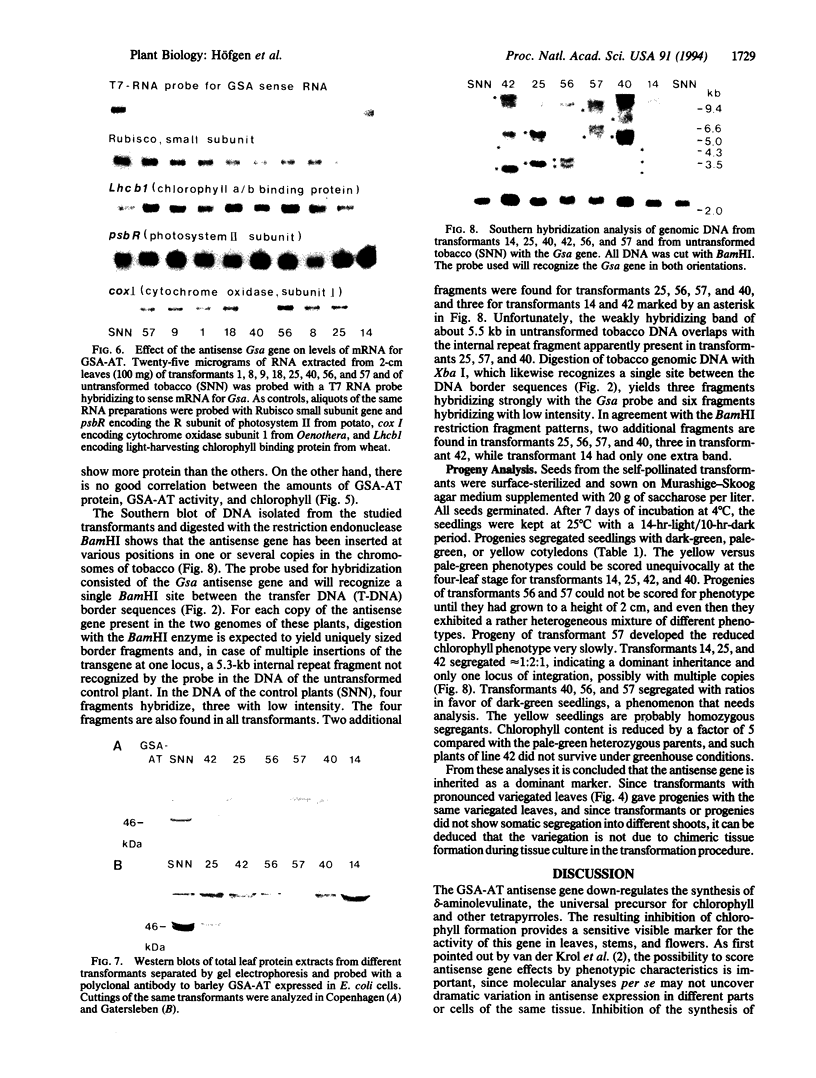
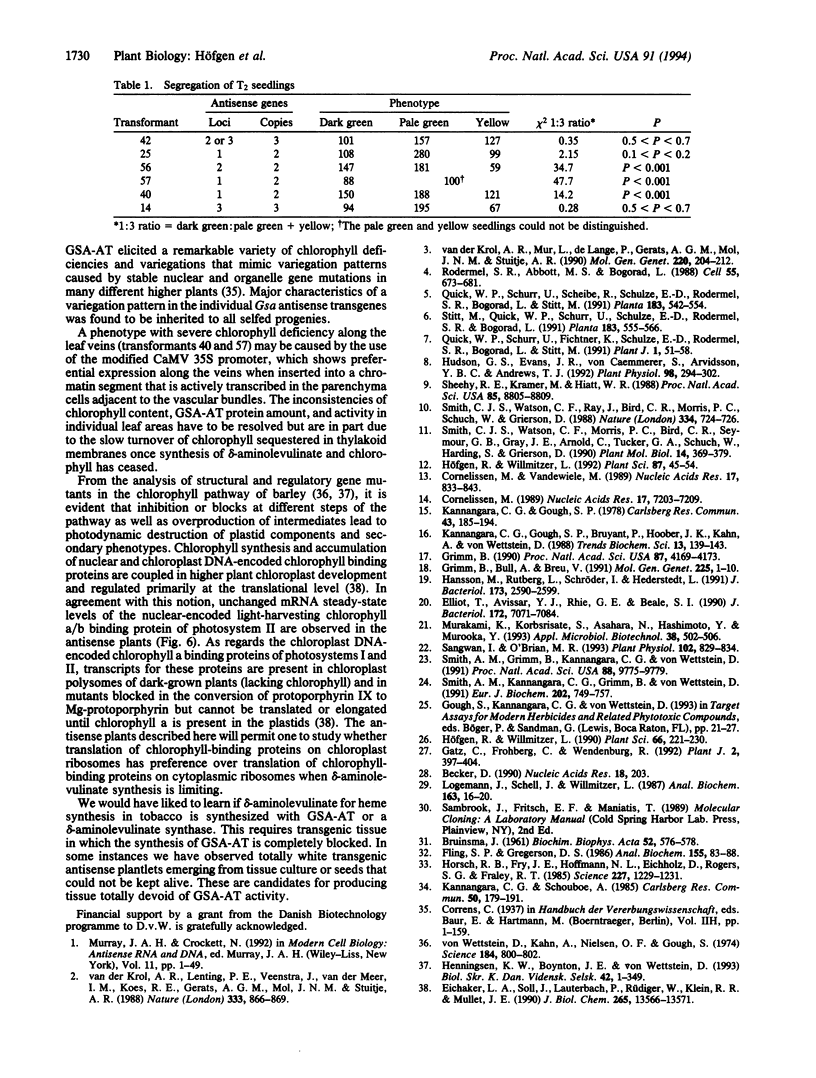
Glutamate 1-semialdehyde aminotransferase [(S)-4-amino-5-oxopentanoate 4,5-aminomutase, EC 5.4.3.8] catalyzes the last step in the conversion of glutamate to delta-aminolevulinate of which eight molecules are needed to synthesize a chlorophyll molecule. Two full-length cDNA clones that probably represent the homeologous Gsa genes of the two tobacco (Nicotiana tabacum) genomes have been isolated. The deduced amino acid sequences of the 468-residue-long precursor polypeptides differ by 10 amino acids. The cDNA sequence of isoenzyme 2 was inserted in reverse orientation under the control of a cauliflower mosaic virus 35S promoter derivative in an expression vector and was introduced by Agrobacterium-mediated transformation into tobacco plants. Antisense gene expression decreased the steady-state mRNA level of glutamate 1-semialdehyde aminotransferase, the translation of the enzyme, and chlorophyll synthesis. Remarkably, partial or complete suppression of the aminotransferase mimics in tobacco a wide variety of chlorophyll variegation patterns caused by nuclear or organelle gene mutations in different higher plants. The antisense gene is inherited as a dominant marker.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- A simple and general method for transferring genes into plants. Science. 1985 Mar 8;227(4691):1229–1231. doi: 10.1126/science.227.4691.1229. [DOI] [PubMed] [Google Scholar]
- Becker D. Binary vectors which allow the exchange of plant selectable markers and reporter genes. Nucleic Acids Res. 1990 Jan 11;18(1):203–203. doi: 10.1093/nar/18.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelissen M. Nuclear and cytoplasmic sites for anti-sense control. Nucleic Acids Res. 1989 Sep 25;17(18):7203–7209. doi: 10.1093/nar/17.18.7203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelissen M., Vandewiele M. Both RNA level and translation efficiency are reduced by anti-sense RNA in transgenic tobacco. Nucleic Acids Res. 1989 Feb 11;17(3):833–843. doi: 10.1093/nar/17.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichacker L. A., Soll J., Lauterbach P., Rüdiger W., Klein R. R., Mullet J. E. In vitro synthesis of chlorophyll a in the dark triggers accumulation of chlorophyll a apoproteins in barley etioplasts. J Biol Chem. 1990 Aug 15;265(23):13566–13571. [PubMed] [Google Scholar]
- Elliott T., Avissar Y. J., Rhie G. E., Beale S. I. Cloning and sequence of the Salmonella typhimurium hemL gene and identification of the missing enzyme in hemL mutants as glutamate-1-semialdehyde aminotransferase. J Bacteriol. 1990 Dec;172(12):7071–7084. doi: 10.1128/jb.172.12.7071-7084.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fling S. P., Gregerson D. S. Peptide and protein molecular weight determination by electrophoresis using a high-molarity tris buffer system without urea. Anal Biochem. 1986 May 15;155(1):83–88. doi: 10.1016/0003-2697(86)90228-9. [DOI] [PubMed] [Google Scholar]
- Gatz C., Frohberg C., Wendenburg R. Stringent repression and homogeneous de-repression by tetracycline of a modified CaMV 35S promoter in intact transgenic tobacco plants. Plant J. 1992 May;2(3):397–404. doi: 10.1111/j.1365-313x.1992.00397.x. [DOI] [PubMed] [Google Scholar]
- Grimm B., Bull A., Breu V. Structural genes of glutamate 1-semialdehyde aminotransferase for porphyrin synthesis in a cyanobacterium and Escherichia coli. Mol Gen Genet. 1991 Jan;225(1):1–10. doi: 10.1007/BF00282635. [DOI] [PubMed] [Google Scholar]
- Grimm B. Primary structure of a key enzyme in plant tetrapyrrole synthesis: glutamate 1-semialdehyde aminotransferase. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4169–4173. doi: 10.1073/pnas.87.11.4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson M., Rutberg L., Schröder I., Hederstedt L. The Bacillus subtilis hemAXCDBL gene cluster, which encodes enzymes of the biosynthetic pathway from glutamate to uroporphyrinogen III. J Bacteriol. 1991 Apr;173(8):2590–2599. doi: 10.1128/jb.173.8.2590-2599.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson G. S., Evans J. R., von Caemmerer S., Arvidsson Y. B., Andrews T. J. Reduction of ribulose-1,5-bisphosphate carboxylase/oxygenase content by antisense RNA reduces photosynthesis in transgenic tobacco plants. Plant Physiol. 1992 Jan;98(1):294–302. doi: 10.1104/pp.98.1.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannangara C. G., Gough S. P., Bruyant P., Hoober J. K., Kahn A., von Wettstein D. tRNA(Glu) as a cofactor in delta-aminolevulinate biosynthesis: steps that regulate chlorophyll synthesis. Trends Biochem Sci. 1988 Apr;13(4):139–143. doi: 10.1016/0968-0004(88)90071-0. [DOI] [PubMed] [Google Scholar]
- Logemann J., Schell J., Willmitzer L. Improved method for the isolation of RNA from plant tissues. Anal Biochem. 1987 May 15;163(1):16–20. doi: 10.1016/0003-2697(87)90086-8. [DOI] [PubMed] [Google Scholar]
- Murakami K., Korbsrisate S., Asahara N., Hashimoto Y., Murooka Y. Cloning and characterization of the glutamate 1-semialdehyde aminomutase gene from Xanthomonas campestris pv. phaseoli. Appl Microbiol Biotechnol. 1993 Jan;38(4):502–506. doi: 10.1007/BF00242945. [DOI] [PubMed] [Google Scholar]
- Rodermel S. R., Abbott M. S., Bogorad L. Nuclear-organelle interactions: nuclear antisense gene inhibits ribulose bisphosphate carboxylase enzyme levels in transformed tobacco plants. Cell. 1988 Nov 18;55(4):673–681. doi: 10.1016/0092-8674(88)90226-7. [DOI] [PubMed] [Google Scholar]
- Sangwan I., O'Brian M. R. Expression of the soybean (Glycine max) glutamate 1-semialdehyde aminotransferase gene in symbiotic root nodules. Plant Physiol. 1993 Jul;102(3):829–834. doi: 10.1104/pp.102.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehy R. E., Kramer M., Hiatt W. R. Reduction of polygalacturonase activity in tomato fruit by antisense RNA. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8805–8809. doi: 10.1073/pnas.85.23.8805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. J., Watson C. F., Morris P. C., Bird C. R., Seymour G. B., Gray J. E., Arnold C., Tucker G. A., Schuch W., Harding S. Inheritance and effect on ripening of antisense polygalacturonase genes in transgenic tomatoes. Plant Mol Biol. 1990 Mar;14(3):369–379. doi: 10.1007/BF00028773. [DOI] [PubMed] [Google Scholar]
- Smith M. A., Grimm B., Kannangara C. G., von Wettstein D. Spectral kinetics of glutamate-1-semialdehyde aminomutase of Synechococcus. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9775–9779. doi: 10.1073/pnas.88.21.9775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. A., Kannangara C. G., Grimm B., von Wettstein D. Characterization of glutamate-1-semialdehyde aminotransferase of Synechococcus. Steady-state kinetic analysis. Eur J Biochem. 1991 Dec 18;202(3):749–757. doi: 10.1111/j.1432-1033.1991.tb16429.x. [DOI] [PubMed] [Google Scholar]
- Wettstein D. V., Kahn A., Nielsen O. F., Gough S. Genetic regulation of chlorophyll synthesis analyzed with mutants in barley. Science. 1974 May 17;184(4138):800–802. doi: 10.1126/science.184.4138.800. [DOI] [PubMed] [Google Scholar]