Abstract
The interpersonal model of loss of control (LOC) eating proposes that socially distressing situations lead to anxious states that trigger excessive food consumption. Self-reports support these links, but the neurobiological underpinnings of these relationships remain unclear. We therefore examined brain regions associated with anxiety in relation to LOC eating and energy intake in the laboratory. Twenty-two overweight and obese (BMIz: 1.9±0.4) adolescent (15.8±1.6y) girls with LOC eating (LOC+, n=10) and without LOC eating (LOC−, n=12) underwent functional magnetic resonance imaging (fMRI) during a simulated peer interaction chatroom paradigm. Immediately after the fMRI scan, girls consumed lunch ad libitum from a 10,934-kcal laboratory buffet meal with the instruction to “let yourself go and eat as much as you want.” Pre-specified hypotheses regarding activation of five regions of interest were tested. Analysis of fMRI data revealed a significant group by peer feedback interaction in the ventromedial prefrontal cortex (vmPFC), such that LOC+ had less activity following peer rejection (vs. acceptance), while LOC− had increased activity (p <.005). Moreover, functional coupling between vmPFC and striatum for peer rejection (vs. acceptance) interacted with LOC status: coupling was positive for LOC+, but negative in LOC− (p <.005). Activity of fusiform face area (FFA) during negative peer feedback from high-value peers also interacted with LOC status (p < .005). A positive association between FFA activation and intake during the meal was observed among only those with LOC eating. In conclusion, overweight and obese girls with LOC eating may be distinguished by a failure to engage regions of prefrontal cortex implicated in emotion regulation in response to social distress. The relationship between FFA activation and food intake supports the notion that heightened sensitivity to incoming interpersonal cues and perturbations in socio-emotional neural circuits may lead to overeating in order to cope with negative affect elicited by social discomfort in susceptible youth.
Keywords: Loss of control eating, adolescents, social distress, anxiety, neural activation, food intake
1. Introduction
Pediatric loss of control (LOC) eating, the subjective experience of being unable to control what or how much one eats, has been shown to predict excessive weight gain,1,2 exacerbated disordered eating,3,4 anxiety and depression,2,3,5,6 and metabolic dysfunction.7 LOC eating often emerges during adolescence8,9 and is more commonly reported by girls (vs. boys) and overweight (vs. non-overweight) youth.10 Among overweight and obese girls, LOC eating has clear consequence for food intake: those with reported LOC, compared to those without LOC, consume more energy at laboratory meals when instructed to “binge” versus when told to “eat normally.”11 Despite these data, only ~50% of youth with LOC eating before age 13 years go on to experience persistent and exacerbated LOC eating patterns during middle to late adolescence and beyond.3,4,12 To improve the identification of high-risk youth with LOC eating and refine intervention targets, research is needed to elucidate the neural mechanisms that may contribute to exacerbated disordered eating and obesity.
Psychosocial correlates may inform the underlying neuropathology that promotes LOC eating. Specifically, LOC eating is associated with poor social functioning13–16 and adverse mood states such as anxiety.17,18 Interpersonal theory offers one mechanism by which LOC eating may lead to excess weight gain and binge eating-type disorders.19 The interpersonal model proposes that hyperphagia associated with LOC episodes may reflect a response to negative affective states induced by interpersonal conflicts.19 Our data from retrospective self-reports20 and ecological momentary assessment in the natural environment21 generally support this theoretical model in youth.
A corollary of the interpersonal model is that susceptibility for both interpersonal problems and LOC episodes result from altered neural engagement in anxiety-sensitive brain regions during social, emotional, and consummatory processes. Indeed, among youth with reported LOC eating, social problems are commonly reported13–16 and may be a particularly potent non-homeostatic modulator of excess food consumption. Although its causes are likely multifactorial, LOC eating may occur in a subset of youth with altered brain function also linked to poor regulation during social conflicts. While brain regions such as the hypothalamus regulate normative homeostatic feeding, neural circuits implicated in tracking the emotional salience of stimuli and the complex processing of affect also impact food intake. For instance, negative affect has been associated with increased responsivity in the striatum and amgydala, brain regions implicated in the reward and threat-based processing, during the anticipated consumption of palatable food22–24 Whereas engagement of lateral and medial aspects of prefrontal cortex (PFC), brain regions implicated in inhibitory control, have been shown to dampen craving and palatable food intake.25 The effect of interpersonal distress on this circuit may override homeostatic regulators of intake, and thereby explain the strong connections between negative affective states and non-homeostatic feeding.26 Therefore, a potential neural diathesis for LOC eating may be promoted by aberrant patterns of brain activation during episodes of social distress.27
Given the robust link between LOC eating and anxiety,17,18 research on the neurobiological mechanisms underlying adolescent mood and anxiety disorders may inform the neural underpinnings of LOC eating. Research in mood-disordered youth has identified aberrant activity within corticolimbic and striatal circuits in response to social provocation.28–36 Together, brain structures within these circuits modulate adolescents’ reactions to social interchanges and play an important role in regulating the attention, emotion, and behavioral responses evoked by potentially distressing social situations. Interestingly, there is a substantial overlap between corticolimbic or striatal brain structures implicated in mood disorders and those with aberrant neural engagement to food cues among obese individuals or those with disordered eating.22,24,37–46 Moreover, youth with LOC eating consistently report greater symptoms of anxiety and depression compared to those without LOC.3,47,48 Therefore, LOC eating may result from altered neural engagement common to both socio-emotional and consummatory processes.
To assess the neural underpinnings of LOC eating using an interpersonal model framework more directly, we studied blood oxygen level-dependent (BOLD) responses in the brain using functional magnetic resonance imaging (fMRI) in overweight and obese adolescent girls with and without LOC eating during a simulated peer interaction chatroom paradigm.28,49 This paradigm has previously been used to identify dysregulated engagement of brain regions subsumed by corticolimbic and striatal circuits among adolescents with and at risk for social anxiety.28,29,50,51 Regions that differ between healthy or low-risk and anxious or high-risk youth in this paradigm include the ventrolateral prefrontal cortex (vlPFC) and ventromedial regions of the prefrontal cortex (vmPFC), as well as the fusiform face area (FFA), amygdala (AMY), and striatum. Engagement of these regions, as well as functional connectivity between vlPFC and AMY and between vmPFC and striatum, likely interact to integrate attention, emotion, and corresponding behavioral responses to social situations.52–54 Immediately following the chatroom paradigm, girls consumed lunch at a laboratory test meal designed to model a LOC episode. Consistent with the interpersonal model,19 we hypothesized that compared to girls without LOC, girls with LOC eating would display a differential pattern of brain activation during peer feedback that reflects susceptibility to social rejection. Furthermore, we expected that brain activation patterns during peer feedback would relate to subsequent eating behavior in the post scan test meal.
2. Materials and Methods
2.1 Participants
Participants were recruited via flyers for a study examining eating behaviors in adolescents (ClinicalTrials.gov ID: NCT00631644). Potential participants were eligible if they were right-handed, female, overweight (body mass index, BMI, ≥ 85th percentile standards for U.S. girls55), between 13 and 17 years of age, had good general health (other than overweight or obesity) as indicated by medical history and physical examination, absence of urine glucose excretion, and normal serum electrolytes, hepatic, and thyroid function. Exclusion criteria were chronic illnesses, pregnancy, ongoing weight-loss treatment, use of medications likely to affect energy intake or brain function, or a full-syndrome DSM-5 psychiatric condition, other than binge eating disorder. Participants were also excluded due to a history of significant neurological injury or insult, or the presence of dental braces, or other metal in or on their body that would preclude safe and successful MRI scanning. Girls provided written assent and parents/guardians gave written consent. The study was approved by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Institutional Review Board.
2.2 Procedure
Participants completed outpatient appointments on two separate days at the NIH Clinical Research Center. Following an overnight fast, participants were screened for eligibility at an initial visit that included a medical history and a physical examination performed by an endocrinologist or nurse practitioner. Height was measured three times to the nearest millimeter by a stadiometer (Holtain, Crymmych, Wales) calibrated before each participant’s measurement. Fasting weight was measured to the nearest 0.1 kg with a calibrated digital scale (Scale-Tronix, Wheaton, IL). Height and weight were used to compute BMI (kg/m2). BMI standard deviation (BMIz) scores for sex and age were calculated according to the Centers for Disease Control and Prevention 2000 standards.55 The Eating Disorder Examination version 14 OD/C.256 was administered to assess presence of LOC eating. LOC eating was defined as a subjective experience of lack of control during reported consumption of ambiguously and/or unambiguously large amounts of food. The Eating Disorder Examination has demonstrated excellent inter-rater reliability and discriminant validity for assessing LOC episodes in adolescents.57 Participants who reported one or more LOC episode in the month prior to assessment were included in the LOC group (LOC+); all others were included in the non-LOC (LOC−) group.
2.3 Chatroom Task
Participants completed the well-established and reliable chatroom task (see work from Guyer and colleagues for detailed methods28,29,49–51,58,59), which is implemented across two laboratory visits. This task was designed to assess brain function during an evocative social context in which adolescents receive positive or negative feedback from high- or low-value peers (see Figure 1). The chatroom task procedures involve the following:
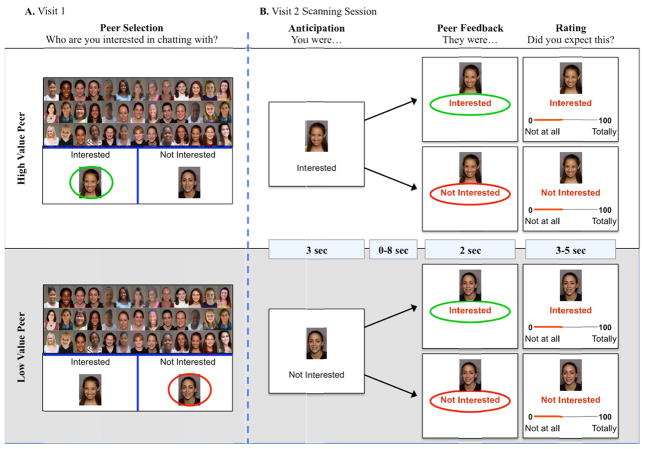
Figure 1.
Depiction of the Chatroom Social Stress Task. A. Participants are asked to categorize peers as 1) high-value: peers with whom they were interested in chatting; and 2) low-value: peers with whom they were not interested in chatting. B. During the fMRI scan, participants learn if their peers are or are not interested in them.
Peer evaluation
After completing screening procedures at their initial outpatient visit, girls were told that they would participate in an online chat session with a peer at their next visit. To prepare for this visit, they were shown 60 photographs of female peers (ages 9–17 years) and were asked to categorize peers as 1) high-value: peers with whom they were interested in chatting; and 2) low-value: peers with whom they were not interested in chatting. Participants were then photographed and told that the 60 purported peers they had just categorized would be shown their picture. Participants were led to believe that purported peers would learn if they had been categorized as high- or low-value before making the same categorization choice for the participant.
Social feedback from peers during fMRI scanning
Following an overnight fast, participants reported to the NIH Clinical Center at 9:30 am for a second outpatient visit to undergo fMRI scanning and other study procedures. Girls consumed no food or drink except water after 10:00 PM on the night before the visit. Two tasks were completed in the scanner. In each task, participants were shown photographs of the previously rated peers. During the first task, participants were asked to guess how interested each peer was in chatting with them. During the second task, participants were reminded that peers were of high- or low-value. Then, they received purported feedback from the high- and low-value peers: the words Interested or Not Interested appeared beneath each photograph. Participants were told that the words indicated how each peer had categorized them. Participants were then asked to indicate how much they expected the feedback they received (0 = not at all; to 100 = totally expected). The two tasks were completed during separate functional runs of fMRI scanning. The interpersonal model of LOC eating implicates stress elicited by a failure to cope with negative interactions in response to social feedback.19 Therefore, brain-based analyses were restricted to data collected during the peer feedback task, obtained in the second functional run.
2.4 fMRI Data Acquisition
Data were acquired on a Siemens MAGNETOM Verio 3T. During receipt of social feedback (functional run 2), 367 functional image volumes were acquired with a T2* echo-planar sequence (34 oblique slices with 2.6 mm thickness; repetition time/echo time (TR/TE) = 2,300/25 ms, flip = 90°; field of view (FOV) = 240 mm, matrix = 64 × 64). To facilitate anatomical localization and co-registration of functional data, a high resolution structural scan was also acquired (sagittal plane) with a T1-weighted magnetization-prepared spoiled gradient-recalled echo sequence (1 mm resolution; echo time/inversion time (TE/TI) = min full/725 ms, flip = 6°; FOV = 220 mm, matrix = 256 × 256).
2.5 Laboratory Test Meal
Immediately following the fMRI scan, a laboratory test meal was administered in a room located in the same building. Each participant was served a large food array (10,934 kcal), varied in macronutrients (54% carbohydrate, 12% protein, 33% fat) and comprised of foods that most children like.60 Participants received a tape-recorded instruction to “Let yourself go and eat as much as you want” to model a LOC eating episode.11 Immediately before, and again, after each test meal, participants completed the psychometrically sound, State Form of the State-Trait Anxiety Inventory for Children61 which measures anxiety “right now, at this very moment.” The amounts of each food and beverage consumed from the meal were measured by using the differences in weight (g) of each item before and after the meal. Energy (kcal) intakes were calculated with data from the U.S. Department of Agriculture National Nutrient Database for Standard Reference (Agricultural Research Service, Beltsville, MD) and food manufacturer nutrient information obtained from food labels. Following the test meal, participants were fully debriefed with regard to the deception involved in the chatroom task.
2.6 Data Analysis
All non-fMRI data analyses were performed with IBM SPSS Statistics (Version 20.0). Data were screened for normality. There were no influential outliers. BMIz was considered in analyses, but since it did not contribute to any model and the results were the same with or without its inclusion (as might be anticipated for studies enrolling only overweight and obese participants), BMIz was removed as a regressor. Self-reported expectation of social feedback during the scanning session was assessed with a repeated measures analysis of variance (ANOVA) with one between-subject factor of group (LOC+, LOC−) and two within-subject factors of peer value (high/low) and peer feedback (positive/negative).
General image processing
All fMRI data analyses were conducted with Analysis of Functional NeuroImages (AFNI) software62 and co-registered to the high resolution structural scan. Data were corrected for slice timing, smoothed (6 mm full width at half maximum), spatially normalized to standard Talairach space, and resampled, resulting in 2.5 mm3 voxels. Temporally adjacent TRs with a Euclidean Norm motion derivative >1 mm were censored and omitted from analyses. Complete data from two additional participants were excluded from analyses due to motion-related artifacts that substantially diminish quality of echoplanar images (≥ 25% censored TRs).
Individual-level fMRI analyses
Separate regressors were created for each type of social feedback event. Events were classified by two criteria: peer value (high/low) and feedback (positive/negative). Thus, four task-specific regressors were modeled: 1) positive feedback from high-value peers; 2) positive feedback from low-values peers; 3) negative feedback from high-value peers; and 4) negative feedback from low-value peers.
Task-specific regressors were convolved with a γ-variate basis function approximating the BOLD response.63 Additional regressors modeled motion residuals and baseline drift. This analysis produced a β-coefficient and associated t-statistic for each regressor at each voxel. Percent signal-change maps were generated by dividing signal intensity at each voxel by the mean voxel intensity, and multiplying by 100.
Group-level fMRI analyses
Whole-brain, group-level analyses assessing the effects of LOC status on hemodynamic response during social feedback were conducted using 3dMVM, an AFNI-based multivariate modeling program (http://afni.nimh.nih.gov/sscc/gangc/MVM.html). This analysis consisted of a repeated measure ANOVA with one between-subject factor of group (LOC+/LOC−), two within-subject factors of peer value (high/low) and peer feedback (positive/negative), and one continuous regressor of total energy intake (mean-centered) during the post scan test meal.
Five a priori regions of interest (ROIs) were considered: vlPFC, vmPFC, fusiform gyrus (which includes FFA), AMY, and striatum. These ROIs were chosen based on prior studies, including those that use the chatroom task, which show that responses in these regions discriminate between high- and low-value peers and/or positive and negative social feedback,28,29,50,51 and were defined anatomically. The vlPFC (762 voxels) is comprised of aspects of inferior frontal gyrus and middle frontal gyrus inferior to z=1. The vmPFC (1146 voxels) is comprised of aspects of superior frontal gyrus, medial frontal gyrus, peri- and subgenual anterior cingulate cortex inferior to z=8 (genu of cingulate). The fusiform gyrus (700 voxels; which includes FFA), AMY (69 voxels) and striatum (688 voxels; dorsal and ventral aspects of the putamen, caudate, and nucleus accumbens) were defined using masks from the Talairach-Tournoux Atlas.
ROI analyses were thresholded by an overall significance level (false detection probability) based on 1,000 Monte Carlo simulations (AFNI, AlphaSim), using a mean estimated spatial correlation of 8.51 × 8.71 × 7.69 mm FWHM, in the respective x, y, and z dimensions. Monte Carlo simulations were performed for each ROI to determine the cluster-size needed to achieve a voxel-specific threshold of p < .005, with an overall family-wise error rate of α < .05. After correcting for the small volume of each ROI, simulations determined the minimum number of contiguous voxels, activated at the p < .005 level, needed to identify significant activity in vlPFC (ke = 14 voxels), vmPFC (ke = 18 voxels), fusiform gyrus (ke 11voxels), AMY (ke = 2 voxels), and striatum (ke = 12; voxels). Activation clusters of interest were extracted and plotted to facilitate interpretation. Exploratory whole-brain analyses for regions not included in ROIs were set at a significance threshold of p < .005, with a cluster extent threshold of ke > 20, and are reported for completeness. Task-based main effect and interactions for peer value and feedback are also reported for completeness using the same threshold as exploratory whole brain analyses (Table 4).
Table 4.
Task effects from exploratory whole brain analyses
| MNI Coordinates | Cluster Size | F | |||
|---|---|---|---|---|---|
| x | y | z | voxels | ||
| Peer Value × Peer Feedback | |||||
| Fusiform Gyrus | 41 | −41 | −19 | 158 | 24.90 |
| Middle Temporal Gyrus | −52 | −55 | −14 | 57 | 23.47 |
| Ventral Striatum extending to Amygdala | −16 | 4 | −7 | 42 | 18.98 |
| Thalamus | −11 | −27 | 8 | 26 | 16.34 |
| Precuneus | 31 | −80 | 33 | 21 | 13.43 |
| Peer Value | |||||
| Parahippocampal Gyrus | 14 | −35 | −3 | 243 | 34.12 |
| Precuneus | −16 | −91 | 40 | 58 | 27.18 |
| Dorsal Anterior Cingulate | −1 | 18 | 35 | 57 | 17.99 |
| Inferior Parietal Lobule | −46 | −36 | 31 | 50 | 51.38 |
| Middle Temporal Gyrus | −34 | −77 | 22 | 43 | 25.90 |
| Superior Temporal Gyrus | 52 | 9 | 2 | 26 | 19.08 |
| Peer Feedback | |||||
| Uncus extending to Amygdala | 21 | 8 | −34 | 24 | 26.43 |
Activation clusters reflect Peer value (High/Low), Peer Feedback (Positive/Negative) interactions or main effects. Whole brain analysis, p < .005, cluster size > 20.
Functional connectivity analyses
There are widespread functional connections between prefrontal cortex and subcortical regions such as AMY and striatum. Strength of this coupling has been implicated in the capacity to regulate affect (reviewed by Ochsner64), and is often disrupted in at risk and patient populations.28,65–67 Thus, exploratory functional connectivity analyses were conducted for regions of prefrontal cortex (seeds) that showed group differences in primary ROI analyses. Psychophysiological interaction (PPI) analyses were therefore performed to determine whether activation in vmPFC during negative, relative to positive feedback, covaried with activation in AMY or striatum to a different extent across groups.
For each participant, mean-adjusted eigenvariate time series data were extracted from the activation cluster in vmPFC that emerged from group-by-social feedback analyses. Time series data were deconvolved with the hemodynamic response function before a PPI term was generated for positive vs. negative social feedback. A random-effects model was calculated to identify group differences in any region showing vmPFC coupling that differed for positive, as compared with negative, social feedback. A significance threshold of p < .005 and cluster extent of 20 was applied to whole-brain functional connectivity analyses.
3. Results
Data are reported from 22 girls who completed the chatroom task and test meal, 10 of whom reported at least one episode of LOC eating in the past month (LOC+). Based upon LOC status, girls did not differ in age, race, BMI metrics, or post-scan/pre-meal state anxiety (Table 1).
Table 1.
Participant Characteristics
| Loss of control (n = 10) | No loss of control (n = 12) | P-value | |
|---|---|---|---|
| Age, y, M±SD | 15.4 ± 1.7 | 16.1 ± 1.4 | .30 |
| Race, % | 30.0% Non-Hispanic White 60.0% Non-Hispanic Black 10.0% Other |
50% Non-Hispanic White 33.4% Non-Hispanic Black 16.6% Other |
.37 |
| BMI*, kg/m2, M±SD | 33.5 ± 9.2 | 32.4 ± 5.1 | .76 |
| BMIz, M±SD | 1.9 ± .5 | 1.9 ± .3 | .98 |
| BMI percentile, M±SD | 95.1 ± 3.7 | 96.1 ± 3.7 | .51 |
| Pre-meal state anxiety, M±SD | 3.9 ± 6.1 | 1.0 ± 1.0 | .11 |
| Intake (kilocalories), M±SD | 1641.40 ± 695.09 | 1322.03 ± 358.95 | .18 |
| Post-meal state anxiety, M±SD | 3.4 ± 2.3 | 1.0 ± 1.0 | .006 |
BMI = body mass index
Manipulation Check
Behavioral responses in the scanner replicated prior findings,28,50 such that there was a significant interaction between Peer Value × Peer Feedback on participants’ report of “expectation” of displayed responses, F(1,21)=18.77, p < .001. Regardless of LOC status, participants reported having expected positive feedback from high-value (M=59.55, SD=15.29) relative to low-value peers (M=41.46, SD=16.73, p < .01); and negative feedback from low-value (M=61.50, SD=11.53) relative to high-value peers (M=44.56, SD=13.46; p < .001). There was no main or interaction effect on self-report for LOC, nor did reported expectations relate to total energy intake across participants, or within LOC+ or LOC− groups when considered separately (all ps ns).
3.1 Group-level fMRI analyses
LOC and brain response
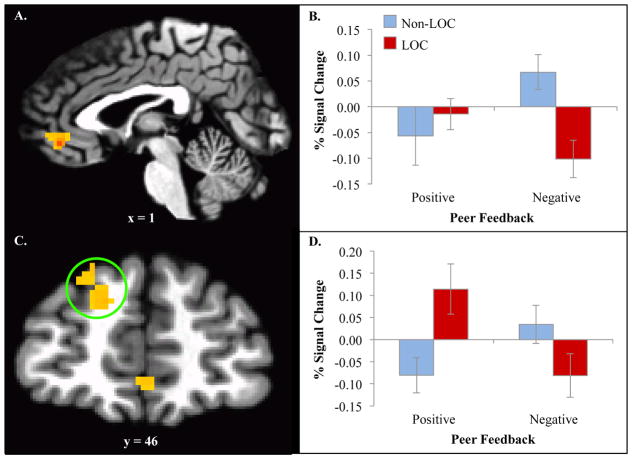
ROI analyses revealed a significant cluster within the vmPFC for the interaction between group (LOC+ versus LOC−) and feedback type (x, y, z = co-ordinates reported in Montreal Neurological Institute standardized space: 1, 43, −5; ke = 41, F = 35.51). Decomposition of this interaction revealed that while both groups responded similarly to positive feedback (t(20)= −.63, p = ns), negative feedback elicited diminished engagement in LOC+, but heighted engagement for LOC− participants (t(20)=3.40, p <.005; Figure 2A–B). Exploratory whole brain analyses revealed a significant cluster for the same interaction within dorsolateral PFC (dlPFC; x, y, z = −14, 51, 40, F = 15.71). Decomposition of this interaction revealed that while LOC− responded similarly to positive and negative feedback (t(10)=−1.74, p = ns), LOC+ had heightened activity to positive, relative to negative, feedback (t(9)=5.34, p <.001; Figure 2C–D). No other main or interaction effect on brain function for group was found in other ROIs. See Table 2 for additional group-level activation clusters from exploratory whole brain analyses.
Figure 2.
Significant clusters for the interaction between group and feedback type A. Ventromedial prefrontal cortex (1, 43, −5; ke = 41, F = 35.51). B. Decomposition showed that both groups responded similarly to positive feedback (t(20)= −.63, p = ns), but negative feedback elicited diminished engagement in LOC+ and heighted engagement for LOC− (t(20)=3.40, p <.005). C. Ventrolateral prefrontal cortex (−14, 51, 40, ke = 61, F = 15.71). D. Decomposition showed that while LOC− responded similarly to positive and negative feedback (t(10)= −1.74, p = ns), LOC+ had heightened activity to positive, relative to negative feedback (t(9)=5.34, p = <.001).
Table 2.
Group-level activation clusters from exploratory whole brain analyses
| MNI Coordinates | Cluster Size | F | |||
|---|---|---|---|---|---|
| x | y | z | voxels | ||
| Group × Peer Value × Peer Feedback | |||||
| - | - | - | - | - | - |
| Group × Peer Value | |||||
| Postcentral Gyrus | 57 | −21 | 20 | 302 | 31.90 |
| Precentral Gyrus | 46 | −13 | 38 | 39 | 15.86 |
| −57 | −16 | 36 | 178 | 36.21 | |
| Insula | −36 | 4 | 10 | 26 | 14.95 |
| −31 | 14 | 5 | 33 | 20.13 | |
| Anterior Cingulate | −4 | 15 | 38 | 30 | 14.36 |
| Inferior Frontal Gyrus | −36 | 30 | 0 | 22 | 14.92 |
| Group × Peer Feedback | |||||
| Superior Frontal Gyrus | −14 | 51 | 40 | 61 | 15.71 |
| Group§ | |||||
| Precentral Gyrus | 49 | −14 | 31 | 119 | 41.56 |
| −46 | −16 | 38 | 32 | 27.34 | |
| Tempero-Parietal Junction | 24 | −73 | 39 | 21 | 24.87 |
Activation clusters reflect Group (LOC+/LOC−), Peer value (High/Low), Peer Feedback (Positive/Negative) interactions or main effects§. Whole brain analysis, p < .005, cluster size > 20.
Functional connectivity analyses
PPI analysis revealed that functional connectivity between the vmPFC seed and striatum (putamen; x, y, z = −24, −4, −5; ke = 35) varied as a function of peer feedback and group (see Figure 3). Negative, relative to positive, feedback resulted in negative vmPFC-striatal coupling in LOC− (t(11)=−2.79, p < .05), but positive coupling in LOC+ (t(9)=4.92, p < .005). Group differences in functional connectivity were also observed in inferior parietal lobule (x, y, z = −46, −68, 38; ke = 77), but were not interrogated further due to lack of a priori hypotheses about this region.
Figure 3.
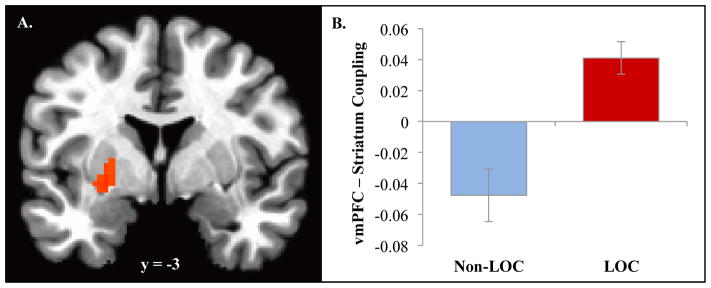
Psychophysiological interaction analysis with ventromedial prefrontal cortex (vmPFC) showed functional connectivity between vmPFC and striatum (putamen; −24, −4, −5; ke = 35) varied by peer feedback and group. Negative, relative to positive, feedback, resulted in negative mPFC-striatal coupling in LOC− (t(11)= −2.79, p < .05), but positive coupling in LOC+ (t(9)=4.92, p < .005).
Relation of neural activation to “LOC” meal energy intake
Two activation clusters in the fusiform gyrus varied by both group and energy intake. Four-way interactions (Group × Peer Feedback × Peer Value × Energy Intake) were observed in bilateral FFA (Right: x, y, z = 31, −47, −13; ke = 13; F = 19.22; Left: x, y, z = −49 −50 −16; ke = 15; F = 24.93; Figure 3). These interactions were primarily driven by group differences during feedback from high-value peers (Fisher’s r-to-z’s: Right = −2.40; p < .05; Left = −2.22; p < .05). For LOC+, greater FFA activity during negative, relative to positive, feedback from high-value peers was positively associated with overall energy intake during the subsequent test meal (r’s: Right = .76, p = .01; Left = .48, p = ns). In contrast, for LOC−, FFA activity during peer feedback did not relate to subsequent energy intake (r’s: Right = .27, p = ns; Left = −.54, p = ns). Group differences were not observed in this region during feedback from low-value peers (Fisher’s r-to-z’s: Right = 1.40; p = ns; Left = .27, p = ns).
A three-way interaction (Group × Peer Value × Energy Intake) was found in the AMY (−21, −5, −23; ke = 7; F=12.25). However, because this interaction did not relate to peer response, the primary experimental condition of interest, it was not further interrogated. See Table 3 for complete group-level activation clusters from exploratory whole brain analyses.
Table 3.
Group-level activation clusters that predict subsequent caloric intake from whole brain analysis
| MNI Coordinates | Cluster Size | F | |||
|---|---|---|---|---|---|
| x | y | z | voxels | ||
| Group × Peer Value × Peer Feedback × Caloric Intake | |||||
| Middle Temporal Gyrus | 57 | −63 | 9 | 29 | 16.61 |
| Group × Peer Value × Caloric Intake | |||||
| Hippocampus | −21 | −13 | −26 | 34 | 17.18 |
| Parahippocampal Gyrus | −34 | −24 | −14 | 30 | 23.64 |
| Precuneus | 24 | −70 | 41 | 22 | 16.1 |
| Posterior Cingulate | −6 | −51 | 15 | 60 | 29.02 |
| −4 | −47 | 35 | 42 | 17.99 | |
| Parahippocampal Gyrus | −34 | −24 | −24 | 30 | 23.64 |
| −21 | −13 | −26 | 25 | 17.18 | |
| Middle Temporal Gyrus | −52 | −42 | −7 | 25 | 21.33 |
| Middle Occipital Gyrus | −49 | −71 | −11 | 33 | 19.83 |
| Group × Peer Feedback × Caloric Intake | |||||
| Temporal Pole | 60 | 1 | 1 | 33 | 18.77 |
| Group × Caloric Intake | |||||
| Cuneus | −6 | −82 | 19 | 75 | 26.15 |
| Middle Temporal Gyrus | −39 | −67 | 19 | 37 | 23.52 |
| Precentral Gyrus | −36 | −22 | 54 | 23 | 27.38 |
Activation clusters reflect Group (LOC+/LOC−), Peer value (High/Low), Peer Feedback (Positive/Negative), Caloric Intake (continuous measure of total intake at test meal) interactions. Whole brain analysis, p < .005, cluster size > 20.
4. Discussion
This study examined potential differences in neural response to peer feedback in girls with and without LOC eating. We found that brain activity during a paradigm known to differentially engage circuitry implicated in social anxiety, differed between girls with and without LOC, and was linked to subsequent food intake. Whereas overweight and obese girls with LOC had diminished vmPFC activation following peer rejection (vs. acceptance), those without LOC had heightened vmPFC activation. Functional connectivity between vmPFC and striatum also varied based upon reported LOC eating. Further, after receiving negative feedback from high-value peers, greater FFA activation was associated with more subsequent energy intake in girls with reported LOC eating, but did not relate to energy intake in those without LOC. Taken together, these preliminary findings support the hypothesis that dysregulated brain response to social evaluation may be linked to overeating in overweight and obese adolescent girls with LOC eating.
Girls with LOC eating exhibited reduced vmPFC engagement in response to peer rejection. The vmPFC has been implicated in numerous aspects of socio-emotional processing, including interpreting social intentions, self-reflection, and affect modulation.68–71 In a social context, healthy individuals generally have heightened engagement of vmPFC following exclusion72–74 and social evaluative threat.75,76 It has been suggested that heightened engagement of vmPFC in this context may, in fact, facilitate the regulation of negative affect typically generated by interpersonal distress.69,71,76 Thus, among the girls in our sample with LOC eating, diminished engagement of vmPFC during negative peer feedback may reflect a failure to engage regulatory mechanisms following peer rejection. This response possibly accounts for data indicating poor social functioning in youth with LOC eating.13–16 Hypoactivity in vmPFC among girls with reported LOC eating may therefore relate to deficits in adjusting and responding to social evaluative outcomes or to emotion regulation more generally. Diminished engagement of the vmPFC may be experienced as alexithymia or the inability to identify or express affect and avoidance in coping with conflicts or articulating emotions.77 Similar cognitive–emotional deficits have been reported by youth with LOC eating during LOC eating episodes.78,79 These data can be interpreted within a Research Domain Criteria framework.80 Girls with LOC eating may have a compromised ability to down-regulate uncomfortable affective states that are a reflection of deficits in negative valence constructs.81 In response, such youth may seek external means of comfort including engaging in excessive food consumption, consistent with problems with the positive valence domain.
Although dorsolateral prefrontal cortex (dlPFC) was not an a priori ROI in this study, it is a brain region that supports regulatory control, and has been linked to eating-related regulatory control among dieters.82,83 Our exploratory whole brain analyses found that activity in the superior frontal gyrus, a region of dlPFC commonly implicated in emotion regulation,84–86 varied as a function of LOC group and valence of social feedback. Specifically, girls with LOC eating had heightened activity in dlPFC during receipt of positive, relative to negative peer feedback; those without LOC did not differentiate between positive and negative feedback. This suggests that unlike dieters, girls with LOC have diminished regulatory control for eating, but like dieters, may engage heightened regulatory processes in contexts distinct from eating. The results may be interpreted as developmental differences in youth with LOC eating who have yet to develop overly restrictive dietary patterns17 reported by adults with binge eating.87,88 Our results also suggest that enhanced emotion regulation in girls with LOC may dampen the potential benefit of positive social feedback, while diminished emotion regulation may increase their susceptibility to the deleterious consequences of negative social feedback. Further work is needed to specifically relate affective response to peer feedback with brain function in adolescent girls with LOC, as well as those with and without restricted eating.
Group differences were also identified in exploratory analyses of functional coupling between PFC and the striatum during peer feedback. Engagement of vmPFC was associated with down-regulated striatal activity in those without LOC, but up-regulated activity in girls with LOC eating. Although evidence from both animal and human studies has linked striatal activity with appetitive behaviors including food consumption,25,89 recent work suggests that the striatum may play a more general role in tracking the affective salience of stimuli.90,91 Up-regulated striatal activity suggests that social feedback carries a heightened salience among girls with LOC eating. The striatum may also shape motivated behavior to obtain or avoid outcomes while influencing learning and habit formation.92–94 Indeed, dysregulated vmPFC-striatal connectivity during peer feedback in the chatroom task contributes to biases in social learning among anxious adolescents.51 Perturbations in corticostriatal circuitry also have been found among individuals with binge-type eating disorders during stimulus-outcome learning paradigms.95–97 Thus, the dysregulated ability to exert executive control over subcortical structures may relate to both aversive and appetitive behaviors. While the precise implications of dysregulated vmPFC-striatal connectivity clearly requires further investigation, the current results suggest one neural mechanism by which poor social functioning may directly relate to LOC eating behavior.
We also found that in girls with LOC eating, brain activity patterns was linked to subsequent energy intake. Specifically, greater engagement of FFA to negative, relative to positive, feedback from high-value peers was linked to higher energy intake for girls with LOC eating, but did not relate to energy intake in those without LOC. This finding parallels previously reported data of heightened FFA engagement following peer rejection among adolescents at risk for social anxiety.50 The FFA demonstrates a specific and selective pattern of engagement to faces and is thought to reflect highly enriched perceptual processing.98,99 The FFA also receives “back projections” from brain regions implicated in affective processing, which are thought to bias responding towards emotionally salient or arousing facial expressions.100,101,36 Thus, among the girls in our sample with LOC eating, heightened engagement of FFA may reflect a bias towards processing negative feedback from high-value peers. This bias, which emerged during only the most threatening of social encounters (negative feedback from high-value peers), also was positively associated with consumption during the test meal in those with LOC eating. However, heightened FFA is also observed in response to food cues or palatable food intake among obese and/or food-deprived individuals.102–105 Thus, it is somewhat difficult to determine whether caloric consumption in those with LOC is specifically related to heightened activity in FFA elicited by highly salient social cues, highly salient food cues encountered at the test buffet, or some combination thereof. For example, heightened engagement of FFA may be an index of poor affect regulation processes, which fail to buffer girls with LOC during negative social encounters. Failure to engage affect regulation processes may promote subsequent self-soothing via dysregulated eating that is potentiated by the elevated salience of food. Further complicating interpretation of these findings is the use of face-based stimuli, which are known to engage FFA.98,99 Further investigation is required to isolate the specific mechanisms that mediate the relationship between FFA and energy consumption in girls with LOC eating.
Contrary to our hypothesis, we found no relationship between AMY activation and LOC eating status or energy intake. Although youth with anxiety disorders have demonstrated AMY hyperactivation in social evaluation paradigms,28,29,50,51 some data indicate that individuals with binge-type eating disorders exhibit AMY hypoactivation AMY when presented with socially threatening stimuli relative to healthy controls.106–108 Such hypo-responsivity has been interpreted as emotional blunting, which is consistent with frequent reports of alexithymia among adolescent girls with LOC eating.109 Therefore, a lack of elevated AMY activity during the chatroom paradigm may be the result of emotional blunting among girls with LOC eating. It is also possible that the negative social feedback was insufficiently evocative to elicit a strong AMY response. Youth with LOC eating often report weight-related teasing from peers and family members,15,110,111 and therefore, receiving feedback that unknown peers were not interested in chatting with them for a study may have seemed relatively mild compared to the social ostracism they habitually experience. Alternatively, all overweight and obese participants, who typically report higher rates of bullying and lower social support than their average-weight peers,112 may have found the chatroom paradigm anxiety-provoking regardless of LOC eating status. Future studies would benefit from inclusion of an average-weight comparison group and participants with full-threshold eating disorder psychopathology to evaluate these hypotheses.
Despite differences in brain activity, LOC did not relate to behavioral differences in self-reported expectation of peer feedback. While potentially puzzling, several factors may help explain this apparent discrepancy. First, it is important to underscore the fact that social interactions have complex temporal dynamics. For example, different psychological processes and corresponding neural circuits may be engaged when determining whether a peer is a desirable partner for a social interaction, subsequent anticipation and then receipt of social evaluation from that peer, and finally reflecting upon the extent to which this feedback was expected. The chatroom paradigm was specifically designed to disentangle the neural circuits engaged during each step of this temporally dynamic process. Individuals with LOC eating commonly report that their eating behavior is elicited by social conflict or rejection.13–16 The present study focused on isolating group differences in brain activity specifically engaged by social rejection, relative to acceptance; brain activity engaged while reflecting on expected feedback was not modeled. However, unlike fMRI studies that measure brain function during passive viewing of stimuli and are unable to determine whether stimuli capture the attention of participants, obtaining these behavioral data provide greater confidence that participants were, indeed, engaged in the task at hand.
Given the proposed role of social rejection in LOC eating, one limitation of the present design is that participants were not asked to rate their emotional response to peer feedback. Group differences may have emerged had such a measure had been implemented. However, the relationship between affect elicited by specific experiences of rejection and subsequent caloric intake in LOC eating in the laboratory are not well documented in youth. Indeed, brain-based data often map on to the expression of clinically relevant behavior more closely than self-report.113–115 Thus, group differences in the neural circuits engaged by social feedback, and their relation to subsequent caloric intake, may give us greater insight into the mechanisms that support LOC eating than self-reported alone. Moreover, self-report measures tend to poorly predict long-term expression of childhood disorders.115 Despite their relatively high incidence rates in childhood and adolescence, both social anxiety116,117 and LOC eating3,118 often remit as youth transition to adulthood. Isolating neural signatures in adolescents more likely to sustain disorders into adulthood would help clinicians distinguish patients at greatest need for interventions, from those most likely to remit without such interventions.115,119,120
Strengths of the current study involve the use of interview assessment to determine LOC eating status, measurement of actual energy intake in the laboratory, and a racially diverse sample. This study also fills a needed knowledge gap in the field since socio-emotional processes have rarely been studied in adolescents with disordered eating. However, the sample size was relatively small. As a result, findings should be considered preliminary and require replication. Nevertheless, we applied stringent voxel and clusterwise False Discovery Rate correction and used theory to drive our analyses. This ensures that, despite the small sample size, future brain-based studies testing interpersonal theory are guided only by findings with appropriate statistical corrections.
Another limitation is that ROIs were defined anatomically, rather than with a functional localizer. A functional localizer approach defines ROIs using brain regions that respond differently to task-based factors, and then quantifies group differences in brain function within these regions. As described in Table 4, numerous brain regions differentially responded to task-based factors in the present study. However, they did not consistently overlap with brain regions where group differences in task-based responses emerged. This inconsistency may relate to the fact that, unlike traditional social evaluation paradigms designed to elicit stress via objectively threatening contexts,121 the chatroom paradigm relies on more subtle meta-cognitive factors to elicit stress. For example, instead of receiving overt and ongoing negative social feedback based on real-time performance, participants receive one-time positive or negative social feedback, purportedly generated by a peer at a prior visit. This feedback can, in turn, be interpreted in light of the peer’s value to the participant, and beliefs about what motivated each peer to provide positive or negative feedback. Group differences in brain function may therefore correspond with regions directly related to task-based factors, or be more indicative of the meta-cognitive processes engaged by task-based factors.
Additionally, participants’ weight history was not assessed. Although youth with LOC eating rarely report a history of weight control efforts or weight loss,17 the adult literature suggests differential neural responses between current and historical dieters and thus may be an important area for future exploration in pediatric samples.122 The lack of a measure of trait anxiety in the current study also precluded our ability to determine whether findings persisted above and beyond this potential confound. However, it is notable that youth with and without LOC eating did not differ in their level of state anxiety immediately following the chatroom task.
5. Conclusions
In conclusion, our findings support the hypothesis that social-emotional neurobiological mechanisms may promote overeating in youth with LOC eating. Consistent with interpersonal theory, findings also suggest that the failure to engage prefrontal regulatory regions when receiving negative social feedback may lead to poor emotion regulation. In adolescent girls with LOC eating, biases towards heightened processing during stressful negative social interactions may promote subsequent overeating as an alternative means to more healthy or non-food-related strategies for coping with socially threatening interactions. Moreover, the present findings lay the foundation for future work aimed at distinguishing neural circuits in youth with LOC eating at greatest risk for sustained symptoms in adulthood, and thus at greatest need for early intervention.
Figure 4.
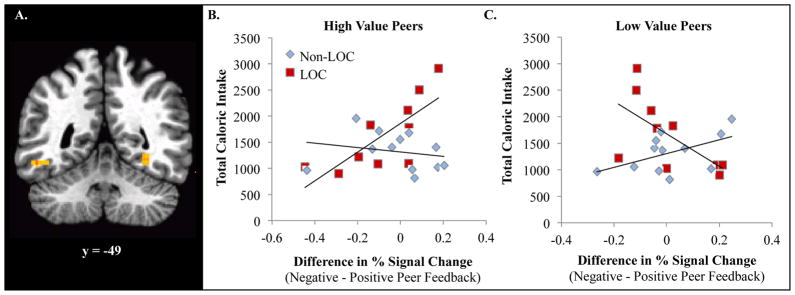
Group differences during feedback from high-value peers (Fisher’s r-to-z’s: Right = −2.40; p < .05; Left = −2.22; p < .05). For LOC+, greater fusiform face area (FFA) activity during negative, relative to positive feedback, from high-value peers was positively associated with greater energy intake during the subsequent test meal (r’s: Right = .76, p = .01; Left = .48, p = ns). For LOC−, FFA activity during peer feedback did not relate to subsequent energy intake (r’s: Right = .27, p = ns; Left = −.54, p = ns).
Acknowledgments
Research Support: NIDDK 1R01DK080906 (to MTK); USUHS grant R072IC (to MTK); Intramural Research Program; NIH, grant 1ZIAHD000641 from the NICHD (to JAY); the Bench to Bedside Program and the Office of Behavioral and Social Sciences Research (OBSSR) of the NIH (to JAY and MTK); Human Imaging Core Facility of the Center for Neuroscience and Regenerative Medicine.
Footnotes
Disclaimers: The opinions and assertions expressed herein are those of the authors and are not to be construed as reflecting the views of the PHS, USUHS or the U.S. Department of Defense.
Trial Registration: ClinicalTrials.gov ID#: NCT00631644
References
- 1.Tanofsky-Kraff M, Yanovski SZ, Schvey NA, Olsen CH, Gustafson J, Yanovski JA. A prospective study of loss of control eating for body weight gain in children at high risk for adult obesity. The International journal of eating disorders. 2009 Jan;42(1):26–30. doi: 10.1002/eat.20580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sonneville KR, Horton NJ, Micali N, et al. Longitudinal Associations Between Binge Eating and Overeating and Adverse Outcomes Among Adolescents and Young AdultsDoes Loss of Control Matter? Binge Eating and Overeating and Adverse Outcomes. JAMA Pediatrics. 2013;167(2):149–155. doi: 10.1001/2013.jamapediatrics.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanofsky-Kraff M, Shomaker LB, Olsen C, et al. A prospective study of pediatric loss of control eating and psychological outcomes. J Abnorm Psychol. 2011 Feb;120(1):108–118. doi: 10.1037/a0021406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hilbert A, Hartmann AS, Czaja J, Schoebi D. Natural course of preadolescent loss of control eating. J Abnorm Psychol. 2013;122(3):684–693. doi: 10.1037/a0033330. [DOI] [PubMed] [Google Scholar]
- 5.Field AE, Sonneville KR, Micali N, et al. Prospective association of common eating disorders and adverse outcomes. Pediatrics. 2012;130(2):e289–295. doi: 10.1542/peds.2011-3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skinner HH, Haines J, Austin SB, Field AE. A Prospective Study of Overeating, Binge Eating, and Depressive Symptoms Among Adolescent and Young Adult Women. J Adolesc Health. 2012;50(5):478–483. doi: 10.1016/j.jadohealth.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanofsky-Kraff M, Shomaker LB, Stern EA, et al. Children’s binge eating and development of metabolic syndrome. Int J Obes (Lond) 2012 Jul;36(7):956–962. doi: 10.1038/ijo.2011.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neumark-Sztainer D, Wall M, Larson NI, Eisenberg ME, Loth K. Dieting and disordered eating behaviors from adolescence to young adulthood: findings from a 10-year longitudinal study. J Am Diet Assoc. 2011;111(7):1004–1011. doi: 10.1016/j.jada.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stice E, Marti CN, Shaw HE, Jaconis M. An 8-year longitudinal study of the natural history of threshold, subthreshold, and partial eating disorders from a community sample of adolescents. J Abnorm Psychol. 2009;118(3):587–597. doi: 10.1037/a0016481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shomaker LB, Tanofsky-Kraff M, Elliott C, et al. Salience of loss of control for pediatric binge episodes: does size really matter? The International journal of eating disorders. 2010 Dec;43(8):707–716. doi: 10.1002/eat.20767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanofsky-Kraff M, McDuffie JR, Yanovski SZ, et al. Laboratory assessment of the food intake of children and adolescents with loss of control eating. Am J Clin Nutr. 2009 Mar;89(3):738–745. doi: 10.3945/ajcn.2008.26886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldschmidt AB, Wall MM, Loth KA, Bucchianeri MM, Neumark-Sztainer D. The course of binge eating from adolescence to young adulthood. Health Psychol. 2013 doi: 10.1037/a0033508. [ePub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Czaja J, Hartmann AS, Rief W, Hilbert A. Mealtime family interactions in home environments of children with loss of control eating. Appetite. 2011;56(3):587–593. doi: 10.1016/j.appet.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 14.Elliott CA, Tanofsky-Kraff M, Shomaker LB, et al. An examination of the interpersonal model of loss of control eating in children and adolescents. Behaviour research and therapy. 2010;48(5):424–428. doi: 10.1016/j.brat.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartmann A, Czaja J, Rief W, Hilbert A. Psychosocial risk factors of loss of control eating in primary school children: a retrospective case-control study. The International journal of eating disorders. 2012;45(6):751–758. doi: 10.1002/eat.22018. [DOI] [PubMed] [Google Scholar]
- 16.Hilbert A, Tuschen-Caffer B, Czaja J. Eating behavior and familial interactions of children with loss of control eating: A laboratory test meal study. Am J Clin Nutr. 2010;91:510–518. doi: 10.3945/ajcn.2009.28843. [DOI] [PubMed] [Google Scholar]
- 17.Tanofsky-Kraff M, Faden D, Yanovski SZ, Wilfley DE, Yanovski JA. The perceived onset of dieting and loss of control eating behaviors in overweight children. The International journal of eating disorders. 2005 Sep;38(2):112–122. doi: 10.1002/eat.20158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morgan CM, Yanovski SZ, Nguyen TT, et al. Loss of control over eating, adiposity, and psychopathology in overweight children. The International journal of eating disorders. 2002 May;31(4):430–441. doi: 10.1002/eat.10038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanofsky-Kraff M, Wilfley DE, Young JF, et al. Preventing excessive weight gain in adolescents: interpersonal psychotherapy for binge eating. Obesity (Silver Spring) 2007 Jun;15(6):1345–1355. doi: 10.1038/oby.2007.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elliott CA, Tanofsky-Kraff M, Shomaker LB, et al. An examination of the interpersonal model of loss of control eating in children and adolescents. Behaviour research and therapy. 2010 May;48(5):424–428. doi: 10.1016/j.brat.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ranzenhofer LM, Engel SG, Crosby RD, et al. Using ecological momentary assessment to examine interpersonal and affective predictors of loss of control eating in adolescent girls. International Journal of Eating Disorders. doi: 10.1002/eat.22333. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bohon C, Stice E. Negative affect and neural response to palatable food intake in bulimia nervosa. Appetite. 2012 Jun;58(3):964–970. doi: 10.1016/j.appet.2012.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rudenga KJ, Sinha R, Small DM. Acute stress potentiates brain response to milkshake as a function of body weight and chronic stress. Int J Obes (Lond) 2013 Feb;37(2):309–316. doi: 10.1038/ijo.2012.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bohon C, Stice E, Spoor S. Female emotional eaters show abnormalities in consummatory and anticipatory food reward: a functional magnetic resonance imaging study. The International journal of eating disorders. 2009 Apr;42(3):210–221. doi: 10.1002/eat.20615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Volkow ND, Wang GJ, Baler RD. Reward, dopamine and the control of food intake: implications for obesity. Trends Cogn Sci. 2011 Jan;15(1):37–46. doi: 10.1016/j.tics.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dallman MF. Stress-induced obesity and the emotional nervous system. Trends Endocrinol Metab. 2010 Mar;21(3):159–165. doi: 10.1016/j.tem.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sabatini MJ, Ebert P, Lewis DA, Levitt P, Cameron JL, Mirnics K. Amygdala gene expression correlates of social behavior in monkeys experiencing maternal separation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007 Mar 21;27(12):3295–3304. doi: 10.1523/JNEUROSCI.4765-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guyer AE, Lau JY, McClure-Tone EB, et al. Amygdala and ventrolateral prefrontal cortex function during anticipated peer evaluation in pediatric social anxiety. Arch Gen Psychiatry. 2008 Nov;65(11):1303–1312. doi: 10.1001/archpsyc.65.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lau JYF, Guyer AE, Tone EB, et al. Neural responses to peer rejection in anxious adolescents: Contributions from the amygdala-hippocampal complex. International Journal of Behavioral Development. 2012 Jan 1;36(1):36–44. doi: 10.1177/0165025411406854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Masten CL, Eisenberger NI, Borofsky LA, McNealy K, Pfeifer JH, Dapretto M. Subgenual anterior cingulate responses to peer rejection: a marker of adolescents’ risk for depression. Dev Psychopathol. 2011 Feb;23(1):283–292. doi: 10.1017/S0954579410000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roberson-Nay R, McClure EB, Monk CS, et al. Increased amygdala activity during successful memory encoding in adolescent major depressive disorder: An FMRI study. Biological psychiatry. 2006 Nov 1;60(9):966–973. doi: 10.1016/j.biopsych.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 32.Telzer EH, Mogg K, Bradley BP, et al. Relationship between trait anxiety, prefrontal cortex, and attention bias to angry faces in children and adolescents. Biol Psychol. 2008 Oct;79(2):216–222. doi: 10.1016/j.biopsycho.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Monk CS, Nelson EE, McClure EB, et al. Ventrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder. Am J Psychiatry. 2006 Jun;163(6):1091–1097. doi: 10.1176/ajp.2006.163.6.1091. [DOI] [PubMed] [Google Scholar]
- 34.Monk CS, Klein RG, Telzer EH, et al. Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. Am J Psychiatry. 2008 Jan;165(1):90–98. doi: 10.1176/appi.ajp.2007.06111917. [DOI] [PubMed] [Google Scholar]
- 35.Killgore WD, Yurgelun-Todd DA. Social anxiety predicts amygdala activation in adolescents viewing fearful faces. Neuroreport. 2005 Oct 17;16(15):1671–1675. doi: 10.1097/01.wnr.0000180143.99267.bd. [DOI] [PubMed] [Google Scholar]
- 36.Lee TH, Sakaki M, Cheng R, Velasco R, Mather M. Emotional arousal amplifies the effects of biased competition in the brain. Soc Cogn Affect Neurosci. 2014 Mar 10; doi: 10.1093/scan/nsu015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yokum S, Ng J, Stice E. Attentional bias to food images associated with elevated weight and future weight gain: an fMRI study. Obesity (Silver Spring) 2011 Sep;19(9):1775–1783. doi: 10.1038/oby.2011.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stice E, Yokum S, Blum K, Bohon C. Weight gain is associated with reduced striatal response to palatable food. J Neurosci. 2010 Sep 29;30(39):13105–13109. doi: 10.1523/JNEUROSCI.2105-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J Abnorm Psychol. 2008 Nov;117(4):924–935. doi: 10.1037/a0013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coletta M, Platek S, Mohamed FB, van Steenburgh JJ, Green D, Lowe MR. Brain activation in restrained and unrestrained eaters: an fMRI study. J Abnorm Psychol. 2009 Aug;118(3):598–609. doi: 10.1037/a0016201. [DOI] [PubMed] [Google Scholar]
- 41.Burger KS, Stice E. Elevated energy intake is correlated with hyperresponsivity in attentional, gustatory, and reward brain regions while anticipating palatable food receipt. Am J Clin Nutr. 2013 Jun;97(6):1188–1194. doi: 10.3945/ajcn.112.055285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burger KS, Stice E. Relation of dietary restraint scores to activation of reward-related brain regions in response to food intake, anticipated intake, and food pictures. Neuroimage. 2011 Mar 1;55(1):233–239. doi: 10.1016/j.neuroimage.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bohon C. Greater emotional eating scores associated with reduced frontolimbic activation to palatable taste in adolescents. Obesity (Silver Spring) 2014 Apr 8; doi: 10.1002/oby.20759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Batterink L, Yokum S, Stice E. Body mass correlates inversely with inhibitory control in response to food among adolescent girls: an fMRI study. Neuroimage. 2010 Oct 1;52(4):1696–1703. doi: 10.1016/j.neuroimage.2010.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jastreboff AM, Sinha R, Lacadie C, Small DM, Sherwin RS, Potenza MN. Neural correlates of stress- and food cue-induced food craving in obesity: association with insulin levels. Diabetes Care. 2013 Feb;36(2):394–402. doi: 10.2337/dc12-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Connolly L, Coveleskie K, Kilpatrick LA, et al. Differences in brain responses between lean and obese women to a sweetened drink. Neurogastroenterol Motil. 2013 Jul;25(7):579–e460. doi: 10.1111/nmo.12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sonneville KR, Horton NJ, Micali N, et al. Longitudinal associations between binge eating and overeating and adverse outcomes among adolescents and young adults: does loss of control matter? JAMA Pediatr. 2013 Feb;167(2):149–155. doi: 10.1001/2013.jamapediatrics.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goossens L, Braet C, Van Vlierberghe L, Mels S. Loss of control over eating in overweight youngsters: the role of anxiety, depression and emotional eating. Eur Eat Disord Rev. 2009 Jan;17(1):68–78. doi: 10.1002/erv.892. [DOI] [PubMed] [Google Scholar]
- 49.Guyer AE, McClure-Tone EB, Shiffrin ND, Pine DS, Nelson EE. Probing the neural correlates of anticipated peer evaluation in adolescence. Child Dev. 2009 Jul-Aug;80(4):1000–1015. doi: 10.1111/j.1467-8624.2009.01313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guyer AE, Benson B, Choate VR, et al. Lasting associations between early-childhood temperament and late-adolescent reward-circuitry response to peer feedback. Dev Psychopathol. 2014 Feb;26(1):229–243. doi: 10.1017/S0954579413000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jarcho JM, Romer AL, Shechner T, et al. Predicting the worst and forgetting the best: unique neural circuits promote social anxiety in adolescents. under review. [Google Scholar]
- 52.Forbes CE, Grafman J. The role of the human prefrontal cortex in social cognition and moral judgment. Annual review of neuroscience. 2010;33:299–324. doi: 10.1146/annurev-neuro-060909-153230. [DOI] [PubMed] [Google Scholar]
- 53.Nelson EE, Leibenluft E, McClure EB, Pine DS. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychological medicine. 2005 Feb;35(2):163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- 54.Lieberman MD. Social cognitive neuroscience: a review of core processes. Annual review of psychology. 2007;58:259–289. doi: 10.1146/annurev.psych.58.110405.085654. [DOI] [PubMed] [Google Scholar]
- 55.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Advance Data. 2000 Jun 8;(314):1–27. [PubMed] [Google Scholar]
- 56.Fairburn CG, Cooper Z, editors. The Eating Disorder Examination. 12. New York: Guilford Press; 1993. [Google Scholar]; Fairburn CG, Wilson GT, editors. Binge eating, nature, assessment and treatment. [Google Scholar]
- 57.Glasofer DR, Tanofsky-Kraff M, Eddy KT, et al. Binge eating in overweight treatment-seeking adolescents. Journal of Pediatric Psychology. 2007 Jan-Feb;32(1):95–105. doi: 10.1093/jpepsy/jsl012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guyer AE, Caouette JD, Lee CC, Ruiz SK. Will they like me? Adolescents’ emotional responses to peer evaluation. Int J Behav Dev. 2014 Mar 1;38(2):155–163. doi: 10.1177/0165025413515627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guyer AE, Choate VR, Pine DS, Nelson EE. Neural circuitry underlying affective response to peer feedback in adolescence. Soc Cogn Affect Neurosci. 2012 Jan;7(1):81–92. doi: 10.1093/scan/nsr043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shomaker LB, Tanofsky-Kraff M, Zocca JM, et al. Eating in the absence of hunger in adolescents: intake after a large-array meal compared with that after a standardized meal. Am J Clin Nutr. 2010 Oct;92(4):697–703. doi: 10.3945/ajcn.2010.29812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Spielberger G, Lushene Vagg, Jacobs . Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologist Press; 1983. [Google Scholar]
- 62.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996 Jun;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 63.Cohen MS. Parametric analysis of fMRI data using linear systems methods. NeuroImage. 1997 Aug;6(2):93–103. doi: 10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]
- 64.Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann N Y Acad Sci. 2012 Mar;1251:E1–24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hardee JE, Benson BE, Bar-Haim Y, et al. Patterns of neural connectivity during an attention bias task moderate associations between early childhood temperament and internalizing symptoms in young adulthood. Biological psychiatry. 2013 Aug 15;74(4):273–279. doi: 10.1016/j.biopsych.2013.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Furman DJ, Hamilton JP, Gotlib IH. Frontostriatal functional connectivity in major depressive disorder. Biol Mood Anxiety Disord. 2011;1(1):11. doi: 10.1186/2045-5380-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Prater KE, Hosanagar A, Klumpp H, Angstadt M, Phan KL. Aberrant amygdala-frontal cortex connectivity during perception of fearful faces and at rest in generalized social anxiety disorder. Depress Anxiety. 2013 Mar;30(3):234–241. doi: 10.1002/da.22014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Frith CD, Frith U. Mechanisms of social cognition. Annu Rev Psychol. 2012;63:287–313. doi: 10.1146/annurev-psych-120710-100449. [DOI] [PubMed] [Google Scholar]
- 69.Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011 Feb;15(2):85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006 Apr;7(4):268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- 71.Flagan T, Beer JS. Three ways in which midline regions contribute to self-evaluation. Front Hum Neurosci. 2013;7:450. doi: 10.3389/fnhum.2013.00450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sebastian CL, Tan GC, Roiser JP, Viding E, Dumontheil I, Blakemore SJ. Developmental influences on the neural bases of responses to social rejection: implications of social neuroscience for education. Neuroimage. 2011 Aug 1;57(3):686–694. doi: 10.1016/j.neuroimage.2010.09.063. [DOI] [PubMed] [Google Scholar]
- 73.Moor BG, Guroglu B, Op de Macks ZA, Rombouts SA, Van der Molen MW, Crone EA. Social exclusion and punishment of excluders: neural correlates and developmental trajectories. Neuroimage. 2012 Jan 2;59(1):708–717. doi: 10.1016/j.neuroimage.2011.07.028. [DOI] [PubMed] [Google Scholar]
- 74.Bolling DZ, Pitskel NB, Deen B, et al. Dissociable brain mechanisms for processing social exclusion and rule violation. Neuroimage. 2011 Feb 1;54(3):2462–2471. doi: 10.1016/j.neuroimage.2010.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Somerville LH, Jones RM, Ruberry EJ, Dyke JP, Glover G, Casey BJ. The medial prefrontal cortex and the emergence of self-conscious emotion in adolescence. Psychol Sci. 2013 Aug;24(8):1554–1562. doi: 10.1177/0956797613475633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hughes BL, Beer JS. Protecting the self: the effect of social-evaluative threat on neural representations of self. J Cogn Neurosci. 2013 Apr;25(4):613–622. doi: 10.1162/jocn_a_00343. [DOI] [PubMed] [Google Scholar]
- 77.Sifneos PE. Alexithymia: past and present. American Journal of Psychiatry. 1996 Jul;153(7 Suppl):137–142. doi: 10.1176/ajp.153.7.137. [DOI] [PubMed] [Google Scholar]
- 78.Tanofsky-Kraff M, Goossens L, Eddy KT, et al. A multisite investigation of binge eating behaviors in children and adolescents. Journal of consulting and clinical psychology. 2007 Dec;75(6):901–913. doi: 10.1037/0022-006X.75.6.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wolkoff LE, Tanofsky-Kraff M, Shomaker LB, et al. Self-reported vs. actual energy intake in youth with and without loss of control eating. Eat Behav. 2011 Jan;12(1):15–20. doi: 10.1016/j.eatbeh.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sanislow CA, Pine DS, Quinn KJ, et al. Developing constructs for psychopathology research: research domain criteria. J Abnorm Psychol. 2010 Nov;119(4):631–639. doi: 10.1037/a0020909. [DOI] [PubMed] [Google Scholar]
- 81.Tanofsky-Kraff M, Engel S, Yanovski JA, Pine DS, Nelson EE. Pediatric disinhibited eating: toward a research domain criteria framework. Int J Eat Disord. 2013 Jul;46(5):451–455. doi: 10.1002/eat.22101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009 May 1;324(5927):646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- 83.Weygandt M, Mai K, Dommes E, et al. The role of neural impulse control mechanisms for dietary success in obesity. NeuroImage. 2013 Dec;83:669–678. doi: 10.1016/j.neuroimage.2013.07.028. [DOI] [PubMed] [Google Scholar]
- 84.Dorfel D, Lamke JP, Hummel F, Wagner U, Erk S, Walter H. Common and differential neural networks of emotion regulation by Detachment, Reinterpretation, Distraction, and Expressive Suppression: A comparative fMRI investigation. NeuroImage. 2014 Jun 30;101C:298–309. doi: 10.1016/j.neuroimage.2014.06.051. [DOI] [PubMed] [Google Scholar]
- 85.Frank DW, Dewitt M, Hudgens-Haney M, et al. Emotion regulation: Quantitative meta-analysis of functional activation and deactivation. Neuroscience and biobehavioral reviews. 2014 Jun 28;45C:202–211. doi: 10.1016/j.neubiorev.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 86.Otto B, Misra S, Prasad A, McRae K. Functional overlap of top-down emotion regulation and generation: An fMRI study identifying common neural substrates between cognitive reappraisal and cognitively generated emotions. Cognitive, affective & behavioral neuroscience. 2014 Sep;14(3):923–938. doi: 10.3758/s13415-013-0240-0. [DOI] [PubMed] [Google Scholar]
- 87.Spurrell EB, Wilfley DE, Tanofsky MB, Brownell KD. Age of onset for binge eating: are there different pathways to binge eating? The International journal of eating disorders. 1997 Jan;21(1):55–65. doi: 10.1002/(sici)1098-108x(199701)21:1<55::aid-eat7>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 88.Abbott DW, de Zwaan M, Mussell MP, et al. Onset of binge eating and dieting in overweight women: implications for etiology, associated features and treatment. Journal of psychosomatic research. 1998 Mar-Apr;44(3–4):367–374. doi: 10.1016/s0022-3999(97)00261-4. [DOI] [PubMed] [Google Scholar]
- 89.Schultz W. Multiple reward signals in the brain. Nat Rev Neurosci. 2000 Dec;1(3):199–207. doi: 10.1038/35044563. [DOI] [PubMed] [Google Scholar]
- 90.Metereau E, Dreher JC. Cerebral correlates of salient prediction error for different rewards and punishments. Cereb Cortex. 2013 Feb;23(2):477–487. doi: 10.1093/cercor/bhs037. [DOI] [PubMed] [Google Scholar]
- 91.Shohamy D. Learning and motivation in the human striatum. Current opinion in neurobiology. 2011 Jun;21(3):408–414. doi: 10.1016/j.conb.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 92.Yin HH, Mulcare SP, Hilario MR, et al. Dynamic reorganization of striatal circuits during the acquisition and consolidation of a skill. Nat Neurosci. 2009 Mar;12(3):333–341. doi: 10.1038/nn.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.O’Doherty JP. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Curr Opin Neurobiol. 2004 Dec;14(6):769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 94.Cavanagh JF, Frank MJ, Allen JJ. Social stress reactivity alters reward and punishment learning. Soc Cogn Affect Neurosci. 2011 Jun;6(3):311–320. doi: 10.1093/scan/nsq041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Balodis IM, Kober H, Worhunsky PD, et al. Monetary reward processing in obese individuals with and without binge eating disorder. Biological psychiatry. 2013;73(9):877–886. doi: 10.1016/j.biopsych.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Celone KA, Thompson-Brenner H, Ross RS, Pratt EM, Stern CE. An fMRI investigation of the fronto-striatal learning system in women who exhibit eating disorder behaviors. NeuroImage. 2011;56(3):1749–1757. doi: 10.1016/j.neuroimage.2011.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Frank GK, Reynolds JR, Shott ME, O’Reilly RC. Altered temporal difference learning in bulimia nervosa. Biological psychiatry. 2011;70(8):728–735. doi: 10.1016/j.biopsych.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci. 1997 Jun 1;17(11):4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Haxby JV, Gobbini MI, Furey ML, Ishai A, Schouten JL, Pietrini P. Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science. 2001 Sep 28;293(5539):2425–2430. doi: 10.1126/science.1063736. [DOI] [PubMed] [Google Scholar]
- 100.Miyahara M, Harada T, Ruffman T, Sadato N, Iidaka T. Functional connectivity between amygdala and facial regions involved in recognition of facial threat. Soc Cogn Affect Neurosci. 2013 Feb;8(2):181–189. doi: 10.1093/scan/nsr085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Furl N, Henson RN, Friston KJ, Calder AJ. Top-down control of visual responses to fear by the amygdala. J Neurosci. 2013 Oct 30;33(44):17435–17443. doi: 10.1523/JNEUROSCI.2992-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Siep N, Roefs A, Roebroeck A, Havermans R, Bonte ML, Jansen A. Hunger is the best spice: an fMRI study of the effects of attention, hunger and calorie content on food reward processing in the amygdala and orbitofrontal cortex. Behav Brain Res. 2009 Mar 2;198(1):149–158. doi: 10.1016/j.bbr.2008.10.035. [DOI] [PubMed] [Google Scholar]
- 103.Kroemer NB, Krebs L, Kobiella A, et al. Fasting levels of ghrelin covary with the brain response to food pictures. Addict Biol. 2013 Sep;18(5):855–862. doi: 10.1111/j.1369-1600.2012.00489.x. [DOI] [PubMed] [Google Scholar]
- 104.Kroemer NB, Krebs L, Kobiella A, et al. (Still) longing for food: insulin reactivity modulates response to food pictures. Hum Brain Mapp. 2013 Oct;34(10):2367–2380. doi: 10.1002/hbm.22071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Stice E, Burger K, Yokum S. Caloric deprivation increases responsivity of attention and reward brain regions to intake, anticipated intake, and images of palatable foods. Neuroimage. 2013 Feb 15;67:322–330. doi: 10.1016/j.neuroimage.2012.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ashworth F, Pringle A, Norbury R, Harmer C, Cowen P, Cooper M. Neural response to angry and disgusted facial expressions in bulimia nervosa. Psychological medicine. 2011;41(11):2375–2384. doi: 10.1017/S0033291711000626. [DOI] [PubMed] [Google Scholar]
- 107.Miyake Y, Okamoto Y, Onoda K, et al. Neural processing of negative word stimuli concerning body image in patients with eating disorders: an fMRI study. NeuroImage. 2010;50(3):1333–1339. doi: 10.1016/j.neuroimage.2009.12.095. [DOI] [PubMed] [Google Scholar]
- 108.Pringle A, Ashworth F, Harmer C, Norbury R, Cooper M. Neural correlates of the processing of self-referent emotional information in bulimia nervosa. Neuropsychologia. 2011;49(12):3272–3278. doi: 10.1016/j.neuropsychologia.2011.07.032. [DOI] [PubMed] [Google Scholar]
- 109.Berger SS, Elliott C, Ranzenhofer LM, et al. Interpersonal problem areas and alexithymia in adolescent girls with loss of control eating. Comprehensive psychiatry. 2014;55(1):170–178. doi: 10.1016/j.comppsych.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Libbey HP, Story MT, Neumark‚ÄêSztainer DR, Boutelle KN. Teasing, disordered eating behaviors, and psychological morbidities among overweight adolescents. Obesity. 2008;16(S2):S24–S29. doi: 10.1038/oby.2008.455. [DOI] [PubMed] [Google Scholar]
- 111.Neumark-Sztainer DR, Wall MM, Haines JI, Story MT, Sherwood NE, van den Berg PA. Shared risk and protective factors for overweight and disordered eating in adolescents. American journal of preventive medicine. 2007;33(5):359–369. e353. doi: 10.1016/j.amepre.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 112.Hayden-Wade HA, Stein RI, Ghaderi A, Saelens BE, Zabinski MF, Wilfley DE. Prevalence, characteristics, and correlates of teasing experiences among overweight children vs. non-overweight peers. Obes Res. 2005 Aug;13(8):1381–1392. doi: 10.1038/oby.2005.167. [DOI] [PubMed] [Google Scholar]
- 113.Berkman ET, Falk EB. Beyond Brain Mapping: Using Neural Measures to Predict Real-World Outcomes. Curr Dir Psychol Sci. 2013 Feb;22(1):45–50. doi: 10.1177/0963721412469394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.McClure EB, Adler A, Monk CS, et al. fMRI predictors of treatment outcome in pediatric anxiety disorders. Psychopharmacology (Berl) 2007 Mar;191(1):97–105. doi: 10.1007/s00213-006-0542-9. [DOI] [PubMed] [Google Scholar]
- 115.Pine DS. Research review: a neuroscience framework for pediatric anxiety disorders. J Child Psychol Psychiatry. 2007 Jul;48(7):631–648. doi: 10.1111/j.1469-7610.2007.01751.x. [DOI] [PubMed] [Google Scholar]
- 116.Beesdo K, Knappe S, Pine DS. Anxiety and anxiety disorders in children and adolescents: developmental issues and implications for DSM-V. The Psychiatric clinics of North America. 2009 Sep;32(3):483–524. doi: 10.1016/j.psc.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pine DS, Cohen P, Gurley D, Brook J, Ma Y. The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Archives of general psychiatry. 1998 Jan;55(1):56–64. doi: 10.1001/archpsyc.55.1.56. [DOI] [PubMed] [Google Scholar]
- 118.Hilbert A, Brauhardt A. Childhood loss of control eating over five-year follow-up. The International journal of eating disorders. 2014 Jun 5; doi: 10.1002/eat.22312. [DOI] [PubMed] [Google Scholar]
- 119.Bress JN, Foti D, Kotov R, Klein DN, Hajcak G. Blunted neural response to rewards prospectively predicts depression in adolescent girls. Psychophysiology. 2013 Jan;50(1):74–81. doi: 10.1111/j.1469-8986.2012.01485.x. [DOI] [PubMed] [Google Scholar]
- 120.Matthews PM, Honey GD, Bullmore ET. Applications of fMRI in translational medicine and clinical practice. Nat Rev Neurosci. 2006 Sep;7(9):732–744. doi: 10.1038/nrn1929. [DOI] [PubMed] [Google Scholar]
- 121.Cavanagh JF, Allen JJ. Multiple aspects of the stress response under social evaluative threat: an electrophysiological investigation. Psychoneuroendocrinology. 2008 Jan;33(1):41–53. doi: 10.1016/j.psyneuen.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 122.Ely AV, Childress AR, Jagannathan K, Lowe MR. Differential reward response to palatable food cues in past and current dieters: a fMRI study. Obesity (Silver Spring) 2014 May;22(5):E38–45. doi: 10.1002/oby.20599. [DOI] [PubMed] [Google Scholar]