Abstract
A human La/SS-B (hLa)-specific TCR/hLa neo-self antigen double transgenic mouse model was developed and used to investigate cellular tolerance and autoimmunity to the ubiquitous RNA-binding La antigen often targeted in systemic lupus erythematosus and Sjögren's syndrome. Extensive thymic clonal deletion of CD4+ T cells occurred in H-2k/k double transgenic mice presenting high levels of the I-Ek-restricted hLa T cell epitope. In contrast, deletion was less extensive in H-2k/b double transgenic mice presenting lower levels of the epitope, and some surviving thymocytes were positively selected as thymic regulatory T cells (tTreg). These mice remained serologically tolerant to hLa and healthy. H-2k/b double transgenic mice deficient of all endogenous Tcra genes, a deficiency known to impair Treg development and function, produced IgG anti-hLa autoantibodies and displayed defective tTreg development. These autoimmune mice had interstitial lung disease characterized by lymphocytic aggregates containing transgenic T cells with an activated, effector memory phenotype. Salivary gland infiltrates were notably absent. Thus, expression of nuclear hLa antigen induces thymic clonal deletion and tTreg selection, and lymphocytic infiltration of the lung is a consequence of La-specific CD4+ T cell autoimmunity.
Introduction
Autoantibodies directed to the RNA-binding antigens Ro/Sjögren's syndrome (SS)4-A and La/SS-B occur frequently in patients with the autoimmune disorders systemic lupus erythematosus (SLE), SS and neonatal lupus erythematosus. Ro and La antibodies are among the earliest specificities detected before SLE diagnosis (1), are heritable traits in SLE patients (2) and closely associate with a type I interferon gene signature in SS (3) and type I IFN activity in SLE (4). Serum La antibodies typically co-occur with Ro antibodies and are used as disease classification markers (5). A lower incidence of both dsDNA antibodies and renal disease has been observed in SLE patients positive for both anti-Ro and anti-La compared to SLE patients having anti-Ro alone (5). SS patients suffer from lymphocytic infiltration of the exocrine glands, resulting in glandular dysfunction and dryness of the eyes and mouth. Primary SS patients with precipitating levels of antibodies to La and Ro are more likely to suffer from extraglandular manifestations of the disease (6), including pulmonary function abnormalities (7).
The high serum concentration (8), class-switched and somatically mutated nature (9) and association with particular HLA class II alleles (10), of human anti-La antibodies in SLE and SS suggest that they are T helper cell dependent. In previous studies we demonstrated incomplete immunologic tolerance to human La (hLa) in healthy mice expressing a hLa transgene (Tg) (11). This tolerance occurred in CD4+ T cells, whereas no tolerance was detectable in hLa-specific B cells from mice expressing hLa (12). Prior studies uncovered evidence of antigen-specific suppressor T lymphocytes specific for the small nuclear RNP A antigen (13) in the periphery of healthy mice. However, thymic mechanisms of CD4+ T cell tolerance to La or other RNA-binding antigens targeted in systemic autoimmunity have not been examined.
TCR Tg mice, in which the phenomenon of allelic exclusion narrows the T cell repertoire to one consisting primarily of a single T cell specificity, have been invaluable for identifying cellular mechanisms of development and tolerance in CD4+ T lymphocytes specific for model antigens, illustrating both thymic clonal deletion (14-16) and thymic regulatory T cell (tTreg) selection (17-19). The outcome of T cell development depends upon TCR affinity and the concentration and level of presentation of the selecting antigenic epitope (20-22). Controlling zygosity of MHC alleles that restrict selecting T cell epitopes has been successfully used in TCR transgenic models as a strategy to alter antigen presentation levels, resulting in altered T cell developmental outcomes (23). Loss of CD4+ T cell tolerance has been observed in TCR transgenic mice specific for myelin basic protein (24, 25) and pancreatic islet antigen (26), but disease phenotypes were observed only in mice additionally deficient in Rag or endogenous Tcr genes (24, 25) or those that were SCID mutant (26). Loss of tolerance and concomitant disease in all of these models was mediated by defective CD4+ T cell-mediated suppression and/or defective tTreg development (27).
In the present study, we investigated cellular mechanisms of CD4+ T cell tolerance and the consequences of its loss for a representative ubiquitous, RNA-binding nuclear antigen, La. We report the creation of TCR Tg mice specific for an immunodominant T cell epitope of the hLa antigen restricted by murine I-Ek and the outcome of crossing of these animals to previously described mice (11) expressing physiologic levels of nuclear hLa antigen, in the presence and absence of endogenous Tcra deficiency. We demonstrate that thymic deletion and tTreg selection are consequences of hLa neo-self antigen expression and that anti-La autoimmunity induced by endogenous Tcra deficiency results in pulmonary pathology. These results suggest that self-antigen-specific T cells contribute to rheumatic lung disease.
Materials and Methods
Mice
Line 3 hLa transgenic (Tg) mice (11) backcrossed to C57BL6/J (B6) more than 12 generations were crossed to B6 mice congenic for H-2k (B6.AK-H2k/FlaEgJ, Jackson Laboratory, Bar Harbor, ME) to generate hLa+/−H-2k/k or hLa+/−H-2k/b mice. B6 Tcra−/− mice (B6.129S2-Tcratm1Mom/J; Jackson Laboratory) were used to generate genotypes on a Tcra−/− background.
Constructs to generate 3B5.8 TCR+/− Tg mice (C57BL/6-Tg(Tcr3B5.8)Adf) were created by cloning the Tcra (TRAV12-2/J17 (Vα8.4/Jα17)) and Tcrb (TRBV4/D1/J2-1 (Vβ10/D1/Jβ2.1)) cDNAs from an I-Ek-restricted hybridoma (3B5.8, generated as described (28)) specific for the hLa67-76 peptide VIVEALSKSK were cloned into human CD2 minigene-driven T cell expression constructs (29) using adapter primers containing XmaI restriction enzyme sites (β chain: (975 bp) 3B5.8 BETA V – 5’-ccc ggg atg ggc tgt agg ctc cta agc tgt gtg gcc ttc-3’ and TCRBC – 5’-tcc cgg gtc agg aat ttt ttt tga cca tgg cca tc-3’; α chain: (870 bp) 3B5.8 Alpha V – 5’-ccc ggg atg aac atg cat cct gtc acc tgc tca gtt c-3’ and CD2 TCR C – 5’-atg gag ctt ggg acc cgg gct ctg tca gtc-3’). SalI- and NotI-cleaved and purified α and β chain constructs were co-microinjected in equimolar amounts into fertilized ova of B6 mice and Tg mice generated.
hLa Tg status was verified by PCR of genomic DNA as described (11, 12). Genomic DNA of 3B5.8 TCR Tg mice was typed using the same primer sets listed above. Tcra wild type and mutant alleles were typed by PCR as recommended by The Jackson Laboratory. H-2 haplotypes were inferred by flow cytometry of PBL using monoclonal antibodies directed to I-Ek (clone 14-4-4S) and I-Ab (clone 25-9-17).
Animals were maintained under pathogen-free barrier conditions in the OMRF Laboratory Animal Resource Center. Mice deficient in endogenous Tcra genes were maintained on water or Napa Nectar™ (Systems Engineering, Napa, CA) containing 0.2 mg/mL trimethoprim and 1.0 mg/mL sulfamethoxazole to prevent opportunistic infections and rectal prolapses. All studies were approved by the OMRF Institutional Animal Care and Use Committee. Unless otherwise indicated, mice were 7-9 wk old at the time of evaluation.
Flow cytometry
Single cell suspensions of whole thymi or spleens from individual mice were prepared using 70 μm nylon mesh, treated with 0.14 M NH4Cl2/17 mM Tris, pH 7.2 to lyse RBC, washed and quantified by hemocytometer using trypan blue exclusion. Lungs were minced, incubated for 30 min at 37°C with 250 Un/mL DNaseI (Sigma) and 1 mg/mL Collagenase D (Roche) in DMEM, sieved through 40 μm nylon mesh, washed and cells quantified as above. Cells (1-2×106 per organ) were stained for multicolor flow cytometry, events collected on an LSRII cytometer (BD Biosciences) and analyzed using FACSDiva (BD Biosciences) or FlowJo (TreeStar, Inc., Ashland, OR) software. Surface markers included anti-CD8a-biotin or -PerCP (clone 53-6.7), -CD25-APC or -biotin (PC61), -Vβ10-PE (B21.5), CD69-biotin (H1.2F3), -CD44-biotin (IM7), -CD4-APC-Cy7 or -APCAlexa750 (RM4-5), -CD62L-APC (MEL-14), Vα2-FITC (B20.1), Vα3.2-FITC (RR3-16), Vα8.3-FITC (B21.14) and Vα11.1/11.2-FITC (RR8-1). Biotinylated antibodies were detected using streptavidin-PE-Texas Red (CalTag/Invitrogen). Intracellular Foxp3 staining was performed using anti-Foxp3-FITC or -APC (clone FJK-16s; eBioscience) according to the manufacturer's protocol. Lymphocyte populations were identified by forward and side scatter. Absolute cell numbers and MFI data were compared using t-test with Welch's correction as necessary or by one-way ANOVA with Tukey's multiple comparison post-test. Comparisons of percentage data were evaluated using the Mann-Whitney U test.
T cell proliferation assay
Microcultures containing nylon-wool-enriched (30) 3B5.8 TCR Tg splenic T cells in a 1:1 ratio with γ-irradiated (2200 rad) splenocytes (APC) from sex- and H-2-matched B6 mice were incubated in triplicate with dilutions of specific (hLa61-84 peptide, endotoxin-depleted 6xHis-tagged hLa or mouse La recombinant proteins, or non-specific (HEL 46-61 peptide or endotoxin-depleted 6xHis-HEL) control Ags in cTCM (MEM with 10% FCS, 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM L-glutamine, and 2 mM Na-pyruvate, 0.1 mM NEAA, and 50 μM β-mercaptoethanol). Cultures were incubated for 72 h at 37°C in 10% CO2 and pulsed with [3H]-thymidine at 1 μCurie/well during the last 18 h. Cultures were harvested onto glass fiber filters and counted by liquid scintillation.
Isolation of thymic DC and medullary thymic epithelial cells (mTEC)
Pooled thymi (8 mice per genotype) were minced in 15 mg/mL Liberase TH and 1.3 mg/mL DNase then disrupted using two cycles of the “m_spleen_02” program on a gentleMACS Dissociator (Milentyi Biotec) with a 15 min incubation at 37 °C between programs. Cells were washed with RPMI/20%FCS/10mM Hepes, resuspended in RPMI 10%FCS/10mM Hepes and passed through a 40 μm filter by gravity. Thymocytes were depleted with biotinylated mAbs to CD3 (clone 145-2C11), CD5 (53-7.3), CD25 (7D4) and erythroid cells (Ter119) followed by Dynabeads® Biotin Binder (Invitrogen) using the manufacturer's instructions. The depleted fractions were labeled with mAbs CD11c-PerCp-Cy5.5 (N418), CD45-APC-Cy7 (30-F11), CD11b-PE-Cy7 (M1/70), Ly-51-Alexa647 (6C3), CD3-BV605 (17A2), and UEA-1-biotin (Vector Laboratories)/SA-PE-Cy5. For constitutive antigen presentation experiments, thymic DC were sorted as FITC autofluorescence−PI−CD3−CD45+CD11b−CD11c+, and mTEC as PI−CD3−CD45−UEA-1+ on a Ly-51 × UEA-1 dotplot as gated in (31) on a MoFlo sorter (Cytomation). For evaluation of MHC expression in separate experiments, anti-I-Ekβ-PE (clone 17-3-3, Santa Cruz Biotechnology) was added to the sort cocktail and large data files collected. Alternatively, mTEC were isolated as above, restained with anti-I-Ekβ and resorted by MoFlo. Data were analyzed by FlowJo version 10.
ELISAs
IgG antibodies to recombinant antigens were measured by ELISA as previously described (28).
Evaluation of constitutive presentation of the 3B5.8 epitope by thymic APC
Graded numbers of thymic DC were cultured in triplicate and 1200 mTEC per well were cultured in duplicate with 5 × 104 3B5.8 T hybridoma cells in 0.2 mL cTCM in round-bottom 96-well tissue culture plates. After 24 h of culture, cell supernatants from individual wells were collected and evaluated for concentrations of IL-2 by ELISA (IL-2 ELISA Set, BD biosciences).
Immunohistochemistry and histological scoring
Single lung lobes of individual mice were inflated with HBSS, fixed in 10% buffered formalin, embedded in paraffin and sectioned (ten 6-μm sections collected at 100 μm intervals per lobe). Slides were stained with hematoxylin and eosin and evaluated by a board-certified veterinary pathologist (SK). Lymphoid aggregates were scored as mild (0.5-1.0), moderate (1.5-2.0), marked (2.5-3.0) or severe (3.5-4.0). Intraalveolar macrophages were scored as the average number per 5 high power fields evaluated. Statistical differences amongst groups were evaluated by one-way ANOVA with Dunn's multiple comparison post-test.
Results
Human La-specific thymocytes are positively selected by H-2k but not H-2b in 3B5.8 TCR Tg mice
B6 mice Tg for both TCR α (Trav12-2/J17; Vα8.4/Jα17) and β (Trbv4/D1/J2-1; Vβ10/D1/Jβ2.1) chain cDNAs isolated from an I-Ek-restricted, hLa aa67-76-specific T cell hybridoma (3B5.8), were crossed with B6 mice congenic for H-2k in order to introduce I-Ek. Flow cytometric analysis showed that 99% of CD4+ PBL from H-2k/b 3B5.8 TCR Tg mice expressed Vβ10, a 10-fold increase over non-Tg littermates (Fig. 1A). The cell surface expression level of Vβ10 on 3B5.8 TCR Tg CD4+ T cells was indistinguishable from that of non-Tg mice (Fig. 1A), indicating a physiologically normal expression level of the Tg TCR. Splenic T cells from H-2k/b or H-2k/k 3B5.8 TCR Tg mice proliferated in a dose-response manner to recombinant human La (hLa) protein but not to recombinant mouse La or non-specific control antigen, while responses from non-Tg littermate mice were near background levels (Fig. 1B). Thus, CD4+ T cells expressing a functional 3B5.8 TCR predominate in H-2k positive 3B5.8 TCR Tg mice.
Figure 1. 3B5.8 TCR (Vβ10)-bearing T cells predominate in the periphery of 3B5.8 TCR Tg mice and respond specifically to hLa antigen in vitro.
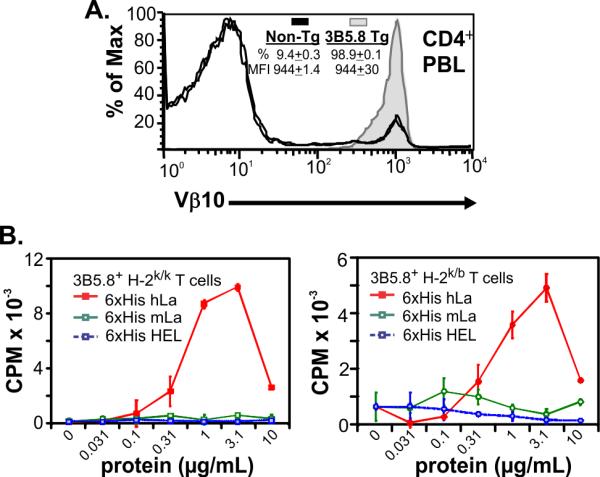
A) Representative Vβ10 histograms of CD4 lymphocyte-gated PBL collected from two 3B5.8 TCR Tg and two non-Tg littermates. Mean ± SEM of CD4+ cells expressing Vβ10 and MFI of Vβ10 values are reported. All mice are H-2k/b. B) Enriched splenic T lymphocytes from H-2k/k (left) and H-2k/b (right) 3B5.8 TCR Tg mice proliferate in response to intact recombinant 6xhis-hLa protein (red) but not to 6xhis-mouse La (green) or irrelevant 6xhis-HEL (blue) protein.
The effect of H-2 on thymic selection of Vβ10+ T cells in 3B5.8 TCR Tg mice was next evaluated. No significant differences in total thymic cellularity of 3B5.8 TCR Tg mice of H-2k/k, H-2k/b or H-2b/b haplotypes were observed (k/k: n=10: 9.3±1.3 × 107 ; k/b, n=8: 12.0±1.8 × 107; b/b, n=3: 11.8±0.6 × 107). However, fractions of CD4+CD8− (CD4 single positive (SP)) Vβ10+ cells were reduced in H-2b/b 3B5.8 TCR Tg mice compared to wild type, non-transgenic mice of H-2k/b (Fig. 2A, upper panels) and H-2k/k haplotypes (not shown). In contrast, substantially increased percentages of Vβ10+ CD4SP thymocytes were observed in both H-2k/b and H-2k/k 3B5.8 Tg mice compared to non-Tg littermate mice of the same H-2 haplotype (Fig. 2A, middle panels). Drastically increased numbers of Vβ10-expressing CD4SP cells in H-2k/b and H-2k/k 3B5.8 Tg mice indicated that there was efficient positive selection of 3B5.8 T cells by H-2k. Conversely, Vβ10+ CD4SP did not develop in H-2b/b 3B5.8 Tg mice (Fig. 2B) despite their abundance at the CD4+CD8+ double positive (DP) stage (Fig. 2C). Similar numbers of Vβ10+ CD4SP were observed in H-2k/b and H-2k/k 3B5.8 TCR Tg mice (Fig. 2B), indicating equivalent positive selection of the Tg T cells by these two haplotypes. Numbers of Vβ10+ DP thymocytes were also significantly elevated in H-2k/b and H-2k/k 3B5.8 TCR Tg mice compared to non-Tg littermates of the same H-2 (Fig. 2C).
Figure 2. Thymic selection of I-Ek-restricted 3B5.8 TCR Tg thymocytes in H-2k/b and H-2k/k mice in the absence or presence of Tg hLa neo-self antigen.
A) Representative Vβ10 lymphocyte-gated CD4 versus CD8 thymic dot plots of non-Tg, 3B5.8 TCR Tg and 3B5.8/hLa double Tg mice of the indicated MHC haplotypes. Quadrant percentages are indicated in the upper right of each panel. B) Absolute numbers of Vβ10+ CD4SP thymocytes. C) Absolute numbers of Vβ10+ CD4+CD8+ double positive (DP) thymocytes. Each symbol represents an individual mouse expressing the Tgs and MHC haplotype indicated. Unfilled symbols=H-2k/b, filled symbols=H-2k/k, x's=H-2b/b ; **p<0.001; ***p<0.0001 by one-way ANOVA with Tukey's multiple comparison test.
No significant differences in the fractions or absolute numbers of Vβ10+CD4+ splenocytes were observed between 3B5.8 TCR Tg mice of the H-2k/b (n=8, 2.23±0.25 × 107) or H-2k/k haplotypes (n=10, 1.77±0.21 × 107). In contrast, 14- to 18-fold fewer Vβ10+CD4+ splenocytes were observed in 3B5.8 TCR Tg H-2b/b mice (n=3, 1.22±0.38 × 106).
Therefore, CD4+ T cells bearing the 3B5.8 TCR are positively selected with similar efficiency by H-2k/k and H-2k/b mice but are not selected in H-2b/b mice.
Thymic Treg differentiation and efficiency of negative selection induced by presence of the hLa neo-self antigen depends on H-2 haplotype
To determine the effect of H-2 haplotype on thymic development of hLa-specific T cells in the presence of the hLa neo-self antigen, percentages and numbers of Vβ10+ CD4SP and DP thymocytes from 3B5.8 TCR Tg mice were compared with those of mice expressing both 3B5.8 and hLa Tgs. The hLa Tg mice express nuclear hLa ubiquitously at levels similar to the natural mouse La antigen (11). 3B5.8/hLa double Tg total thymocyte numbers were significantly reduced compared to that of 3B5.8 single Tg mice on both H-2k/k (2.01 ± 0.39 ×107 versus 9.35 ± 1.35 × 107, p < 0.0001) and H-2k/b (5.71 ± 0.80 × 107 versus 12.0 × 107, p=0.0018) backgrounds. Significantly reduced percentages (Fig. 2A, lower panels) and absolute numbers (Fig. 2B) of 3B5.8 thymocytes were observed at the CD4SP stage in double Tg mice compared to 3B5.8 single Tg littermates in both the H-2k/b and H-2k/k models, consistent with thymic clonal deletion. Deletion was also detected at the DP stage (Fig. 2C), although it was less efficient in H-2k/b compared to H-2k/k double Tg mice, as there was only a 2-fold decrease in H-2k/b DP Vβ10+ thymocytes compared to a 6-fold decrease in H-2k/k 3B5.8/hLa double Tg versus H-2 matched 3B5.8 single Tg mice. Vβ10 MFI on Vβ10+ CD4SP thymocytes of both H-2k/k and H-2k/b double Tg mice was reduced compared to that 3B5.8 single Tg mice of the same haplotype (not shown), suggesting encounter with specific I-Ek/hLa MHC/peptide complexes in vivo.
These trends were also apparent in the periphery, as splenic numbers of CD4+CD8−Vβ10+ 3B5.8 T cells were significantly reduced in 3B5.8/hLa double Tg mice of both H-2 haplotypes (3B5.8 versus 3B5.8/hLa: H-2k/b, 2.23 ± 0.25 × 107 versus 8.96 ± 1.06 × 105, p<0.0001; H-2k/k, 1.77 ± 0.20 × 107 versus 6.09 ± 0.86 × 105, p<0.0001).
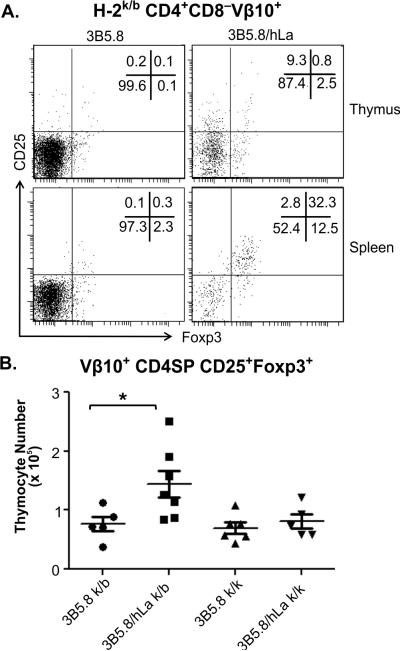
To assess the development of tTreg, CD25 and Foxp3 expression was assessed in thymic Vβ10+ cells. Cells co-expressing CD25 and Foxp3 in the spleen, which may include a combination of peripherally expanded tTreg and de novo-differentiated peripheral Treg, were also measured. Percentages (Fig. 3A) and absolute numbers (Fig. 3B) of CD4SP Vβ10+CD25+Foxp3+ thymocytes were significantly increased in H-2k/b, 3B5.8/hLa double Tg mice compared to 3B5.8 single Tg littermates. This phenomenon was observed only in H-2k/b but not H-2k/k mice (Fig. 3B). Percentages of Vβ10+ splenocytes with a Treg phenotype were drastically increased in 3B5.8/hLa double Tg mice compared to 3B5.8 single Tg littermates (Fig. 3A); however, their absolute numbers were not elevated since spleens of double Tg mice were significantly smaller than that of 3B5.8 single Tg mice. Development of tTreg in response to nuclear hLa expression could be induced by a mechanism of endogenous Vα TCR inclusion. To evaluate this possibility, a cocktail of available monoclonal antibodies specific for five different endogenous TCR Vα's was used to assess tTreg. Although a small number of thymic CD4SP of hLa/3B5.8 double Tg mice expressed these endogenous Vα, the Vα-included cells did not develop into tTreg (Suppl. Fig. 1). Sorted Vβ10+CD4+CD25+ splenocytes from H-2k/b 3B5.8/hLa double Tg mice were evaluated for their capacity to suppress proliferation of Vβ10+CD4+ splenic effector T cells (Teff) cells sorted from 3B5.8 single Tg mice in the presence of irradiated APC and hLa 61-84 peptide, which contains the 3B5.8 epitope. The CD25+ population isolated from 3B5.8/hLa double Tg mice had mild but detectable suppressive activity and was anergic to peptide stimulation (Suppl. Fig. 2A).
Figure 3. Positive selection of Vβ10+CD25+Foxp3+ Treg in H-2k/b but not H-2k/k mice double Tg for the 3B5.8 TCR and the hLa neo-self antigen.
A) Representative Foxp3 and CD25 staining of Vβ10+CD4+CD8− thymocytes and splenocytes from, H-2k/b mice double Tg for the 3B5.8 TCR and the hLa neo-self antigen. B) Absolute numbers of Vβ10+CD4+CD8−CD25+Foxp3+ thymocytes in H-2k/b or H-2k/k mice that are either single Tg for the 3B5.8 TCR (3B5.8) or double Tg for the 3B5.8 TCR and the hLa neo-self antigen (3B5.8/hLa). *p<0.05 by one-way ANOVA with Tukey's multiple comparison test.
Thus, in the presence of the hLa neo-self antigen, CD4+ T cells expressing the 3B5.8 hLa-specific TCR are strongly deleted in H-2k/k mice, are less efficiently deleted in H-2k/b mice, and a portion of the surviving cells in the H-2k/b model are positively selected as tTreg.
More effective 3B5.8 epitope presentation by APC expressing two alleles of the selecting MHC
Since T cells bearing the 3B5.8 receptor are not selected by H-2b/b, differences in efficiency of negative selection and capacity to positively select natural Treg in H-2k/b versus H-2k/k mice are likely due to differences in MHC class II presentation of the hLa epitope recognized by the 3B5.8 TCR. To investigate this, thymic DC and mTEC were isolated from pooled thymi of groups of mice of H-2k/k, H-2k/b and H-2b/b haplotype and evaluated for expression of I-Ek. Both thymic DC and mTEC from H-2k/k mice expressed higher cell surface levels of I-Ek than mice of H-2k/b haplotype, as expected (Figure 4A). To determine if these thymic APC constitutively present the 3B5.8 T cell epitope, thymic DC and mTEC were isolated from groups of hLa Tg and non-Tg littermate mice of haploytpe H-2k/k or H-2k/b and tested for the capacity to stimulate the 3B5.8 hybridoma in vitro. Thymic DC from both H-2k/k and H-2k/b hLa Tg mice stimulated IL-2 release from the 3B5.8 hybridoma in a dose-response fashion, while thymic DC from non-Tg mice did not (Figure 4B, left panel). Thymic DC from H-2k/k hLa Tg mice stimulated greater IL-2 release compared to thymic DC from H-2k/b hLa Tg mice (Figure 4B, left panel). Although only small numbers of mTEC could be isolated from any group of mice (median 2500, range 1200-5000 cells for seven independent groups of 8 mice each), constitutive expression of the 3B5.8 epitope by mTEC from hLa Tg H-2k/k mice was suggested by induction of a miniscule, but detectable amount of IL-2 from the 3B5.8 T cell hybridoma (Figure 4B, right panel). Increased expression of cell surface I-Ek and elevated constitutive expression of the 3B5.8 T cell epitope were also evident in peripheral APC (Suppl. Fig. 3). Thus, the 3B5.8 epitope is presented less effectively in thymi of H-2k/b mice compared to H-2k/k mice, and this inefficient presentation is occurs concurrently with positive selection of tTreg and less effective thymic clonal deletion.
Figure 4. Higher I-Ek expression by thymic DC and mTEC and more efficient constitutive presentation of the 3B5.8 hLa epitope by thymic DC from H-2k/k compared to H-2k/b mice.
A) Gating strategy (upper panels) and I-Ek expression (lower panels) of thymic DC (parent gate is CD45+FITC autofluorescence−CD3−) and mTEC (parent gate is CD45−CD3−) using pools of thymi (n=8 mice/group) depleted of DN, DP and SP. Results representative of two independent experiments. B) Constitutive presentation of the 3B5.8 T cell epitope by thymic DC (left) or mTEC (right) isolated from pooled thymi of hLa Tg mice of the indicated H-2 haplotype. IL-2 production by the 3B5.8 T cell hybridoma after 24 h of co-incubation with graded concentrations of thymic DC or 1200 cells per well mTEC is shown.
Deletion of the endogenous Tcra locus in H-2k/b 3B5.8 TCR/hLa neo-self antigen double Tg mice is permissive for thymic clonal deletion but results in defective Treg selection
Defective Treg selection and/or function and autoimmune pathology has been demonstrated in other TCR Tg mouse models of CD4+ autoimmunity if the mice were deficient in endogenous Tcr genes, Rag genes or were Scid mutant. Thymic T cell development was evaluated in Tcra-deficient H-2k/b 3B5.8/hLa neo-self antigen double Tg mice to determine whether similar Treg defects would be observed. Total thymocyte numbers were reduced in Tcra−/− double (3.77±0.77 × 107, n=11) compared to single Tg mice (12.84±1.27, n=11; p<0.0001). Efficient thymic clonal deletion of CD4+Vβ10+ thymocytes was suggested by their significantly reduced numbers in Tcra−/− 3B5.8./hLa double Tg mice (Fig. 5A). In contrast and unlike Tcra-intact double Tg mice, increased absolute numbers of CD4+Vβ10+Foxp3+CD25+ thymocytes were not observed in Tcra−/− double Tg mice, indicating a lack of positive selection of tTreg as expected (Fig. 5B).
Figure 5. Normal clonal deletion but defective natural Treg selection in 3B5.8/hLa double Tg mice deficient in endogenous Tcra genes.
A) CD4 versus CD8 dotplots of Vβ10+ thymocytes from representative mice of the indicated genotype (left panels) and absolute numbers of Vβ10+ CD4SP thymocytes in Tcra−/− 3B5.8/hLa double Tg mice compared to 3B5.8 single Tg littermates (far right panel). N=5 mice/group. ** p<0.01. Similar results were observed in two experiments using independent groups of mice. B) Foxp3 versus CD25 dotplots of Vβ10+, CD4 single positive (SP) thymocytes from representative mice (left panels) and absolute numbers of thymocytes with a Vβ10+CD4SP Foxp3+CD25+ phenotype. N=5 mice/group. Similar results were observed in two experiments using independent groups of mice.
Absolute numbers of Foxp3+CD25+ T cells in spleens of Tcra−/− 3B5.8xhLa double Tg mice were not elevated compared to 3B5.8 single Tg littermates (not shown); however, some peripheral CD4+Vβ10+Foxp3+CD25+ cells were still detectable. To determine whether these cells had suppressive activity, splenic CD4+Vβ10+CD25+ T cells of Tcra−/− 3B5.8/hLa double Tg mice were sorted and co-cultured with CD4+Vβ10+CD25− 3B5.8 Teff cells from Tcra−/− 3B5.8 single Tg mice in the presence of irradiated, T cell deficient APC and hLa61-84 peptide and T cell proliferation measured. Proliferation of 3B5.8 Teff cells was not reduced by co-culture with CD4+Vβ10+CD25+ T cells of Tcra−/− 3B5.8/hLa double Tg mice (Suppl. Fig. 2B), indicating an absence of detectable suppressive activity. Therefore, selection of tTreg is deficient in Tcra−/− 3B5.8/hLa double Tg mice, and suppressive activity of Treg found in the periphery of Tcra−/− 3B5.8/hLa double Tg mice—which may include expanded tTreg and peripherally differentiated Treg—is defective.
Deletion of the endogenous Tcra locus in H-2k/b 3B5.8 TCR/hLa neo-self antigen double Tg mice results in anti-La autoimmunity and lung pathology
Although the absolute numbers of splenic Thy1.2+CD4+Vβ10+ T cells were severely reduced in Tcra-deficient double Tg mice compared to their single Tg littermates (3B5.8 Tg 218.7±31.9 × 105 versus 4.4±0.5 × 105, p<0.0001), evaluation of these cells for expression of CD44 and CD62L revealed conversion to the effector/memory phenotype (Fig. 6A). These cells also expressed higher levels of CD69 in double Tg mice (MFI 821±116) compared to 3B5.8 single Tg littermates (418±48; p=0.01).
Figure 6. Spontaneous autoimmunity in Tcra-deficient 3B5.8xhLa double Tg mice.
A) Representative CD62L/CD44 flow cytometry profiles of CD4+Vβ10+ splenocytes from Tcra−/− TCR single Tg (3B5.8) or double Tg (3B5.8/hLa) mice (left panel) and increased fraction of CD4+Vβ10+ effector/memory splenocytes in 3B5.8/hLa double Tg (n=8) compared to 3B5.8 single Tg (n=7) mice (right panel). Results are combined from two separate experiments testing littermates at 16 and 21 wk of age. Plots depict mean±SEM with differences evaluated by t-test. B) End-point titers of serum IgG antibodies binding recombinant 6xhis-hLa (upper panels) or irrelevant control 6xhis-PA antigen (lower panels) in mice of the indicated transgenic genotypes and age groups as determined by ELISA. 6-7 wk and 8-9 wk samples are from the same groups of mice (n=6, 9, 8 and 1, for 3B5.8, 3.5.8/hLa, Non-Tg and hLa, respectively). 12-15 wk samples (n=7, 10, 11 and 7, for 3B5.8, 3.5.8/hLa, Non-Tg and hLa, respectively) and 20-28 wk samples (n=4, 5, 6 and 3, for 3B5.8, 3.5.8/hLa, Non-Tg and hLa, respectively) are from independent groups of mice. Panels are grouped by Tcra-deficient Tg genotype.
To determine whether deletion of the endogenous Tcra locus in 3B5.8 TCR/hLa double Tg mice results in serologic autoimmunity, serum samples from Tcra−/− 3B5.8 single Tg and Tcra−/− 3B5.8/hLa double Tg mice of various ages were evaluated for antibodies to recombinant 6xhis-hLa/SS-B antigen. Strikingly, in contrast to the endogenous Tcra-intact double Tg model, all Tcra−/− 3B5.8/hLa double Tg mice developed high-titer serum IgG antibodies to recombinant hLa antigen but not to an irrelevant foreign antigen from Bacillus anthracis (6xhis-PA) that is immunogenic in H-2kmice (32) (Fig. 6B). Anti-hLa antibodies were prominent by 6 wk of age in all tested Tcra−/− double Tg mice and were substantially increased in the same animals by 8 wk of age. Separate litters of Tcra−/− double Tg mice aged to either 12-15 wk or 20-28 wk had sustained, high-titer anti-hLa autoantibodies similar to those of 8 wk old mice. No antibodies to recombinant mouse Ro60-MBP antigen were detectable by ELISA or Western blot (not shown), indicating a lack of intermolecular B cell epitope spreading in this model.
Multiple organs of 16-22 wk old mice were evaluated for evidence of autoimmune pathology. Interstitial, often peribronchial, lymphocytic accumulations were consistently observed in lung tissue of Tcra−/− 3B5.8/hLa double Tg mice but not their 3B5.8 single Tg or non-Tg littermates (Fig. 7A and B). Numbers of intraalveolar macrophages were slightly increased in 3B5.8/hLa double Tg mice but this did not significantly differ from non-Tg Tcra−/− mice of the same age and H-2 haplotype (Fig. 7B). Small lymphocytic infiltrations were occasionally but inconsistently observed surrounding the hepatic portal vein in 3B5.8/hLa double Tg mice, but lymphocytic infiltration of salivary gland tissue was notably absent (not shown). Flow cytometric analysis of lung cell infiltrates revealed drastically increased fractions of CD4+Vβ10+ T cells of effector memory phenotype (Fig. 7C) expressing elevated cell surface levels of CD69 (3B5.8/hLa Tg: 2101±276 versus 3B5.8 Tg: 871±122; p=0.003) in double Tg mice compared to 3B5.8 single Tg littermates. As also observed in spleens (data not shown), some CD4+ T cells from the lungs of 3B5.8/hLa double Tg mice down-regulated cell surface Vβ10 (Fig.7D, left panel). Notably, among CD4+ T lymphocytes isolated from spleen (not shown) and lung tissues (Figure 7D, right panel) of Tcra−/− double Tg mice, only those Vβ10+ cells expressing the hLa TCR were activated, as evidenced by their selective upregulation of CD69. Moreover, CD4+Vβ10+ T cells isolated from lungs of 3B5.8 single Tg mice did not express elevated CD69, indicating that CD4+ T cell activation in lungs was induced by the hLa neo-self antigen and not other bystander mechanism(s) (Fig. 7D, right panel). The presence of these infiltrates did not affect the survival of Tcra−/− 3B5.8xhLa double Tg mice compared to Tcra−/− 3B5.8 single Tg or hLa single Tg littermates (not shown).
Figure 7. Autoimmune lung involvement in Tcra-deficient 3B5.8xhLa double Tg mice.
A) Representative hematoxylin and eosin stained lung tissue sections from 16wk old Tcra−/− 3B5.8 single Tg (left) or 3B5.8/hLa double Tg (right) mice. The right panel depicts a peribronchial lymphoid aggregate. B) Quantification of lymphoid aggregates (left) and intraalveolar macrophages (right) from 16-21 wk old Tcra-deficient mice of the indicated genotypes (3B5.8 n=7, 3B5.8/hLa n=9, Non-Tg n=4). Differences were assessed by one-way ANOVA with Dunn's multiple comparison post-test. C) Representative CD62L/CD44 flow cytometry profiles of CD4+Vβ10+ T cells isolated from lungs of endogenous Tcra-deficient TCR single Tg (3B5.8) or double Tg (3B5.8/hLa) mice (left panel) and increased fraction of lung CD4+Vβ10+ effector/memory T cells in 3B5.8/hLa double Tg mice (right panel). Results are combined from two separate experiments testing littermates at 16 and 21 wk of age (3B5.8 n=7, 3B5.8/hLa n=9). Plots depict mean±SEM with differences evaluated by t-test. D) Representative histograms depicting reduced Vβ10 expression on CD4+ T cells isolated from lungs of Tcra-deficient double Tg (3B5.8/hLa) mice compared to their single Tg (3B5.8) littermates (left panel) and expression of CD69 on subpopulations of Vβ10+ or Vβ10− T cells from individual mice of the indicated genotype (right panel). Plots depict mean±SEM. ****p<0.0001 by one-way ANOVA with Tukey's multiple comparison post-test.
Therefore, deficiency of endogenous Tcra genes in mice double Tg for the hLa-specific 3B5.8 TCR and the hLa neo-self antigen results in defective selection of tTreg, defective suppressive activity of Treg isolated from the periphery, serologic anti-La autoimmunity and accumulation of activated, effector memory T cells in lung tissue.
Discussion
This study highlights thymic mechanisms of immunologic tolerance to the class of ubiquitous, RNA-binding self antigens that are frequent targets of systemic autoimmunity in rheumatic diseases and demonstrates pathologic consequences of CD4+ T cell autoimmunity to La. Using the first TCR Tg mouse model specific for a clinically relevant, RNA-binding nuclear antigen, we show that thymic clonal deletion and tTreg differentiation are induced by nuclear expression of the La antigen and are thus mechanisms of normal thymic tolerance to this class of antigen. A previous study documented evidence for nRNP A-specific suppressor T cells in the periphery of healthy mice, but did not determine whether tTreg, which contribute substantially to the peripheral Treg pool, are positively selected (13). Thus, the present study is the first demonstration of tTreg positive selection in response to an RNA-binding nuclear antigen.
Thymic DC isolated from H-2k/k and H-2k/b hLa Tg mice constitutively presented the hLa epitope recognized by the hLa-specific 3B5.8 TCR. This is in stark contrast to an earlier study concluding that thymic DC were incapable presenting nuclear antigens (33). In that study bone marrow derived cells from thymus did not constitutively present nuclear β-galactosidase, nor was cross-presentation of the antigen by thymic DC detected. In this respect our findings are similar to those of Datta and colleagues who clearly showed constitutive presentation of nucleosomal histone peptide by thymic DC in a healthy mouse model of nucleosome-induced thymic clonal deletion (34). Features that may promote efficient thymic DC presentation of nuclear antigens may include altered direct presentation pathways secondary to the nucleic acid binding character of the nuclear La and nucleosome antigens and/or the true ubiquitous expression of these antigens, which could surpass a necessary threshold of cellular material available for cross-presentation by thymic DC.
In addition, positive selection of tTreg in lower peptide-presenting H-2k/b but not higher peptide-presenting H-2k/k 3B5.8/hLa double Tg mice provides clear support for the avidity model of Treg selection (35, 36). Specifically, positive selection of CD4SP, in the absence of expression of the hLa neo-self antigen, was equivalent in the two models, thus establishing the avidity model of tTreg selection without the potential factor of altered tuning (37) at early stages of development that has confounded other studies (38). Although endogenous Vα inclusion in tTreg from 3B5.8/hLa double Tg mice was not detected using available Vα antibodies, the possibility that hLa expression promoted inclusion of a different endogenous Vα as a mechanism further promoting tTreg differentiation cannot be ruled out. Expression of dual TCRs using the Tg Vβ10 could theoretically reduce cell surface expression levels of the La-specific TCR and promote tTreg by lowering antigen-specific TCR signaling.
Pathologic consequences of La-specific autoimmunity, if any, have been difficult to ascertain in human rheumatic disease, since this antigenic specificity nearly always co-occurs with humoral autoimmunity directed to the Ro antigen. Prior studies have not reported pathology after immunization of normal mice with human or mouse La proteins or peptides in adjuvant (39, 40). This may be the result of incomprehensive evaluation of tissue pathology and/or the presence of effective tolerance mechanisms that control infiltration of organs by effector T cells. Elimination of endogenous TCR gene rearrangements or endogenous TCR loci in other TCR/neo-self antigen double transgenic mouse models resulted in loss of tolerance and disease secondary to failed immunosuppression by CD4+ T cells. Thus, we used deletion of endogenous Tcra genes as a tool to impair tTreg development and/or other forms of tolerance in 3B5.8xhLa double Tg mice in order to observe the effect of anti-La autoimmunity in the CD4+ T cell compartment. Elimination of endogenous Tcra genes precluded positive selection of tTreg in 3B5.8/hLa double Tg mice as expected. Though elimination of endogenous Tcra genes impaired tTreg positive selection in hLa Tg mice, other mechanisms in addition to loss of tTreg may contribute to or be responsible for induction of autoimmunity in Tcra−/− hLa/3B5.8 TCR double Tg mice, including potential cellular or functional loss of other thymic-derived suppressor populations we did not measure or peripherally generated Tregs. Regardless of the mechanism, however, this maneuver resulted in early, specific and highly penetrant serologic and cellular autoimmunity to the La antigen.
Intermolecular epitope spreading to Ro was not observed in autoimmune Tcra−/− 3B5.8/hLa double Tg mice. This could be a consequence of poor physical interaction between hLa and mouse Ro antigens. Using mouse cells transfected with a hLa expression construct, a hLa-specific monoclonal antibody could co-immunoprecipitate mouse Ro protein, but the efficiency of Ro pull down was significantly less than could be observed in human cells (41).
The Tcra-deficient 3B5.8 TCR/hLa double Tg mouse model provided us with a unique opportunity to observe potential pathologic consequences of La-specific cellular and humoral autoimmunity in the absence of Ro. Although the pathologic consequences following immunization of mice with La antigen have not previously been reported, we reasoned that defective selection of tTreg and/or defective CD4-mediated immunosuppression, as had been demonstrated in other models, might promote pathology owing to unchecked T cell autoreactivity. Since immunization of BALB/c mice with peptides of 60 kD Ro induced serologic epitope spreading to the La antigen and focal lymphocytic infiltrates of salivary glands and other features of SS (42), observation of exocrinopathy was a potential outcome. The La autoimmune mice displayed no inflammation of salivary glands; however, peribronchial accumulations of activated, effector/memory CD4+ T lymphocytes expressing the La TCR were consistently observed in the lungs. An essentially identical T cell phenotype was observed among CD4+ T lymphocytes of the spleen, reflecting systemic autoimmunity expected for this ubiquitous antigen. This activated phenotype was restricted to hLa-specific T cells since there was no T cell activation or pathology in Tcra−/− 3B5.8 TCR single Tg mice or activation of cells from Tcra−/− 3B5.8 TCR/hLa double Tg mice that had downregulated Vβ10. Peng, et al. observed that although non-specific bystander activation of T cells could induce low levels of polyreactive autoantibodies, only activation by bona fide self antigen could induce cellular infiltration of target organs on the MRLlpr/lpr lupus background (43). Detection of autoreactivity only in the presence of cognate antigen, high titer hLa autoantibodies, and lack of responses to Ro60 antigen in the present report indicate that the responses were not polyreactive. Together, these findings suggest that the pulmonary pathology observed in this model is a consequence of autoimmunity rather than non-specific mechanisms of La-specific T cell activation secondary to homeostatic expansion or other bystander mechanisms.
We recently observed both IgG anti-La autoantibodies and similar peribronchial lymphocytic infiltrates in the B6.Sle1.Yaa model of lupus-like autoimmunity (44). Interestingly, similar peribronchial accumulations of CD4+ T lymphocytes have been observed in primary SS (45) and were assumed to occur secondary to exocrinopathy. Our study suggests that such pathology can occur in the absence of prior exocrinopathy and may be associated with systemic autoantigen-specific T lymphocytes. In support of this, there is a case report of pulmonary pseudolymphoma preceding SS by two years (46). Lymphoid interstitial lung disease in SS is diverse and can occur as follicular bronchiolitis, as observed in our model, to more diffuse lymphocytic interstitial pneumonia (LIP) most prominent in relation to bronchioles with usually mild fibrosis, to pseudolymphoma with nodular infiltrates (47, 48). Interestingly, restrictive pulmonary disease in SS was associated with antibodies to Ro and La (7), and a highly significant association was observed between anti-La antibodies in SS and internal organ manifestations including in lungs and liver (49).
This study demonstrates that thymic clonal deletion and tTreg differentiation are cellular mechanisms of CD4+ T cell tolerance to a clinically relevant, ubiquitous, nuclear antigen, provides new support for the avidity model of tTreg selection, and identifies a novel link between La-specific cellular autoimmunity and lymphoid interstitial lung disease
Supplementary Material
Acknowledgments
The authors thank Jacob Bass and Diana Hamilton of the OMRF Flow Cytometry Core Facility, Beverly Hurt of the OMRF Graphics Resource Center and Kathryn Bryant for assistance. The authors are grateful to D. Kioussis for providing the CD2 minigene expression construct and to Jim McCluskey and Tom Gordon for providing human La Tg mice and vectors encoding recombinant La proteins used in this study.
Footnotes
This work was supported by National Institutes of Health (NIH) grants R01AI48097, K02AI051647 and P50AR060804 to ADF. JCY was supported by an National Institute of Allergy and Infectious Diseases Kirschstein-NRSA Institutional Trainee fellowship (T32 AI007633) followed by an American Heart Association Predoctoral fellowship. The contents are the sole responsibility of the authors and do not necessarily represent the official views of the NIH.
Non-standard abbreviations: hLa, human La/SS-B; SLE, systemic lupus erythematosus; SS, Sjögren's syndrome; Teff, effector T cell; Tg, transgene or transgenic; Treg, regulatory T cell
References
- 1.Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, Harley JB. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349:1526–1533. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 2.Ramos PS, Kelly JA, Gray-McGuire C, Bruner GR, Leiran AN, Meyer CM, Namjou B, Espe KJ, Ortmann WA, Reichlin M, Langefeld CD, James JA, Gaffney PM, Behrens TW, Harley JB, Moser KL. Familial aggregation and linkage analysis of autoantibody traits in pedigrees multiplex for systemic lupus erythematosus. Genes Immun. 2006;7:417–432. doi: 10.1038/sj.gene.6364316. [DOI] [PubMed] [Google Scholar]
- 3.Emamian ES, Leon JM, Lessard CJ, Grandits M, Baechler EC, Gaffney PM, Segal B, Rhodus NL, Moser KL. Peripheral blood gene expression profiling in Sjogren's syndrome. Genes Immun. 2009;10:285–296. doi: 10.1038/gene.2009.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niewold TB, Hua J, Lehman TJ, Harley JB, Crow MK. High serum IFN-alpha activity is a heritable risk factor for systemic lupus erythematosus. Genes Immun. 2007;8:492–502. doi: 10.1038/sj.gene.6364408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reichlin M. Significance of the Ro antigen system. J Clin Immunol. 1986;6:339–348. doi: 10.1007/BF00915372. [DOI] [PubMed] [Google Scholar]
- 6.Pease CT, Charles PJ, Shattles W, Markwick J, Maini RN. Serological and immunogenetic markers of extraglandular primary Sjogren's syndrome. Br J Rheumatol. 1993;32:574–577. doi: 10.1093/rheumatology/32.7.574. [DOI] [PubMed] [Google Scholar]
- 7.Martinez-Cordero E, Andrade-Ortega L, Martinez-Miranda E. Pulmonary function abnormalities in patients with primary Sjogren's syndrome. J Investig Allergol Clin Immunol. 1993;3:205–209. [PubMed] [Google Scholar]
- 8.Gordon TP, Greer M, Reynolds P, Guidolin A, McNeilage LJ. Estimation of amounts of anti-La(SS-B) antibody directed against immunodominant epitopes of the La(SS-B) autoantigen. Clinical and experimental immunology. 1991;85:402–406. doi: 10.1111/j.1365-2249.1991.tb05739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raats JM, Roeffen WF, Litjens S, Bulduk I, Mans G, van Venrooij WJ, Pruijn GJ. Human recombinant anti-La (SS-B) autoantibodies demonstrate the accumulation of phosphoserine-366-containing la isoforms in nucleoplasmic speckles. Eur J Cell Biol. 2003;82:131–141. doi: 10.1078/0171-9335-00304. [DOI] [PubMed] [Google Scholar]
- 10.Dudek NL, Maier S, Chen ZJ, Mudd PA, Mannering SI, Jackson DC, Zeng W, Keech CL, Hamlin K, Pan ZJ, Davis-Schwarz K, Workman-Azbill J, Bachmann M, McCluskey J, Farris AD. T cell epitopes of the La/SSB autoantigen in humanized transgenic mice expressing the hLa class II haplotype DRB1*0301/DQB1*0201. Arthritis Rheum. 2007;56:3387–3398. doi: 10.1002/art.22870. [DOI] [PubMed] [Google Scholar]
- 11.Keech CL, Farris AD, Beroukas D, Gordon TP, McCluskey J. Cognate T cell help is sufficient to trigger anti-nuclear autoantibodies in naive mice. J Immunol. 2001;166:5826–5834. doi: 10.4049/jimmunol.166.9.5826. [DOI] [PubMed] [Google Scholar]
- 12.Aplin BD, Keech CL, de Kauwe AL, Gordon TP, Cavill D, McCluskey J. Tolerance through indifference: autoreactive B cells to the nuclear antigen La show no evidence of tolerance in a transgenic model. J Immunol. 2003;171:5890–5900. doi: 10.4049/jimmunol.171.11.5890. [DOI] [PubMed] [Google Scholar]
- 13.Kawahata K, Misaki Y, Komagata Y, Setoguchi K, Tsunekawa S, Yoshikawa Y, Miyazaki J, Yamamoto K. Altered expression level of a systemic nuclear autoantigen determines the fate of immune response to self. J Immunol. 1999;162:6482–6491. [PubMed] [Google Scholar]
- 14.Murphy KM, Heimberger AB, Loh DY. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo. Science (New York, N.Y. 1990;250:1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 15.Vasquez NJ, Kaye J, Hedrick SM. In vivo and in vitro clonal deletion of double-positive thymocytes. The Journal of experimental medicine. 1992;175:1307–1316. doi: 10.1084/jem.175.5.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Viret C, He X, Janeway CA., Jr. On the self-referential nature of naive MHC class II-restricted T cells. J Immunol. 2000;165:6183–6192. doi: 10.4049/jimmunol.165.11.6183. [DOI] [PubMed] [Google Scholar]
- 17.Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, Naji A, Caton AJ. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nature immunology. 2001;2:301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 18.Apostolou I, Sarukhan A, Klein L, von Boehmer H. Origin of regulatory T cells with known specificity for antigen. Nature immunology. 2002;3:756–763. doi: 10.1038/ni816. [DOI] [PubMed] [Google Scholar]
- 19.Kawahata K, Misaki Y, Yamauchi M, Tsunekawa S, Setoguchi K, Miyazaki J, Yamamoto K. Generation of CD4(+)CD25(+) regulatory T cells from autoreactive T cells simultaneously with their negative selection in the thymus and from nonautoreactive T cells by endogenous TCR expression. J Immunol. 2002;168:4399–4405. doi: 10.4049/jimmunol.168.9.4399. [DOI] [PubMed] [Google Scholar]
- 20.Bogen B, Gleditsch L, Weiss S, Dembic Z. Weak positive selection of transgenic T cell receptor-bearing thymocytes: importance of major histocompatibility complex class II, T cell receptor and CD4 surface molecule densities. European journal of immunology. 1992;22:703–709. doi: 10.1002/eji.1830220313. [DOI] [PubMed] [Google Scholar]
- 21.Sebzda E, Wallace VA, Mayer J, Yeung RS, Mak TW, Ohashi PS. Positive and negative thymocyte selection induced by different concentrations of a single peptide. Science (New York, N.Y. 1994;263:1615–1618. doi: 10.1126/science.8128249. [DOI] [PubMed] [Google Scholar]
- 22.Alam SM, Travers PJ, Wung JL, Nasholds W, Redpath S, Jameson SC, Gascoigne NR. T-cell-receptor affinity and thymocyte positive selection. Nature. 1996;381:616–620. doi: 10.1038/381616a0. [DOI] [PubMed] [Google Scholar]
- 23.Berg LJ, Frank GD, Davis MM. The effects of MHC gene dosage and allelic variation on T cell receptor selection. Cell. 1990;60:1043–1053. doi: 10.1016/0092-8674(90)90352-f. [DOI] [PubMed] [Google Scholar]
- 24.Van de Keere F, Tonegawa S. CD4(+) T cells prevent spontaneous experimental autoimmune encephalomyelitis in anti-myelin basic protein T cell receptor transgenic mice. The Journal of experimental medicine. 1998;188:1875–1882. doi: 10.1084/jem.188.10.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olivares-Villagomez D, Wang Y, Lafaille JJ. Regulatory CD4(+) T cells expressing endogenous T cell receptor chains protect myelin basic protein-specific transgenic mice from spontaneous autoimmune encephalomyelitis. The Journal of experimental medicine. 1998;188:1883–1894. doi: 10.1084/jem.188.10.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanagawa O, Militech A, Vaupel BA. Regulation of diabetes development by regulatory T cells in pancreatic islet antigen-specific TCR transgenic nonobese diabetic mice. J Immunol. 2002;168:6159–6164. doi: 10.4049/jimmunol.168.12.6159. [DOI] [PubMed] [Google Scholar]
- 27.Hori S, Haury M, Coutinho A, Demengeot J. Specificity requirements for selection and effector functions of CD25+4+ regulatory T cells in anti-myelin basic protein T cell receptor transgenic mice. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:8213–8218. doi: 10.1073/pnas.122224799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan ZJ, Davis K, Maier SM, Bachmann MP, Kim-Howard XR, Keech C, Gordon TP, McCluskey J, Farris AD. Neo-epitopes are required for immunogenicity of the La/SS-B nuclear antigen in the context of late apoptotic cells. Clinical and experimental immunology. 2006;143:237–248. doi: 10.1111/j.1365-2249.2005.03001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhumabekov T, Corbella P, Tolaini M, Kioussis D. Improved version of a human CD2 minigene based vector for T cell-specific expression in transgenic mice. J Immunol Methods. 1995;185:133–140. doi: 10.1016/0022-1759(95)00124-s. [DOI] [PubMed] [Google Scholar]
- 30.Julius MH, Simpson E, Herzenberg LA. A rapid method for the isolation of functional thymus-derived murine lymphocytes. European journal of immunology. 1973;3:645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- 31.Williams KM, Mella H, Lucas PJ, Williams JA, Telford W, Gress RE. Single cell analysis of complex thymus stromal cell populations: rapid thymic epithelia preparation characterizes radiation injury. Clin Transl Sci. 2009;2:279–285. doi: 10.1111/j.1752-8062.2009.00128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dumas EK, Nguyen ML, Cox PM, Rodgers H, Peterson JL, James JA, Farris AD. Stochastic humoral immunity to Bacillus anthracis Protective Antigen: Identification of anti-peptide IgG correlating with seroconversion to Lethal Toxin neutralization. Vaccine. 2013;31:1856–1863. doi: 10.1016/j.vaccine.2013.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oukka M, Colucci-Guyon E, Tran PL, Cohen-Tannoudji M, Babinet C, Lotteau V, Kosmatopoulos K. CD4 T cell tolerance to nuclear proteins induced by medullary thymic epithelium. Immunity. 1996;4:545–553. doi: 10.1016/s1074-7613(00)80481-1. [DOI] [PubMed] [Google Scholar]
- 34.Michaels MA, Kang HK, Kaliyaperumal A, Satyaraj E, Shi Y, Datta SK. A defect in deletion of nucleosome-specific autoimmune T cells in lupus-prone thymus: role of thymic dendritic cells. J Immunol. 2005;175:5857–5865. doi: 10.4049/jimmunol.175.9.5857. [DOI] [PubMed] [Google Scholar]
- 35.Maloy KJ, Powrie F. Regulatory T cells in the control of immune pathology. Nature immunology. 2001;2:816–822. doi: 10.1038/ni0901-816. [DOI] [PubMed] [Google Scholar]
- 36.Hinterberger M, Aichinger M, da Costa OP, Voehringer D, Hoffmann R, Klein L. Autonomous role of medullary thymic epithelial cells in central CD4(+) T cell tolerance. Nature immunology. 2010;11:512–519. doi: 10.1038/ni.1874. [DOI] [PubMed] [Google Scholar]
- 37.Grossman Z, Singer A. Tuning of activation thresholds explains flexibility in the selection and development of T cells in the thymus. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:14747–14752. doi: 10.1073/pnas.93.25.14747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wirnsberger G, Hinterberger M, Klein L. Regulatory T-cell differentiation versus clonal deletion of autoreactive thymocytes. Immunol Cell Biol. 2011;89:45–53. doi: 10.1038/icb.2010.123. [DOI] [PubMed] [Google Scholar]
- 39.Topfer F, Gordon T, McCluskey J. Intra- and inter-molecular spreading of autoimmunity involving the nuclear self-antigens La (SS-B) and Ro (SS-A). Proceedings of the National Academy of Sciences of the United States of America. 1995;92:875–879. doi: 10.1073/pnas.92.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Farris AD, Brown L, Reynolds P, Harley JB, James JA, Scofield RH, McCluskey J, Gordon TP. Induction of autoimmunity by multivalent immunodominant and subdominant T cell determinants of La (SS-B). J Immunol. 1999;162:3079–3087. [PubMed] [Google Scholar]
- 41.Keech CL, Gordon TP, Reynolds P, McCluskey J. Expression and functional conservation of the human La(SS-B) nuclear autoantigen in murine cell lines. J Autoimmun. 1993;6:543–555. doi: 10.1006/jaut.1993.1045. [DOI] [PubMed] [Google Scholar]
- 42.Scofield RH, Asfa S, Obeso D, Jonsson R, Kurien BT. Immunization with short peptides from the 60-kDa Ro antigen recapitulates the serological and pathological findings as well as the salivary gland dysfunction of Sjogren's syndrome. J Immunol. 2005;175:8409–8414. doi: 10.4049/jimmunol.175.12.8409. [DOI] [PubMed] [Google Scholar]
- 43.Peng SL, Fatenejad S, Craft J. Induction of nonpathologic, humoral autoimmunity in lupus-prone mice by a class II-restricted, transgenic alpha beta T cell. Separation of autoantigen-specific and -nonspecific help. J Immunol. 1996;157:5225–5230. [PubMed] [Google Scholar]
- 44.Maier-Moore JS, Horton CG, Mathews SA, Confer AW, Lawrence C, Pan Z, Coggeshall KM, Farris AD. Interleukin-6 deficiency corrects nephritis, lymphocyte abnormalities, and secondary Sjogren's syndrome features in lupus-prone Sle1.Yaa mice. Arthritis Rheumatol. 2014;66:2521–2531. doi: 10.1002/art.38716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Papiris SA, Saetta M, Turato G, La Corte R, Trevisani L, Mapp CE, Maestrelli P, Fabbri LM, Potena A. CD4-positive T-lymphocytes infiltrate the bronchial mucosa of patients with Sjogren's syndrome. Am J Respir Crit Care Med. 1997;156:637–641. doi: 10.1164/ajrccm.156.2.9610076. [DOI] [PubMed] [Google Scholar]
- 46.Pagani JJ, Collins JD, Reza MJ. Sjogren syndrome presenting as pulmonary pseudolymphoma: report of a case. J Natl Med Assoc. 1979;71:677–678. [PMC free article] [PubMed] [Google Scholar]
- 47.Deheinzelin D, Capelozzi VL, Kairalla RA, Barbas Filho JV, Saldiva PH, de Carvalho CR. Interstitial lung disease in primary Sjogren's syndrome. Clinical-pathological evaluation and response to treatment. Am J Respir Crit Care Med. 1996;154:794–799. doi: 10.1164/ajrccm.154.3.8810621. [DOI] [PubMed] [Google Scholar]
- 48.Fox RI, Howell FV, Bone RC, Michelson P. Primary Sjogren syndrome: clinical and immunopathologic features. Semin Arthritis Rheum. 1984;14:77–105. doi: 10.1016/0049-0172(84)90001-5. [DOI] [PubMed] [Google Scholar]
- 49.Locht H, Pelck R, Manthorpe R. Diagnostic and prognostic significance of measuring antibodies to alpha-fodrin compared to anti-Ro-52, anti-Ro-60, and anti-La in primary Sjogren's syndrome. J Rheumatol. 2008;35:845–849. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.