Abstract
Myelodysplastic syndromes (MDS) are clonal hematopoietic disorders characterized by cytopenias, ineffective hematopoiesis, myelodysplasia, and an increased risk of acute myeloid leukemia (AML). While sporadic MDS is primarily a disease of the elderly, MDS in children and young and middle-aged adults is frequently associated with underlying genetic predisposition syndromes. In addition to the classic hereditary bone marrow failure syndromes (BMFS) such as Fanconi Anemia and Dyskeratosis Congenita, in recent years there has been an increased awareness of non-syndromic familial MDS/AML predisposition syndromes such as those caused by mutations in GATA2, RUNX1, CEBPA, and SRP72 genes. Here, we will discuss the importance of recognizing an underlying genetic predisposition syndrome a patient with MDS, will review clinical scenarios when genetic predisposition should be considered, and will provide a practical overview of the common BMFS and familial MDS/AML syndromes which may be encountered in adult patients with MDS.
Keywords: Myelodysplastic syndromes, MDS, bone marrow failure syndromes, genetic predisposition, familial MDS, familial leukemia, Fanconi Anemia, Dyskeratosis Congenita, GATA2, MonoMac, Emberger Syndrome, RUNX1, FPD/AML, CEBPA, SRP72, Diamond-Blackfan Anemia, Shwachman-Diamond Syndrome, Severe Congenital Neutropenia, Congenital Amegakaryocytic Thrombocytopenia
1) Introduction
Ms. A is a 22-year-old female who presented with progressive fatigue and pancytopenia. Complete blood count revealed a white blood cell count of 2.7 • 103/µl, haemoglobin of 9.1 g/dl with an elevated mean corpuscular volume of 115 fl, and a platelet count of 86 • 103/µl, with a low absolute neutrophil count of 490 • 103/µl. Past medical history was notable for frequent bacterial infections, a cervical conisation procedure for Human Papilloma Virus (HPV)-associated cervical intraepithelial neoplasia and lymphedema of her left lower extremity as a child. There were no toxic exposures, and she was taking no medications. Family history was unremarkable. On physical examination, Ms. A was a well-appearing young woman of normal stature, with no abnormal physical findings. A bone marrow aspirate and biopsy revealed a hypocellular marrow with erythroid dysplasia and an expansion of myeloblasts, consistent with MDS. Cytogenetic studies revealed monosomy 7. Due to the patient’s young age, an inherited BMFS was suspected. Testing for Dyskeratosis Congenita and Fanconi Anemia was negative. Based on her presentation, she was clinically diagnosed with Emberger Syndrome. After GATA2 genetic testing became available, a de novo pathogenic GATA2 gene mutation was confirmed. She and her family received genetic counselling. No other family members were affected. She elected to proceed with fertility preservation, and subsequently underwent a bone marrow transplant. She is currently doing well.
The 2008 World Health Organization (WHO) classification defines myelodysplastic syndromes as a group of clonal hematopoietic stem cell disorders characterized by cytopenias, dysplasia in one or more myeloid cell lines, ineffective hematopoiesis, and increased risk of acute myeloid leukemia (AML)[1]. Historically defined by blast percentage and dysplastic morphology, MDS is now known to be driven by the sequential acquisition of clonal genetic changes through somatic mutations as well as gain or loss of chromosomal regions. Recurrent mutations found in MDS disrupt key regulatory pathways including RNA splicing (SFSB1, SRSF2, U2AF1 and ZRSR2), epigenetic modifier genes (TET2, DNMT3A, IDH1/IDH2, ASXL1, EZH2, and SETBP1), regulators of transcription (RUNX1, BCOR, ETV6), DNA repair (TP53), signaling pathways (NRAS, KRAS, CBL, JAK2, FLT3, NF1), and cohesins (STAG2)[2].
The incidence of MDS increases with age, with 86% of MDS patients diagnosed over the age of 60 years; the median age at diagnosis is 76 years[3]. Based on the 2001 Surveillance, Epidemiology, and End Results (SEER) data, the incidence of MDS in patients younger than 40 is estimated at 0.14 per 100,000, but rises to over 36 per 100,000 in patients 80 years and older[4]. In children, MDS is exceedingly rare, with an annual incidence of 0.8 to 4 cases per million[5]. Sporadic, or primary MDS, the most common type of MDS diagnosed in the elderly, is thought to be multifactorial and to arise due to the cumulative age-related genetic damage. In contrast, secondary MDS can frequently be traced to cytotoxic exposures, such as alkylating agents and topoisomerase inhibitors, radiation, and certain environmental and occupational toxins such as benzene, agricultural chemicals and solvents[6]. In pediatric patients, MDS is strongly associated with cytotoxic exposures, classic hereditary BMFS, nonsyndromic familial MDS/AML predisposition syndromes, or long history of acquired aplastic anemia[5] (Figure 1). With the increasing knowledge of hereditary BMFS and familial MDS/AML syndromes, there is a growing appreciation of their contribution to the development of MDS in the younger adult patients (Figure 2). In this review, we will discuss the importance of recognizing an underlying genetic predisposition syndrome in an MDS patient, will review clinical scenarios when genetic predisposition should be considered, and will provide a practical overview of the common hereditary BMFS and familial MDS/AML syndromes that may be encountered in adult patients with MDS.
Figure 1. Primary and Secondary Myelodysplastic Syndromes.
Figure 2. A Schematic Diagram of Relative Incidence of Primary MDS and MDS Secondary to Genetic Predisposition Syndromes.
Primary MDS (dashed line, gray bar), the most common type of MDS diagnosed in the elderly, is thought to be multifactorial and to arise due to the cumulative age-related genetic damage. In contrast, in children and younger adults, MDS is strongly associated with cytotoxic exposures (not shown), and genetic predisposition syndromes (solid line, black bar), including the classic hereditary BMFS and the nonsyndromic familial MDS/AML syndromes.
2) The critical importance of recognizing an underlying genetic predisposition syndrome in a patient with MDS
In addition to having implications for genetic counselling of a patient and their family, a diagnosis of an underlying genetic predisposition syndrome in a patient with MDS can be of immediate practical importance for selecting MDS therapy, considering hematopoietic stem cell transplantation (HSCT), and for long-term cancer surveillance and prognosis (Table 1). For the patient in the clinical vignette above, having an accurate genetic diagnosis allowed for genetic counselling, was important for donor selection, and gave her an option of pre-implantation diagnostics in the future.
Table 1.
Clinical Significance of an Underlying Genetic Predisposition Syndrome in a Patient with MDS
| Choice of MDS therapy |
| Considerations for HSCT |
| Timing of transplant |
| Choice of conditioning regimen |
| Donor selection |
| Unique transplant-related complications |
| Prognosis |
| Cancer surveillance |
| Pre-implantation genetics |
| Genetic counseling of patient and family |
First, in contrast to sporadic or primary MDS, where the MDS treatment plan is guided by the prognostic risk stratification, current MDS prognostic models have excluded patients with known genetic predisposition syndromes, and in most cases, would greatly underestimate their risk of progression[7]. Second, patients with an underlying BMFS or familial MDS/AML predisposition present with MDS at younger age and commonly have hypocellular marrow; importantly, although these characteristics were found to be predictive of response to immunosuppressive therapy in patients with primary MDS[8–11], patients with genetic predisposition syndromes will not achieve long-term remission with immunosuppression, and this approach is likely to result in infectious complications and delay of definitive therapy. Third, long-term outcomes of MDS secondary to BMFS and familial MDS/AML predisposition are historically poor, driven by progressive cytopenias, infectious complications and evolution to acute leukemia; for these patients, hematopoietic stem cell transplantation (HSCT) should be conscientiously considered earlier in the disease course[12]. Any consideration for cytotoxic “debulking” therapy prior to transplant must be carefully weighed with the risks of treatment-related toxicity, which can be significant in patients with BMFS, such as Fanconi Anemia.
Further, a diagnosis of hereditary BMFS or familial AML/MDS predisposition adds several considerations to pre-transplant planning and post-HSCT care of an MDS patient, particularly to the choice of a conditioning regimen, selection of HSCT donor, and unique transplant-related toxicities. Whereas in selecting the conditioning regimen, fit younger adults with primary MDS are frequently offered myeloablative conditioning with a goal of reducing relapse and improving disease-free survival[13], patients with hereditary BMFS may have improved survival and lower transplant-related mortality with reduced-intensity conditioning[12]. Patients with Fanconi Anemia were shown to have improved HSCT outcomes with fludarabine-containing nonmyeloablative regimens incorporating T-cell depletion[12]. Similarly, in patients with Dyskeratosis Congenita and Shwachman-Diamond Syndrome, in whom traditional myeloablative regimens have been associated with prohibitively high treatment-related complications, reduced-intensity conditioning may be better tolerated[14–16]. In selecting a potential HSCT donor, accurate diagnosis of an underlying genetic predisposition syndrome allows to exclude sibling donors who may be occult carriers of the same genetic syndrome. Inadvertent selection of clinically silent, adult sibling donors with Dyskeratosis Congenita have been reported, leading to poor stem cell mobilization, delayed engraftment, and increased mortality[17]. Similarly, inadvertent selection of a clinically silent sibling donor with Diamond-Blackfan Anemia caused non-engraftment[18]. Unique transplant-related complications in patients with BMFS include graft failure and pulmonary complications in patients with Dyskeratosis Congenita[14–15], hypersensitivity to radiation and DNA crosslinking agents, high rates of GVHD and endocrinopathies in Fanconi Anemia[12], and cardiopulmonary complications in Shwachman-Diamond Syndrome[16]. Finally, although HSCT may cure MDS and restore normal hematopoiesis, patients with an underlying genetic predisposition syndrome may be at an increased risk of non-hematologic malignancies. In selected populations, such as patients with Fanconi Anemia, where an estimated risk of solid tumors approaches 75% by the age of 45 years[19–20], or Dyskeratosis Congentia, where the actuarial risk of cancer is estimated at 40% by the age of 50 years[21], a comprehensive lifetime cancer surveillance program, genetic counselling and screening of family members are paramount.
Because of these unique treatment considerations, MDS patients with an underlying hereditary BMFS or familial MDS/AML should be evaluated and treated in specialized centres with expertise in care of patients with bone marrow failure (BMF).
3) When to consider hereditary bone marrow failure and familial MDS/AML syndromes in the evaluation of MDS?
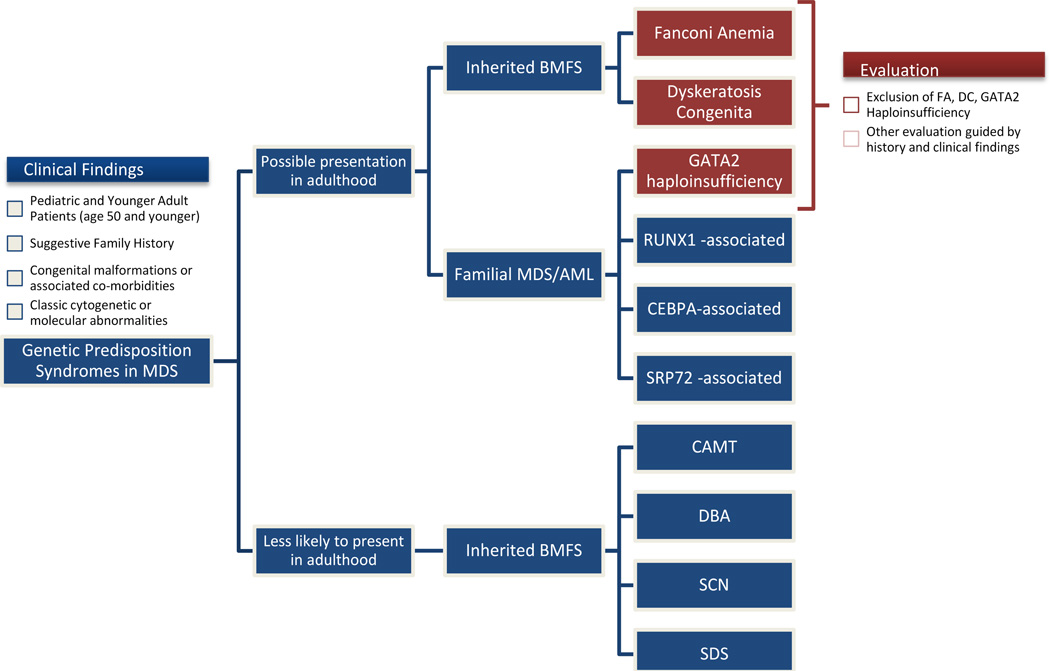
Although the majority of patients with classic hereditary BMFS are diagnosed in childhood, some patients have no or only subtle extrahematopoietic manifestations and may present in adulthood with MDS. Other common adult presentations include cytopenias, BMF, AML, or solid tumors; not infrequently, the diagnosis is uncovered due to excessive toxicity to chemotherapy or radiation[17, 22–29]. Because of the potential for life-threatening toxicities with inappropriate therapy, all pediatric and young adults presenting with MDS should be systematically evaluated for an underlying genetic predisposition syndrome. Although it is difficult to specify an exact age range when genetic screening is most fruitful, we recommend testing to exclude Fanconi Anemia and Dyskeratosis Congenita (because of increased treatment-related toxicities) and GATA2 haploinsufficiency (most common familial MDS/AML predisposition syndrome in adults) in all MDS patients under the age of 50 years, who have no history of cytotoxic exposures (Figure 3, Table 2). Additional evaluation should be guided by the clinical and family history.
Figure 3. Proposed Algorithm for Evaluation of Genetic Predisposition Syndromes in a Patient with MDS.
Because of a risk of life-threatening toxicities with inappropriate therapy, all pediatric and younger adult patients presenting with MDS should be systematically evaluated for an underlying predisposition syndrome. We recommend that Fanconi Anemia, Dyskeratosis Congenita and GATA2 haploinsufficiency should be ruled out in all MDS patients under the age of 50 years who do not have a history of cytotoxic chemotherapy. Clinical history suggestive of an underlying genetic predisposition syndrome includes congenital anomalies and associated clinical conditions, suggestive family history, and classic cytogenetic and molecular findings. Genetic syndromes that are more likely to be encountered as a new diagnosis in an adult patient with MDS including Fanconi Anemia, Dyskeratosis Congenita and familial MDS/AML syndromes—GATA2 haploinsufficiency, RUNX1-associated FAP/AML, familial AML with mutated CEBPA, and familial aplastic anemia/MDS with SRP72 mutation. Although isolated cases of adult presentations of Congenital Amegakaryocytic Thrombocytopenia (CAMT), Diamond-Blackfan Anemia (DBA), Severe Congenital Neutropenia (SCN) and Shwachman-Diamond Syndrome (SDS) have been reported, a new presentation of these syndromes in adult patients is highly unusual.
Table 2.
Clinical Findings Suggestive of a Possible Genetic Predisposition Syndrome in a Patient with MDS
|
Clinical characteristics suggestive of a possible genetic predisposition syndrome include short stature, mucocutaneous findings (e.g. café-au-lait spots, nail dystrophy, premature graying, or skin hypopigmentation), congenital malformations (e.g. cardiac defects or skeletal abnormalities), and associated clinical conditions (e.g. pulmonary fibrosis or endocrinopathies); however, these findings may be subtle or absent. In patients undergoing chemotherapy and radiation, a history of unusually delayed count recovery as well as other unexpected toxicities can point to an underlying DNA repair or telomere maintenance disorder. A meticulous family history may reveal family members with MDS or leukemia presenting at a young age, or may be notable for relatives with cytopenias, solid tumors, congenital malformations, or associated clinical conditions such as pulmonary fibrosis or premature ovarian failure; a history of consanguinity may point to a possible autosomal recessive disorder. Importantly, phenotypic heterogeneity, incomplete penetrance, autosomal recessive and sporadic cases, and somatic mosaicism may obscure the presence of an underlying genetic syndrome. Finally, the presence of signature cytogenetic, and molecular characteristics, or a new diagnosis of AML with morphologic findings of underlying myelodysplasia in a young adult should prompt an investigation of an occult genetic predisposition syndrome. Classic cytogenetic findings seen in genetic predisposition syndromes include duplications of chromosome regions 1q or 3q seen in Fanconi Anemia, isochromosome 7q characteristic of Shwachman-Diamond Syndrome, and isolated monosomy 7 common in GATA2 haploinsufficiency and a number of other disorders. Molecularly, bi-allelic disruption of genes such as RUNX1 and CEBPA, which are implicated both in sporadic AML as well as in familial MDS/AML syndromes, may indicate a leukemogenic “second-hit” somatic mutation in a patient with an inherited disruption in the affected gene[30].
4) What genetic predisposition syndromes are associated with MDS?
There is a growing number of genetic predisposition syndromes associated with MDS, including the classic syndromic BMFS as well as the pure familial MDS/AML syndromes; we refer the reader to several recent in-depth reviews of individual syndromes[31–41]. Here, we will focus on the practical information pertinent to evaluating an adult patient with MDS (Table 3, Figure 3). We will first present genetic syndromes that can present de novo in an adult patient, including BMFS (Fanconi Anemia, Dyskeratosis Congenita) and familial MDS/AML syndromes (GATA2 haploinsufficiency, RUNX1-associated familial platelet disorder with propensity to myeloid malignancy, familial AML with mutated CEBPA, and familial aplastic anemia/MDS with SRP72 mutation). We will then finish with four classic BMFS syndromes associated with MDS which are less likely to present in adulthood (Congenital Amegakaryocytic Thrombocytopenia, Diamond-Blackfan Anemia, Severe Congenital Neutropenia and Shwachman-Diamond Syndrome).
Table 3.
Inherited Bone Marrow Failure and MDS/AML Predisposition Syndromes
| Syndrome | Pathogenesis | Inheritance | Known Genes (Inheritance) |
Non-Hematologic Findings | Screening Test | Cytogenetic Abnormalities |
Risk of MDS/AML |
|---|---|---|---|---|---|---|---|
|
Congenital Amegakaryocytic Thrombocytopenia |
Thrombopoietin signaling and stem cell maintenance |
AR | MPL | None | MPL gene sequencing |
Monosomy 7, Trisomy 8 |
Increased, rate unknown |
|
Diamond-Blackfan Anemia |
Ribosome biogenesis | AD, XLR |
RPS19, RPS17, RPS24,RPL35A, RPL5,RPL11, RPS7,RPS26, RPS10 (AD); GATA1 (XLR) |
Small stature, congenital anomalies (e.g. craniofacial, cardiac, skeletal, genitourinary) |
Elevated erythrocyte adenosine deaminase and Haemoglobin F; genetic testing |
N/A | 1–20% |
|
Dyskeratosis Congenita |
Telomere maintenance |
XLR, AD, AR |
DKC1 (XLR); TERT, TERC, TINF2,RTEL1 (AD); NOP10, NHP2,WRAP53, RTEL1, TERT, CTC1 (AR). |
Nail dystrophy, abnormal skin pigmentation, oral leukoplakia, pulmonary fibrosis, hepatic fibrosis, cancer predisposition |
Telomere length measurement |
N/A | 30% |
| Fanconi Anemia | Interstrand crosslink DNA repair |
AR, XLR |
FANCA, FANCC, FANCD1/BRCA2, FANCD2, FANCE, FANCF, FANCG/XRCC9, FANCI, FANCJ/BACH1/BRIP1, FANCL, FANCM, FANCN/PALB2, FANCO/RAD51C, FANCP/SLX4, FANCQ(ERCC4) (AR); FANCB (XLR). |
Thumb and radius anomalies, café-au-lait spots, short stature, endocrinopathies, microcephaly, GU anomalies, hip dysplasia, cancer predisposition |
Chromosome breakage studies |
Dup 1q+, 3q+, −Deletion 7/7q, Deletion 11q, cryptic RUNX1 rearrangements |
40% |
|
Severe Congenital Neutropenia |
Various, including unfolded protein response, increased apoptosis of myeloid cells, impaired myeloid differentiation |
AD, AR, XLR |
ELANE, CSF3R, GFI1 (AD); HAX1, G6PC3 (AR); WAS (XLR) |
HAX1: neurodevelopmental; G6PC3: cardiac and other |
Gene sequencing; characteristic maturation arrest in myeloid differentiation. |
21–40% | |
|
Shwachman- Diamond Syndrome |
Ribosome biogenesis, mitotic spindle stability, and stress response |
AR | SBDS | Short stature, pancreatic insufficiency, skeletal abnormalities |
Isochr(7)(q10), del(20)(q11) |
8.1–36% | |
|
Familial acute myelogenous leukemia with mutated CEBPA |
Transcription regulation |
AD | CEBPA | None |
CEBPA sequencing of non-leukemic cells |
N/A | 100% |
|
GATA2 Haploinsufficiency |
Transcription regulation |
AD | GATA2 | Monocytopenia, nontuberculous mycobacterial and viral infections, lymphedema |
GATA2 sequencing; bone marrow morphology and flow cytometry |
Monosomy 7, Trisomy 8 |
50% |
|
RUNX1-associated familial platelet disorder with propensity to myeloid malignancy |
Transcription regulation |
AD | RUNX1 | None |
RUNX1 sequencing, including deletion and duplication testing |
Trisomy 21 | 35% |
|
Familial aplastic anemia/ myelodysplastic syndrome with SRP72 mutation |
SRP72 transcription factor |
AD | SRP72 | Congenital nerve deafness | SRP72 sequencing | N/A | unknown |
The disorders listed are the most common genetic predisposition syndromes for MDS/AML; the list is growing and additional syndromes are likely to be added in coming years. AD, autosomal dominant; AR, autosomal recessive; XLR, X-linked recessive; N/A, not available
Although disease-specific screening remains the standard-of-care for many syndromic BMFS (Table 3), targeted sequencing of known MDS predisposition genes using next-generation sequencing panels will soon enable rapid and comprehensive clinical testing for multiple MDS predisposition genes. As genetic testing becomes a routine part of the clinical MDS evaluation, specialized knowledge of genetic testing will become increasingly more important to accurately interpret and integrate test results into the clinical decision-making. Potential pitfalls of next-generation sequencing include the limitations of a particular panel’s coverage, false-negative results due to inability to detect deletions and duplications, and the difficulty in distinguishing pathogenic mutations from benign polymorphisms.
4.1) Fanconi Anemia
Fanconi Anemia (FA) is a genetic syndrome categorized by congenital anomalies, BMF and predisposition to cancer, with an estimated incidence of 1 in 100,000 births and autosomalrecessive or X-linked recessive inheritance[32]. To date, sixteen genes have been linked to FA, all a part of the common FA pathway that mediates interstrand crosslink DNA repair and homologous recombination[32]. Many patients exhibit congenital anomalies, including skeletal defects, classically abnormal thumb or radius, short stature, café-au-lait spots, endocrinopathies, and premature ovarian failure. Up to 40% of FA patients have no abnormal physical findings[31]. BMF is the most common manifestation, present in 75–90% of FA patients, with an estimated risk of evolution to MDS or AML of 50%[42]. Cytogenetic abnormalities characteristic of FA include the gain of 1q23–32 and 3q26[43]. Although most FA patients are diagnosed in childhood, patients can present in adulthood with BMF, MDS/AML, solid tumors, and increased toxicity from chemotherapy and radiation[22–24]. The actuarial risk of solid tumors in FA approaches 75% by the age of 50 years[42]. FA is diagnosed by demonstrating hypersensitivity to DNA interstrand crosslinking agents diepoxybutane and mitomycin C in a chromosomal breakage study[31]. Up to 15% of FA patients may have false-negative or ambiguous results due to somatic mosaicism of peripheral blood lymphocytes; this can be overcome by testing skin fibroblasts instead of peripheral blood [44]. After establishing FA diagnosis, specific affected genes can be identified by FA complementation testing or gene sequencing[31].
4.2) Dyskeratosis Congenita
Dyskeratosis Congenita (DC) is an inherited BMFS classically characterized by a triad of abnormal skin pigmentation, leukoplakia and nail dystrophy[34], with an estimated incidence of 1 per 1,000,000 births[45]. To date, eleven genes involved in telomere maintenance have been linked to DC pathogenesis, accounting for 60% of DC patients; DC can be inherited in X-linked recessive, autosomal-dominant or autosomal-recessive pattern[33]. Clinical manifestations can range from the most severe, such as the Hoyeraal-Hreidarsson Syndrome (intrauterine growth retardation, developmental delay, microcephaly, cerebellar hypoplasia, immunodeficiency, and aplastic anemia) and the Revesz syndrome (bilateral exudative retinopathy, BM hypoplasia, nail dystrophy, fine hair, cerebellar hypoplasia, and growth retardation), to the more subtle presentations in adulthood including pancytopenia, pulmonary fibrosis, or cutaneous changes[17, 22–28, 34]. The cumulative risk of MDS in DC approaches 33% by the age of 40 years; 40% of patients develop solid tumors by the age of 50 years[21]. DC is diagnosed by demonstrating abnormally short telomere lengths by flow fluorescence in situ hybridization (FISH) on peripheral blood lymphocytes[34].
4.3) GATA2 Haploinsufficiency Syndrome
Haploinsufficiency of the hematopoietic transcription factor GATA2 underlies a multifaceted MDS predisposition syndrome previously described as MonoMAC syndrome, dendritic cell, monocyte, B and NK lymphoid deficiency, familial MDS/AML, and Emberger syndrome[40]. The incidence of GATA2 haploinsufficiency is unknown. In the analysis of 57 GATA2 mutated patients at the National Institutes of Health (NIH), the median age at presentation was 20 years (range 5 months to 78 years), with 21% of patients presenting with MDS/AML at diagnosis. Other findings were viral infections (including HPV) (32%), non-tuberculous mycobacterial infections (28%), lymphedema (9%), and fungal infections (4%). Notably, 50% and 16% of patients were asymptomatic at the age of 20 years and 40 years, respectively. Overall, the risk of MDS/AML in patients with GATA2 deficiency is estimated at 50%[46]. Compared to patients with primary MDS, patients with GATA2 haploinsufficiency tend to be younger and are more likely to have hypocellular MDS[26, 46]. Most frequent cytogenetic abnormalities are trisomy 8 (24%) and monosomy 7 (16%)[40]. There is emerging data that somatic mutations in ASXL1, found in 29% of GATA2 -associated MDS/AML, predispose to a proliferative chronic myelomonocytic leukemia and may benefit for earlier HSCT[47]. There is likely an increased risk of solid tumors, including those associated with viral infections, such as HPV-associated squamous cell carcinoma, and other cancers including breast, pancreatic, renal cell and desmoid[40]. GATA2 haploinsufficiency syndrome is diagnosed by GATA2 gene sequencing.
4.4) RUNX1-associated Familial Platelet Disorder With Propensity to Myeloid Malignancy (FPD/AML)
RUNX1-associated FPD/AML is an autosomal-dominant disorder caused by germline mutations in the transcription factor RUNX1; its incidence is unknown[48–49]. Patients classically present with lifelong mild-to-moderate thrombocytopenia and a family history of MDS/AML at a young age; a history of bleeding may be elicited in some cases[48–50]. In a study of ten families with two or more family members affected diagnosed with MDS/AML, five of the ten families were found to carry germline RUNX1 mutations, with an estimated risk for MDS/AML of 35% in affected members. A thrombocytopenia prodrome may not recognized, or patients may carry a presumed diagnosis of chronic immune thrombocytopenia[48]. Progression to MDS/AML can be associated with acquisition of a second mutation in RUNX1 or an acquisition of trisomy 21[51]. RUNX1 FPD/AML syndrome is diagnosed by genetic sequencing in conjunction with ancillary cytogenetic techniques to detect deletions and duplications.
4.5) Familial Acute Myelogenous Leukemia With Mutated CEBPA
Familial AML with mutated CEBPA is an autosomal-dominant genetic disorder caused a germline mutation in the granulocytic differentiation factor CEBPA. Exemplified by the original description of a pedigree with three family members presenting with AML at ages of 10, 18, and 30 years[52], patients with germline CEBPA mutations come to medical attention either at the time of AML diagnosis or due to a family history of AML at a young age; there is a nearcomplete penetrance, and non-hematologic manifestations have not been reported. Although the incidence of germline CEBPA mutations is unknown, a screen of 187 consecutive AML patients revealed two patients (1%) who carried a germline CEBPA mutation; both were found to have “second-hit” somatic CEBPA mutations[30]. Of note, somatic CEBPA mutations are found in 5–14% of patients with an presumed primary AML, of whom 20% have bi-allelic CEBPA disruption and should be screened for germline CEBPA mutations[53]. The diagnosis is established by CEBPA gene sequencing of non-leukemic tissue, in order to avoid false-positive diagnosis due to somatic CEBPA alterations.
4.6) Familial aplastic anemia/MDS with SRP72 mutation
Familial aplastic anemia/MDS with SRP72 mutation is an autosomal dominant familial MDS syndrome caused by a mutation in SRP72, a component of the signal recognition particle necessary for protein processing[54]. Originally identified in a family with several adolescent or young adult family members affected by cytopenias or MDS, the SRP72 mutation was subsequently identified in screen of 96 BMF patients in one additional family where the affected family members were diagnosed with MDS at 39 and 76 years of age. The clinical phenotype of this syndrome is incompletely defined[54]. The diagnosis is established by SRP72 gene sequencing.
4.7) Congenital Amegakaryocytic Thrombocytopenia (CAMT)
CAMT is an autosomal recessive BMFS caused by the lack of the functional thrombopoietin receptor c-Mpl; approximately 100 cases of CAMT have been reported to date [35]. Patients typically present with severe thrombocytopenia before the first year of life, followed by progressive BMF due to the hematopoietic stem and progenitor cell exhaustion[35]. A presentation with familial aplastic anemia without a preceding thrombocytopenic phase has also been described[55]. No congenital anomalies have been definitively linked to CAMT; however, strabismus, cardiac and brain malformations were seen in isolated cases[35]. There is an increased risk of evolution to MDS/AML, and of acquisition of clonal cytogenetic abnormalities[56]. The diagnosis is established by identifying homozygous or compound heterozygous mutations in MPL.
4.8) Diamond-Blackfan Anemia (DBA)
DBA is an inherited BMFS characterized by red cell failure, congenital anomalies and increased incidence of cancer[36], with an incidence of 5–10 per one million births[57]. Classically thought to be caused by haploinsufficiency of one of a number of ribosomal proteins, a recent study identified mutations in GATA-1 erythroid transcription factor in an X-linked, DBA-like syndrome[58]. To date, mutations in ten genes have been linked to DBA, accounting for 60% of DBA cases. Although the majority of patients present with anemia in the first year of life, subtle cases of DBA can present in adulthood or can remain clinically unapparent[18, 29, 59]. Approximately half of DBA patients have congenital anomalies including craniofacial, skeletal, genitourinary and cardiac[36]. DBA patients have a 287-fold increased risk of MDS and a 28-fold increased risk of AML; there a 5.4-fold higher risk of all cancers compared to the general population[60]. The diagnosis of DBA is established in the presence of a macrocytic anemia, erythroid aplasia, and laboratory testing showing an elevated fetal haemoglobin (HbF) and erythrocyte adenosine deaminase (eADA)[36]. With the improving understanding of genetic defects in DBA and with the increasing availability of genetic testing, we expect that molecular diagnosis will soon become a clinical standard for the majority of DBA patients.
4.9) Severe Congenital Neutropenia (SCN)
SCN is a heterogeneous group of genetic disorders leading to peripheral neutropenia and increased risk of infections, with an estimated incidence of 1 per 100,000 to 200,000 births[38–39]. Originally described by Kostmann in 1956[61], the now eponymous autosomal dominant syndrome is caused mutations in the neutrophil elastase gene ELANE, leading to myeloid differentiation arrest at the promyelocyte stage[38]. More recently, SCN has been linked to mutations in other genes, including CSF3R, HAX1, G6PC3, GFI1, and WAS[38–39]. Classically, patients present in early childhood with non-cyclical neutropenia, aphthous stomatitis and bacterial infections[38–39]. Clinical severity can vary, and a delayed presentation of Kostmann syndrome with recurrent stomatitis and neutropenia in young adulthood has been reported[62]. The risk of evolution to MDS/AML is estimated at 21%–40% at 10 years, with an increased risk in patients on long-term granulocyte colony stimulating factor (G-CSF) therapy, particularly in poorly-responding patients[63–65]. Somatic gain-of-function mutations in CSF3R are frequently seen, and may cooperate with downstream mutations such as RUNX1 to further dysregulate CSF signaling and promote leukemogenesis[66–68]. SCN is diagnosed based on clinical presentation, bone marrow findings, and molecular sequencing of the SCN-associated genes.
4.10) Shwachman-Diamond Syndrome (SDS)
SDS is an autosomal recessive BMFS, characterized by exocrine pancreatic insufficiency and BMF, with an incidence of 1 in 77,000 to 200,000 births[37]. Over 90% of cases have been linked to bi-allelic mutations in the Shwachman–Bodian–Diamond Syndrome (SBDS) gene, which is involved in ribosome assembly and stress response[37]. Patients typically present in childhood with cytopenias, pancreatic insufficiency, failure to thrive, skeletal anomalies, and mental retardation; late presentations with hypoplastic MDS and AML in young adulthood have been documented[69]. The risk of evolution to MDS is 8.1% and 36% at 10 and 30 years, respectively[37]. Classic cytogenetic abnormalities in SDS include del(20)(q11) and isochromosome i(7)(q10) (a region containing the SBDS gene); these may be transitory and frequently have an indolent phenotype[70–72]. The incidence of cancer in SDS is unknown, although there are emerging reports of solid tumors in SDS patients. SDS is diagnosed clinically based on the hematologic and pancreatic findings, followed by molecular confirmation of homozygous or compound heterozygous mutations in the SBDS gene[37].
5) Summary
In conclusion, hereditary BMFS and familial MDS/AML syndromes are a heterogeneous group of genetic disorders which may present with MDS in both pediatric and adult patients. A diagnosis of a genetic predisposition syndrome in a patient with MDS carries important critical implications for decisions on MDS therapy and HSCT, cancer surveillance, and genetic counselling. Because of the risk for life-threatening toxicities with inappropriate therapy, all pediatric and adult MDS patients under the age of 50 years, without a history indicative of treatment-related MDS, should be evaluated for an underlying predisposition syndrome and tested to exclude FA, DC and GATA2 haploinsufficiency. With the increased integration of molecular testing and next-generation sequencing in clinical practice, we anticipate that more adult patients presenting with MDS will be diagnosed with a genetic predisposition syndrome; cautious interpretation of any identified gene mutations will be an ongoing challenge.
Practice Points.
In children and younger adults, MDS is strongly associated with cytotoxic exposures, classic hereditary BMFS and non-syndromic familial MDS/AML syndromes.
A diagnosis of a genetic predisposition syndrome in a patient with MDS may have immediate practical implications for decisions for MDS therapy, HSCT considerations (including timing of transplant, donor selection, conditioning regimen, and expected toxicities), cancer surveillance, and genetic counselling of patient and their family.
Because of the risk for life-threatening toxicities with inappropriate therapy, all pediatric and adult MDS patients under the age of 50 years, without a history indicative of treatment-related MDS, should be evaluated for an underlying predisposition syndrome and tested to exclude FA, DC and GATA2 haploinsufficiency.
Clinical history suggestive of an underlying genetic predisposition syndrome includes a personal or family history of cytopenias, congenital anomalies and associated clinical conditions, family history of MDS/AML at a young age, and signature cytogenetic and molecular findings.
With the increased integration of molecular testing and next-generation sequencing in clinical practice, we anticipate that more adult patients presenting with MDS will be diagnosed with a genetic predisposition syndrome; cautious interpretation of any identified gene mutations will be an ongoing challenge.
Research Agenda.
Identification of genes and pathways underlying development of MDS
Identification of familial MDS/AML predisposition syndromes
Identification of modifier genes that may determine the penetrance and age of onset of BMF and MDS in patients with a genetic predisposition syndrome
Epidemiologic studies of predisposition syndromes, including disease penetrance
Optimal surveillance and timing of HSCT in patients with MDS predisposition syndromes
Identification of therapeutic targets to prevent progression, and to treat and reverse disease
Acknowledgements
We thank all patients for participating in studies of bone marrow failure at the Children's Hospital of Philadelphia and the Hospital of the University of Pennsylvania. This work is supported by the NIH 5-T32-HL-07439-34 grant to D.B., and the NCI NIH R01 CA105312, NIDDK DK084188 and the Buck Family Chair in Hematology to M.B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors have no conflict of interest.
References
- 1.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H. Lyon, France: IARC Press; 2008. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. [Google Scholar]
- 2.Cazzola M, Della Porta MG, Malcovati L. The genetic basis of myelodysplasia and its clinical relevance. Blood. 2013;122:4021–4034. doi: 10.1182/blood-2013-09-381665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ma X, Does M, Raza A, Mayne ST. Myelodysplastic syndromes: incidence and survival in the United States. Cancer. 2007;109:1536–1542. doi: 10.1002/cncr.22570. [DOI] [PubMed] [Google Scholar]
- 4.Rollison DE, Howlader N, Smith MT, Strom SS, Merritt WD, Ries LA, et al. Epidemiology of myelodysplastic syndromes and chronic myeloproliferative disorders in the United States, 2001–2004, using data from the NAACCR and SEER programs. Blood. 2008;112:45–52. doi: 10.1182/blood-2008-01-134858. [DOI] [PubMed] [Google Scholar]
- 5.Glaubach T, Robinson LJ, Corey SJ. Pediatric myelodysplastic syndromes: they do exist! J Pediatr Hematol Oncol. 2014;36:1–7. doi: 10.1097/MPH.0000000000000046. [DOI] [PubMed] [Google Scholar]
- 6.Sekeres MA. The epidemiology of myelodysplastic syndromes. Hematol Oncol Clin North Am. 2010;24:287–294. doi: 10.1016/j.hoc.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 7.Bejar R. Prognostic models in myelodysplastic syndromes. Hematology Am Soc Hematol Educ Program. 2013;2013:504–510. doi: 10.1182/asheducation-2013.1.504. [DOI] [PubMed] [Google Scholar]
- 8.Broliden PA, Dahl IM, Hast R, Johansson B, Juvonen E, Kjeldsen L, et al. Antithymocyte globulin and cyclosporine A as combination therapy for low-risk non-sideroblastic myelodysplastic syndromes. Haematologica. 2006;91:667–670. [PubMed] [Google Scholar]
- 9.Lim ZY, Killick S, Germing U, Cavenagh J, Culligan D, Bacigalupo A, et al. Low IPSS score and bone marrow hypocellularity in MDS patients predict hematological responses to antithymocyte globulin. Leukemia. 2007;21:1436–1441. doi: 10.1038/sj.leu.2404747. [DOI] [PubMed] [Google Scholar]
- 10.Sloand EM, Wu CO, Greenberg P, Young N, Barrett J. Factors affecting response and survival in patients with myelodysplasia treated with immunosuppressive therapy. J Clin Oncol. 2008;26:2505–2511. doi: 10.1200/JCO.2007.11.9214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Passweg JR, Giagounidis AA, Simcock M, Aul C, Dobbelstein C, Stadler M, et al. Immunosuppressive therapy for patients with myelodysplastic syndrome: a prospective randomized multicenter phase III trial comparing antithymocyte globulin plus cyclosporine with best supportive care--SAKK 33/99. J Clin Oncol. 2011;29:303–9. doi: 10.1200/JCO.2010.31.2686. [DOI] [PubMed] [Google Scholar]
- 12.MacMillan ML, Wagner JE. Haematopoeitic cell transplantation for Fanconi anaemia - when and how? Br J Haematol. 2010;149:14–21. doi: 10.1111/j.1365-2141.2010.08078.x. [DOI] [PubMed] [Google Scholar]
- 13.Vaughn JE, Scott BL, Deeg HJ. Transplantation for myelodysplastic syndromes 2013. Curr Opin Hematol. 2013;20:494–500. doi: 10.1097/MOH.0b013e328364f547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ayas M, Nassar A, Hamidieh AA, Kharfan-Dabaja M, Othman TB, Elhaddad A, et al. Reduced intensity conditioning is effective for hematopoietic SCT in dyskeratosis congenita-related BM failure. Bone Marrow Transplant. 2013;48:1168–1172. doi: 10.1038/bmt.2013.35. [DOI] [PubMed] [Google Scholar]
- 15.Gadalla SM, Sales-Bonfim C, Carreras J, Alter BP, Antin JH, Ayas M, et al. Outcomes of allogeneic hematopoietic cell transplantation in patients with dyskeratosis congenita. Biol Blood Marrow Transplant. 2013;19:1238–1243. doi: 10.1016/j.bbmt.2013.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhatla D, Davies SM, Shenoy S, Harris RE, Crockett M, Shoultz L, et al. Reduced-intensity conditioning is effective and safe for transplantation of patients with Shwachman-Diamond syndrome. Bone marrow transplantation. 2008;42:159–165. doi: 10.1038/bmt.2008.151. [DOI] [PubMed] [Google Scholar]
- 17.Fogarty PF, Yamaguchi H, Wiestner A, Baerlocher GM, Sloand E, Zeng WS, et al. Late presentation of dyskeratosis congenita as apparently acquired aplastic anaemia due to mutations in telomerase RNA. Lancet. 2003;362:1628–1630. doi: 10.1016/S0140-6736(03)14797-6. [DOI] [PubMed] [Google Scholar]
- 18.Orfali RF, Wynn RF, Stevens RF, Chopra R, Ball SE. Failure of red cell production following allogenic BMT for Diamond Blackfan anaemia (DBA) illustrates functional significance of high erythrocyte adenosine deaminase (eADA) activity in the donor [abstract] Blood. 1999;94 Abstract 414. [Google Scholar]
- 19.Alter BP. Cancer in Fanconi anemia, 1927–2001. Cancer. 2003;97:425–440. doi: 10.1002/cncr.11046. [DOI] [PubMed] [Google Scholar]
- 20.Alter BP, Greene MH, Velazquez I, Rosenberg PS. Cancer in Fanconi anemia. Blood. 2003;101:2072. doi: 10.1182/blood-2002-11-3597. [DOI] [PubMed] [Google Scholar]
- 21.Alter BP, Giri N, Savage SA, Rosenberg PS. Cancer in dyskeratosis congenita. Blood. 2009;113:6549–6557. doi: 10.1182/blood-2008-12-192880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zatterale A, Calzone R, Renda S, Catalano L, Selleri C, Notaro R, et al. Identification and treatment of late onset Fanconi's anemia. Haematologica. 1995;80:535–538. [PubMed] [Google Scholar]
- 23.Huck K, Hanenberg H, Gudowius S, Fenk R, Kalb R, Neveling K, et al. Delayed diagnosis and complications of Fanconi anaemia at advanced age--a paradigm. Br J Haematol. 2006;133:188–197. doi: 10.1111/j.1365-2141.2006.05998.x. [DOI] [PubMed] [Google Scholar]
- 24.Bremer M, Schindler D, Gross M, Dork T, Morlot S, Karstens JH. Fanconi's anemia and clinical radiosensitivity report on two adult patients with locally advanced solid tumors treated by radiotherapy. Strahlenther Onkol. 2003;179:748–753. doi: 10.1007/s00066-003-1099-8. [DOI] [PubMed] [Google Scholar]
- 25.Reimann C, Kloeckener-Gruissem B, Niemeyer CM, Vanscheidt W. Late manifestation of dyskeratosis congenita presenting as chronic dermal ulcer in a 37-year-old man. J Eur Acad Dermatol Venereol. 2008;22:897–898. doi: 10.1111/j.1468-3083.2007.02530.x. [DOI] [PubMed] [Google Scholar]
- 26.Calvo KR, Vinh DC, Maric I, Wang W, Noel P, Stetler-Stevenson M, et al. Myelodysplasia in autosomal dominant and sporadic monocytopenia immunodeficiency syndrome: diagnostic features and clinical implications. Haematologica. 2011;96:1221–1225. doi: 10.3324/haematol.2011.041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vinh DC, Patel SY, Uzel G, Anderson VL, Freeman AF, Olivier KN, et al. Autosomal dominant and sporadic monocytopenia with susceptibility to mycobacteria, fungi, papillomaviruses, and myelodysplasia. Blood. 2010;115:1519–1529. doi: 10.1182/blood-2009-03-208629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Powell JB, Dokal I, Carr R, Taibjee S, Cave B, Moss C. X-linked dyskeratosis congenita presenting in adulthood with photodamaged skin and epiphora. Clin Exp Dermatol. 2014;39:310–314. doi: 10.1111/ced.12272. [DOI] [PubMed] [Google Scholar]
- 29.Balaban EP, Buchanan GR, Graham M, Frenkel EP. Diamond-Blackfan syndrome in adult patients. Am J Med. 1985;78:533–538. doi: 10.1016/0002-9343(85)90352-3. [DOI] [PubMed] [Google Scholar]
- 30.Pabst T, Eyholzer M, Haefliger S, Schardt J, Mueller BU. Somatic CEBPA mutations are a frequent second event in families with germline CEBPA mutations and familial acute myeloid leukemia. J Clin Oncol. 2008;26:5088–5093. doi: 10.1200/JCO.2008.16.5563. [DOI] [PubMed] [Google Scholar]
- 31.Kee Y, D'Andrea AD. Molecular pathogenesis and clinical management of Fanconi anemia. J Clin Invest. 2012;122:3799–3806. doi: 10.1172/JCI58321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kottemann MC, Smogorzewska A. Fanconi anaemia and the repair of Watson and Crick DNA crosslinks. Nature. 2013;493:356–363. doi: 10.1038/nature11863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ballew BJ, Savage SA. Updates on the biology and management of dyskeratosis congenita and related telomere biology disorders. Expert Rev Hematol. 2013;6:327–337. doi: 10.1586/ehm.13.23. [DOI] [PubMed] [Google Scholar]
- 34.Dokal I. Dyskeratosis congenita. Hematology Am Soc Hematol Educ Program. 2011;2011:480–486. doi: 10.1182/asheducation-2011.1.480. [DOI] [PubMed] [Google Scholar]
- 35.Ballmaier M, Germeshausen M. Congenital amegakaryocytic thrombocytopenia: clinical presentation, diagnosis, and treatment. Semin Thromb Hemost. 2011;37:673–681. doi: 10.1055/s-0031-1291377. [DOI] [PubMed] [Google Scholar]
- 36.Vlachos A, Muir E. How I treat Diamond-Blackfan anemia. Blood. 2010;116:3715–3723. doi: 10.1182/blood-2010-02-251090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Myers KC, Davies SM, Shimamura A. Clinical and molecular pathophysiology of Shwachman-Diamond syndrome: an update. Hematol Oncol Clin North Am. 2013;27:117–128. doi: 10.1016/j.hoc.2012.10.003. ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horwitz MS, Corey SJ, Grimes HL, Tidwell T. ELANE mutations in cyclic and severe congenital neutropenia: genetics and pathophysiology. Hematol Oncol Clin North Am. 2013;27:19–41. doi: 10.1016/j.hoc.2012.10.004. vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boztug K, Klein C. Genetics and pathophysiology of severe congenital neutropenia syndromes unrelated to neutrophil elastase. Hematol Oncol Clin North Am. 2013;27:43–60. doi: 10.1016/j.hoc.2012.11.004. vii. [DOI] [PubMed] [Google Scholar]
- 40.Spinner MA, Sanchez LA, Hsu AP, Shaw PA, Zerbe CS, Calvo KR, et al. GATA2 deficiency: a protean disorder of hematopoiesis, lymphatics, and immunity. Blood. 2014;123:809–821. doi: 10.1182/blood-2013-07-515528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nickels EM, Soodalter J, Churpek JE, Godley LA. Recognizing familial myeloid leukemia in adults. Ther Adv Hematol. 2013;4:254–269. doi: 10.1177/2040620713487399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosenberg PS, Greene MH, Alter BP. Cancer incidence in persons with Fanconi anemia. Blood. 2003;101:822–826. doi: 10.1182/blood-2002-05-1498. [DOI] [PubMed] [Google Scholar]
- 43.Quentin S, Cuccuini W, Ceccaldi R, Nibourel O, Pondarre C, Pages MP, et al. Myelodysplasia and leukemia of Fanconi anemia are associated with a specific pattern of genomic abnormalities that includes cryptic RUNX1/AML1 lesions. Blood. 2011;117:e161–e170. doi: 10.1182/blood-2010-09-308726. [DOI] [PubMed] [Google Scholar]
- 44.Soulier J, Leblanc T, Larghero J, Dastot H, Shimamura A, Guardiola P, et al. Detection of somatic mosaicism and classification of Fanconi anemia patients by analysis of the FA/BRCA pathway. Blood. 2005;105:1329–1336. doi: 10.1182/blood-2004-05-1852. [DOI] [PubMed] [Google Scholar]
- 45.Dokal I, Vulliamy T, Mason P, Bessler M. Clinical utility gene card for: dyskeratosis congenita. Eur J Hum Genet. 2011;19 doi: 10.1038/ejhg.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dickinson RE, Milne P, Jardine L, Zandi S, Swierczek SI, McGovern N, et al. The evolution of cellular deficiency in GATA2 mutation. Blood. 2014;123:863–874. doi: 10.1182/blood-2013-07-517151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.West RR, Hsu AP, Holland SM, Cuellar-Rodriguez J, Hickstein DD. Acquired ASXL1 mutations are common in patients with inherited GATA2 mutations and correlate with myeloid transformation. Haematologica. 2014;99:276–2781. doi: 10.3324/haematol.2013.090217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Owen CJ, Toze CL, Koochin A, Forrest DL, Smith CA, Stevens JM, et al. Five new pedigrees with inherited RUNX1 mutations causing familial platelet disorder with propensity to myeloid malignancy. Blood. 2008;112:4639–4645. doi: 10.1182/blood-2008-05-156745. [DOI] [PubMed] [Google Scholar]
- 49.Jongmans MC, Kuiper RP, Carmichael CL, Wilkins EJ, Dors N, Carmagnac A, et al. Novel RUNX1 mutations in familial platelet disorder with enhanced risk for acute myeloid leukemia: clues for improved identification of the FPD/AML syndrome. Leukemia. 2010;24:242–246. doi: 10.1038/leu.2009.210. [DOI] [PubMed] [Google Scholar]
- 50.Beri-Dexheimer M, Latger-Cannard V, Philippe C, Bonnet C, Chambon P, Roth V, et al. Clinical phenotype of germline RUNX1 haploinsufficiency: from point mutations to large genomic deletions. Eur J Hum Genet. 2008;16:1014–1018. doi: 10.1038/ejhg.2008.89. [DOI] [PubMed] [Google Scholar]
- 51.Preudhomme C, Renneville A, Bourdon V, Philippe N, Roche-Lestienne C, Boissel N, et al. High frequency of RUNX1 biallelic alteration in acute myeloid leukemia secondary to familial platelet disorder. Blood. 2009;113:5583–5587. doi: 10.1182/blood-2008-07-168260. [DOI] [PubMed] [Google Scholar]
- 52.Smith ML, Cavenagh JD, Lister TA, Fitzgibbon J. Mutation of CEBPA in familial acute myeloid leukemia. N Engl J Med. 2004;351:2403–2407. doi: 10.1056/NEJMoa041331. [DOI] [PubMed] [Google Scholar]
- 53.Renneville A, Mialou V, Philippe N, Kagialis-Girard S, Biggio V, Zabot MT, et al. Another pedigree with familial acute myeloid leukemia and germline CEBPA mutation. Leukemia. 2009;23:804–806. doi: 10.1038/leu.2008.294. [DOI] [PubMed] [Google Scholar]
- 54.Kirwan M, Walne AJ, Plagnol V, Velangi M, Ho A, Hossain U, et al. Exome sequencing identifies autosomal-dominant SRP72 mutations associated with familial aplasia and myelodysplasia. Am J Hum Genet. 2012;90:888–892. doi: 10.1016/j.ajhg.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walne AJ, Dokal A, Plagnol V, Beswick R, Kirwan M, de la Fuente J, et al. Exome sequencing identifies MPL as a causative gene in familial aplastic anemia. Haematologica. 2012;97:524–528. doi: 10.3324/haematol.2011.052787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maserati E, Panarello C, Morerio C, Valli R, Pressato B, Patitucci F, et al. Clonal chromosome anomalies and propensity to myeloid malignancies in congenital amegakaryocytic thrombocytopenia (OMIM 604498) Haematologica. 2008;93:1271–1273. doi: 10.3324/haematol.12748. [DOI] [PubMed] [Google Scholar]
- 57.Willig TN, Niemeyer CM, Leblanc T, Tiemann C, Robert A, Budde J, et al. Identification of new prognosis factors from the clinical and epidemiologic analysis of a registry of 229 Diamond-Blackfan anemia patients. DBA group of Societe d'Hematologie et d' Immunologie Pediatrique (SHIP), Gesellshaft fur Padiatrische Onkologie und Hamatologie (GPOH), and the European Society for Pediatric Hematology and Immunology (ESPHI) Pediatr Res. 1999;46:553–561. doi: 10.1203/00006450-199911000-00011. [DOI] [PubMed] [Google Scholar]
- 58.Sankaran VG, Ghazvinian R, Do R, Thiru P, Vergilio JA, Beggs AH, et al. Exome sequencing identifies GATA1 mutations resulting in Diamond-Blackfan anemia. J Clin Invest. 2012;122:2439–2443. doi: 10.1172/JCI63597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fargo JH, Kratz CP, Giri N, Savage SA, Wong C, Backer K, et al. Erythrocyte adenosine deaminase: diagnostic value for Diamond-Blackfan anaemia. Br J Haematol. 2013;160:547–554. doi: 10.1111/bjh.12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vlachos A, Rosenberg PS, Atsidaftos E, Alter BP, Lipton JM. Incidence of neoplasia in Diamond Blackfan anemia: a report from the Diamond Blackfan Anemia Registry. Blood. 2012;119:3815–3819. doi: 10.1182/blood-2011-08-375972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kostmann R. Infantile genetic agranulocytosis; agranulocytosis infantilis hereditaria. Acta Paediatr Suppl. 1956;45:1–78. [PubMed] [Google Scholar]
- 62.Cho HK, Jeon IS. Different Clinical Phenotypes in Familial Severe Congenital Neutropenia Cases with Same Mutation of the ELANE Gene. J Korean Med Sci. 2014;29:452–455. doi: 10.3346/jkms.2014.29.3.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rosenberg PS, Zeidler C, Bolyard AA, Alter BP, Bonilla MA, Boxer LA, et al. Stable long-term risk of leukaemia in patients with severe congenital neutropenia maintained on G-CSF therapy. Br J Haematol. 2010;150:196–199. doi: 10.1111/j.1365-2141.2010.08216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rosenberg PS, Alter BP, Bolyard AA, Bonilla MA, Boxer LA, Cham B, et al. The incidence of leukemia and mortality from sepsis in patients with severe congenital neutropenia receiving long-term G-CSF therapy. Blood. 2006;107:4628–4635. doi: 10.1182/blood-2005-11-4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rosenberg PS, Alter BP, Link DC, Stein S, Rodger E, Bolyard AA, et al. Neutrophil elastase mutations and risk of leukaemia in severe congenital neutropenia. Br J Haematol. 2008;140:210–213. doi: 10.1111/j.1365-2141.2007.06897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Skokowa J, Steinemann D, Katsman-Kuipers JE, Zeidler C, Klimenkova O, Klimiankou M, et al. Cooperativity of RUNX1 and CSF3R mutations in severe congenital neutropenia: a unique pathway in myeloid leukemogenesis. Blood. 2014;123:2229–2237. doi: 10.1182/blood-2013-11-538025. [DOI] [PubMed] [Google Scholar]
- 67.Link DC, Kunter G, Kasai Y, Zhao Y, Miner T, McLellan MD, et al. Distinct patterns of mutations occurring in de novo AML versus AML arising in the setting of severe congenital neutropenia. Blood. 2007;110:1648–1655. doi: 10.1182/blood-2007-03-081216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Beekman R, Valkhof MG, Sanders MA, van Strien PM, Haanstra JR, Broeders L, et al. Sequential gain of mutations in severe congenital neutropenia progressing to acute myeloid leukemia. Blood. 2012;119:5071–5077. doi: 10.1182/blood-2012-01-406116. [DOI] [PubMed] [Google Scholar]
- 69.Myers KC, Bolyard AA, Otto B, Wong TE, Jones AT, Harris RE, et al. Variable clinical presentation of shwachman-diamond syndrome: update from the north american shwachman-diamond syndrome registry. J Pediatr. 2014;164:866–870. doi: 10.1016/j.jpeds.2013.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dror Y, Durie P, Ginzberg H, Herman R, Banerjee A, Champagne M, et al. Clonal evolution in marrows of patients with Shwachman-Diamond syndrome: a prospective 5-year follow-up study. Exp Hematol. 2002;30:659–669. doi: 10.1016/s0301-472x(02)00815-9. [DOI] [PubMed] [Google Scholar]
- 71.Minelli A, Maserati E, Nicolis E, Zecca M, Sainati L, Longoni D, et al. The isochromosome i(7)(q10) carrying c.258+2t>c mutation of the SBDS gene does not promote development of myeloid malignancies in patients with Shwachman syndrome. Leukemia. 2009;23:708–711. doi: 10.1038/leu.2008.369. [DOI] [PubMed] [Google Scholar]
- 72.Maserati E, Pressato B, Valli R, Minelli A, Sainati L, Patitucci F, et al. The route to development of myelodysplastic syndrome/acute myeloid leukaemia in Shwachman-Diamond syndrome: the role of ageing, karyotype instability, and acquired chromosome anomalies. Br J Haematol. 2009;145:190–197. doi: 10.1111/j.1365-2141.2009.07611.x. [DOI] [PubMed] [Google Scholar]