Abstract
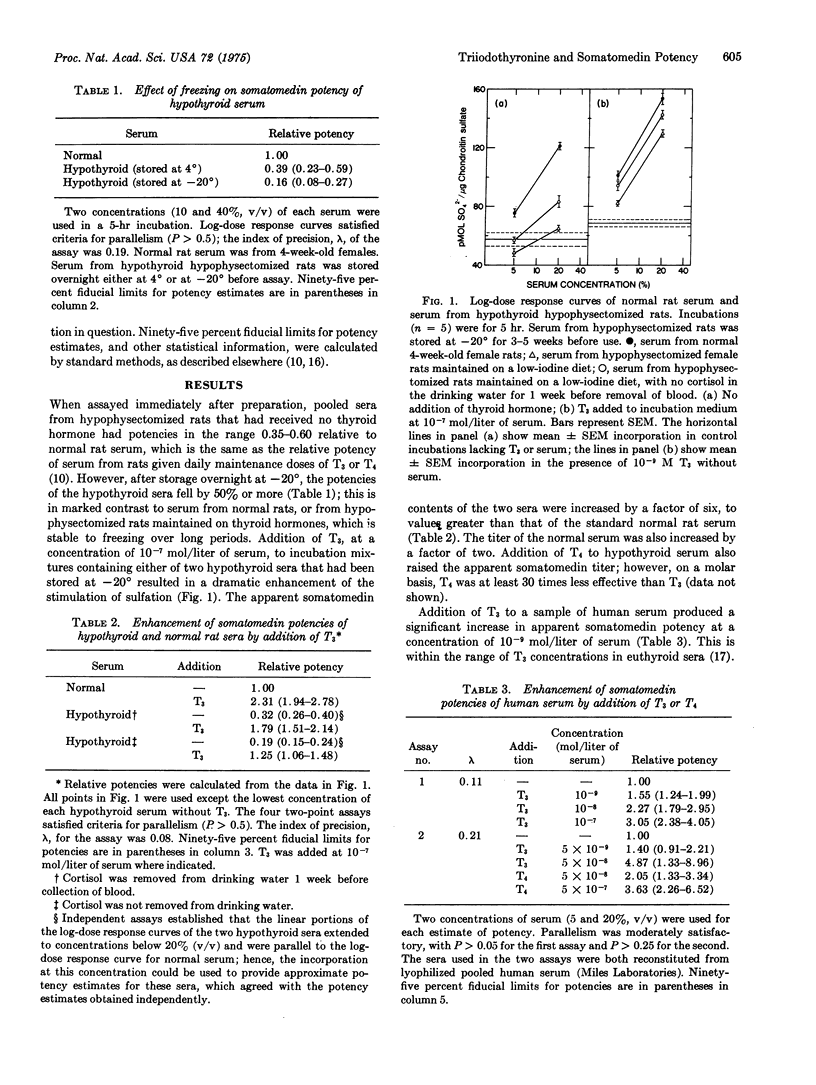
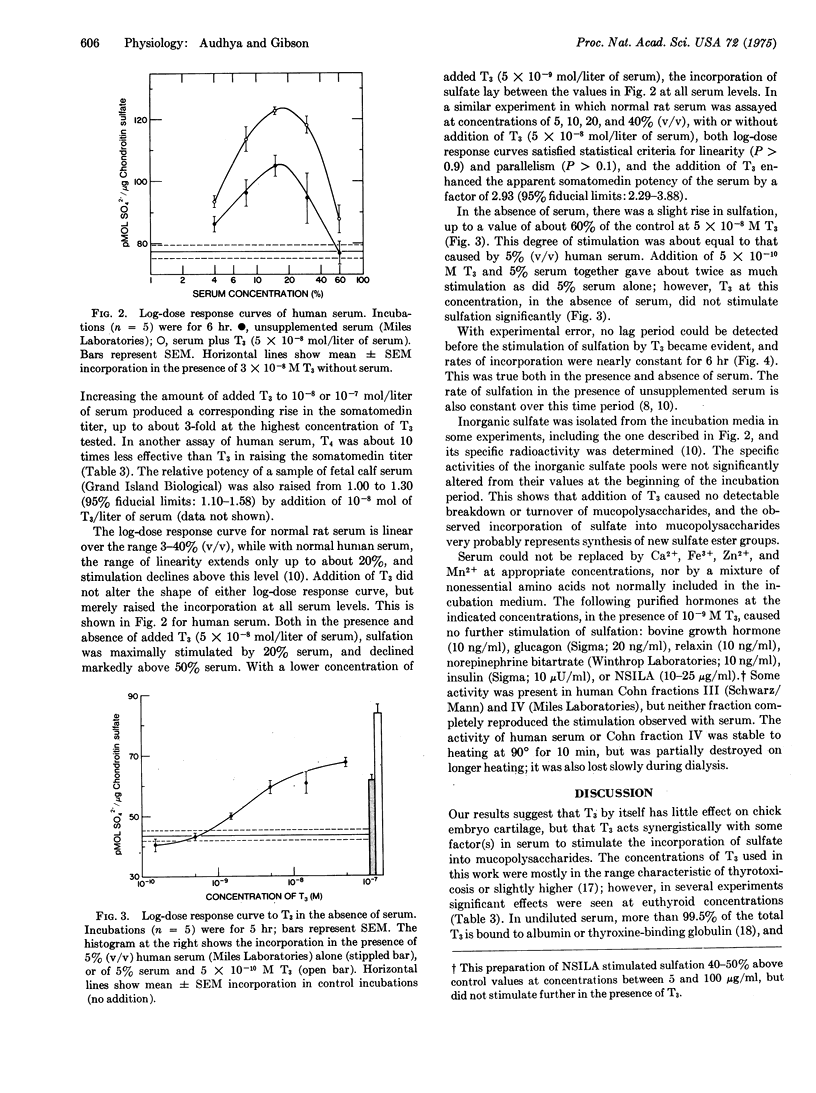
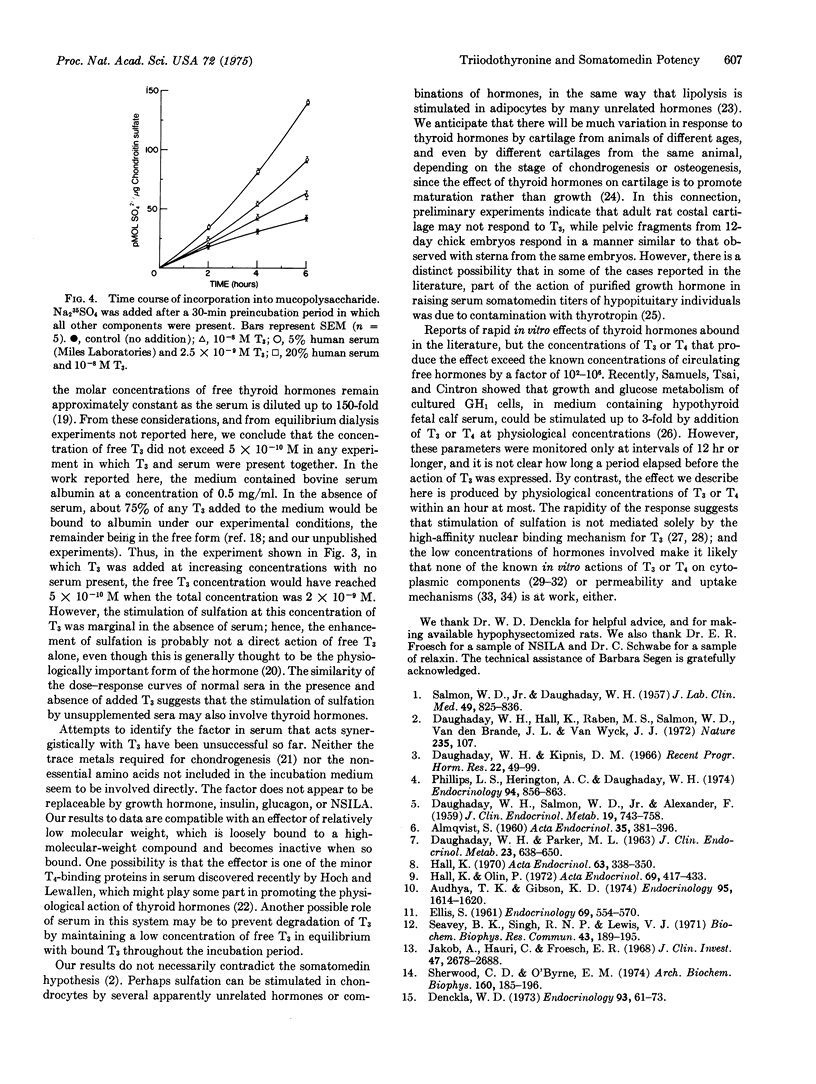
Somatomedin potencies of sera were assayed by following sulfation of mucopolysaccharides in chick embryo sterna in vitro. Apparent potencies of sera from hypophysectomized rats, maintained on a low-iodine diet, were restored to levels above normal by addition to the incubation medium of L-triiodothyronine at 10-7 mol/liter of serum. The potencies of normal rat, human, and fetal calf sera were raised 1.3- to 3-fold by addition of triiodothyronine at 10-9-10-7 mol/liter of serum. L-Thyroxine was about 10 times less effective than triiodothyronine. Triiodothyronine alone did not stimulate sulfation to nearly the same extent as triiodothyronine plus serum, even at higher concentrations. Serum could not be substituted in this system by any of six purified hormones, nor by trace metals or amino acids not included in the incubation mixture. It is concluded that triiodothyronine, in combination with a factor in serum, causes a rapid stimulation of sulfation in chick embryo cartilage, and that thyroid hormones may be involved in the action of normal serum on this tissue.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALMQVIST S. Studies on sulfation factor (SF) activity of human serum. Effect of human growth hormone on SF levels in pituitary dwarfism. Acta Endocrinol (Copenh) 1960 Nov;35:381–396. doi: 10.1530/acta.0.xxxv0381. [DOI] [PubMed] [Google Scholar]
- Adamson L. F., Ingbar S. H. Selective alteration by triiodothyronine of amino acid transport in embryonic bone. Endocrinology. 1967 Dec;81(6):1362–1371. doi: 10.1210/endo-81-6-1362. [DOI] [PubMed] [Google Scholar]
- Audhya T. K., Gibson K. D. Serum inorganic sulfate and apparent somatomedin activity in an assay using chick embryo cartilage. Endocrinology. 1974 Dec;95(6):1614–1620. doi: 10.1210/endo-95-6-1614. [DOI] [PubMed] [Google Scholar]
- DAUGHADAY W. H., PARKER M. L. Sulfation factor measurement as an aid in the recognition of pituitary dwarfism. J Clin Endocrinol Metab. 1963 Jul;23:638–650. doi: 10.1210/jcem-23-7-638. [DOI] [PubMed] [Google Scholar]
- DAUGHADAY W. H., SALMON W. D., Jr, ALEXANDER F. Sulfation factor activity of sera from patients with pituitary disorders. J Clin Endocrinol Metab. 1959 Jul;19(7):743–758. doi: 10.1210/jcem-19-7-743. [DOI] [PubMed] [Google Scholar]
- Daughaday W. H., Hall K., Raben M. S., Salmon W. D., Jr, van den Brande J. L., van Wyk J. J. Somatomedin: proposed designation for sulphation factor. Nature. 1972 Jan 14;235(5333):107–107. doi: 10.1038/235107a0. [DOI] [PubMed] [Google Scholar]
- Daughaday W. H., Kipnis D. M. The growth-promoting and anti-insulin actions of somatotropin. Recent Prog Horm Res. 1966;22:49–99. doi: 10.1016/b978-1-4831-9825-5.50005-1. [DOI] [PubMed] [Google Scholar]
- Denckla W. D. Minimal O 2 consumption as an index of thyroid status: standardization of method. Endocrinology. 1973 Jul;93(1):61–73. doi: 10.1210/endo-93-1-61. [DOI] [PubMed] [Google Scholar]
- ELLIS S. Studies on the serial extraction of pituitary proteins. Endocrinology. 1961 Sep;69:554–570. doi: 10.1210/endo-69-3-554. [DOI] [PubMed] [Google Scholar]
- GREEN K., MATTY A. J. Action of thyroxine on active transport in isolated membranes of Bufo bufo. Gen Comp Endocrinol. 1963 Jun;3:244–252. doi: 10.1016/0016-6480(63)90019-4. [DOI] [PubMed] [Google Scholar]
- HOCH F. L. Biochemical actions of thyroid hormones. Physiol Rev. 1962 Oct;42:605–673. doi: 10.1152/physrev.1962.42.4.605. [DOI] [PubMed] [Google Scholar]
- Hall K., Olin P. Sulphation factor activity and growth rate during long-term treatment of patients with pituitary dwarfism with human growth hormone. Acta Endocrinol (Copenh) 1972 Mar;69(3):417–433. doi: 10.1530/acta.0.0690417. [DOI] [PubMed] [Google Scholar]
- Hall K. Quantative determination of the sulphation factor activity in human serum. Acta Endocrinol (Copenh) 1970 Feb;63(2):338–350. doi: 10.1530/acta.0.0630338. [DOI] [PubMed] [Google Scholar]
- Hoch H., Lewallen C. G. Low affinity binding of thyroxine to proteins of human serum. J Clin Endocrinol Metab. 1974 Apr;38(4):663–673. doi: 10.1210/jcem-38-4-663. [DOI] [PubMed] [Google Scholar]
- Jakob A., Hauri C., Froesch E. R. Nonsuppressible insulin-like activity in human serum. 3. Differentiation of two distinct molecules with nonsuppressible ILA. J Clin Invest. 1968 Dec;47(12):2678–2688. doi: 10.1172/JCI105951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OPPENHEIMER J. H., SURKS M. I. DETERMINATION OF FREE THYROXINE IN HUMAN SERUM: A THEORETICAL AND EXPERIMENTAL ANALYSIS. J Clin Endocrinol Metab. 1964 Aug;24:785–793. doi: 10.1210/jcem-24-8-785. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H., Koerner D., Schwartz H. L., Surks M. I. Specific nuclear triiodothyronine binding sites in rat liver and kidney. J Clin Endocrinol Metab. 1972 Aug;35(2):330–333. doi: 10.1210/jcem-35-2-330. [DOI] [PubMed] [Google Scholar]
- Phillips L. S., Herington A. C., Daughaday W. H. Somatomedin stimulation of sulfate incorporation in porcine costal cartilage discs. Endocrinology. 1974 Mar;94(3):856–863. doi: 10.1210/endo-94-3-856. [DOI] [PubMed] [Google Scholar]
- Rudman D., DiGirolamo M. Comparative studies on the physiology of adipose tissue. Adv Lipid Res. 1967;5:35–117. [PubMed] [Google Scholar]
- SALMON W. D., Jr, DAUGHADAY W. H. A hormonally controlled serum factor which stimulates sulfate incorporation by cartilage in vitro. J Lab Clin Med. 1957 Jun;49(6):825–836. [PubMed] [Google Scholar]
- Samuels H. H., Tsai J. S., Cintron R. Thyroid hormone action: a cell-culture system responsive to physiological concentrations of thyroid hormones. Science. 1973 Sep 28;181(4106):1253–1256. doi: 10.1126/science.181.4106.1253. [DOI] [PubMed] [Google Scholar]
- Samuels H. H., Tsai J. S. Thyroid hormone action in cell culture: domonstration of nuclear receptors in intact cells and isolated nuclei. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3488–3492. doi: 10.1073/pnas.70.12.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seavey B. K., Singh R. N., Lewis U. J., Geschwind I. I. Bovine growth hormone: evidence for two allelic forms. Biochem Biophys Res Commun. 1971 Apr 2;43(1):189–195. doi: 10.1016/s0006-291x(71)80105-5. [DOI] [PubMed] [Google Scholar]
- Sherwood C. D., O'Byrne E. M. Purification and characterization of porcine relaxin. Arch Biochem Biophys. 1974 Jan;160(1):185–196. doi: 10.1016/s0003-9861(74)80025-1. [DOI] [PubMed] [Google Scholar]
- Sokoloff L., Roberts P. A., Januska M. M., Kline J. E. Mechanisms of stimulation of protein synthesis by thyroid hormones in vivo. Proc Natl Acad Sci U S A. 1968 Jun;60(2):652–659. doi: 10.1073/pnas.60.2.652. [DOI] [PMC free article] [PubMed] [Google Scholar]


