Abstract
Background
The reduction of the nicotine content of cigarettes to non-addicting levels is a potential federal regulatory intervention to reduce the prevalence of cigarette smoking and related disease. Many clinical trials on the effects and safety of nicotine reduction are ongoing. An important methodological concern is non-compliance with reduced nicotine content cigarettes in the context of freely available conventional cigarettes. We propose two approaches using biomarkers to estimate non-compliance in smokers of very low nicotine content (VLNC) cigarettes in a clinical trial.
Methods
Data from 50 subjects in a study of gradual nicotine reduction were analyzed. Using plasma cotinine concentrations measured at baseline and while smoking VLNC cigarettes, we compared within-subject ratios of plasma cotinine comparing usual brand to VLNC in relation to nicotine content of these cigarettes. In another approach we used nicotine pharmacokinetic data to estimate absolute plasma cotinine/cigarettes per day (CPD) threshold values for compliance based on the nicotine content of VLNC.
Results
The two approaches showed concordance indicating at least 60% non-compliance with smoking VLNC. In a sensitivity analysis assuming extreme compensation and extreme values for nicotine metabolic parameters, non-compliance was still at least 40%, much higher than self-reported non-compliance.
Conclusion
Biomarker analysis demonstrates a high degree of non-compliance with smoking VLNC cigarettes, indicating that smokers are supplementing these with conventional cigarettes.
Impact
We propose a practical approach to assessing compliance with smoking VLNC in clinical trials of nicotine reduction.
Keywords: Cigarette smoking, reduced nicotine content cigarettes, nicotine reduction, cotinine, biomarkers
Introduction
Reducing or eliminating cigarette smoking and the use of other forms of combustible tobacco would have an enormous effect in reducing tobacco-related mortality and morbidity. (1) One approach to decreasing smoking prevalence would be to reduce the nicotine content in cigarettes to non-addicting levels on a nationwide scale. (2) This would potentially reduce the level of addiction, prevent adolescents from becoming addicted adult smokers, and promote smoking cessation. Nicotine reduction has been proposed as a national tobacco regulatory intervention and has been discussed as a potential “tobacco end game strategy”. (3–5) The FDA has the authority to reduce the nicotine content of cigarettes (so long as it is not reduced to zero) as granted by the 2009 Family Smoking Prevention and Tobacco Control Act. (6) It should be noted that reduced nicotine content cigarettes are not the same as “low tar and nicotine” cigarettes, The latter are engineered to be low yield by machine testing, but in fact deliver as much tar and nicotine as higher yield cigarettes.
Several clinical trials have been published and others are underway to examine smokers’ responses to switching to reduced nicotine content cigarettes (RNCs), particularly with respect to subjective effects and potential compensatory smoking. (7–10) These clinical trials recruited volunteer smokers, not interested in quitting, who agreed to smoke RNCs provided through the study, but this is in the context of readily available higher nicotine content cigarettes on the market. In such studies, assessing the subjects’ compliance with smoking only RNCs is essential to quantifying the effects of the relationship between RNCs and outcomes of interest. The impact of non-compliance on study results will need to be considered by the FDA in evaluating the potential impact of regulation of nicotine content of cigarettes. Some subjects admit to smoking regular tobacco cigarettes along with the RNCs, but others do not. Thus far, the assessment of compliance has been limited to self-report, which may not be accurate due to a variety of factors. The aim of this paper is to describe an approach for estimating biochemically (using measurements of plasma cotinine) whether smokers are being compliant with smoking very low nicotine content research cigarettes.
Materials and Methods
We evaluated compliance to smoking very low nicotine content (VLNC) cigarettes in two ways. One was an analysis of changes in cotinine levels within subjects in comparison to expected changes based on changes in nicotine content of cigarettes. The second was a theoretical estimation of plasma cotinine concentrations from VLNC based on known pharmacokinetics of nicotine and cotinine.
Data from a clinical trial of smokers switching from conventional cigarettes to RNCs were used for this analysis. The methods and results of the trial have been published previously. (7) In brief, healthy subjects were randomized to a progressive reduction of nicotine content of cigarettes over six months or to a control group who continued to smoke their usual brand cigarettes. Those in the RNC group smoked their usual brand followed by research cigarettes containing 10, 6, 4, 2 and 0.5 mg nicotine, smoked for one month each. Our analysis focuses on the 50 subjects in the RNC group who completed the six month tapering phase of the study and were still smoking. Three subjects of the 53 who completed this phase of the study were excluded because they reported not smoking. The VLNC cigarette (0.5 mg nicotine content) condition was the focus of this analysis. These subjects provided cigarette consumption and biomarker data at baseline, while smoking their usual brand of cigarettes, and at six months, when smoking VLNCs.
Results
Empirical analysis of noncompliance
Cotinine is the major proximate metabolite of nicotine and is widely used as a biomarker of nicotine intake from tobacco. (11) On average 80% of nicotine is converted to cotinine, but there is considerable individual variability in the percent conversion. When cotinine is compared within subjects, inter-individual variability in metabolism is not an issue, thus changes in cotinine levels over time accurately reflect an individual’s change in nicotine intake.
Typical conventional tobacco cigarettes contain 10–15 mg nicotine per cigarette rod. On average the systemic intake of nicotine is about 1 mg, but because of individual differences in intensity of smoking some smokers take in smaller amounts of nicotine and others as much as 3 mg per cigarette. (12) Thus, the absolute systemic bioavailability for nicotine from a cigarette is typically about 10% but can be as high as 30% or possibly more with high intensity smoking.
To assess compliance we examined within-subject changes in plasma cotinine levels normalized for cigarette consumption, comparing baseline [plasma cotinine/CPD] with the [plasma cotinine/CPD] after one month of smoking VLNCs.
Based on the decrease of nicotine content from 10 mg (assumed content of the usual brand) to 0.5 mg (VLNC cigarette), the predicted ratio of plasma cotinine/CPD comparing the VLNC vs baseline conditions with no compensation would be 0.5 mg/10 mg =0.05. Conservatively allowing for a 4-fold increase in bioavailability from 10 to 40% due to extreme compensation, we estimate an upper limit ratio of 0.2. Thus any smoker with a ratio of [plasma cotinine/CPD VLNC]/[plasma cotinine/CPD baseline] ratio exceeding 0.2 would indicate non-compliance.
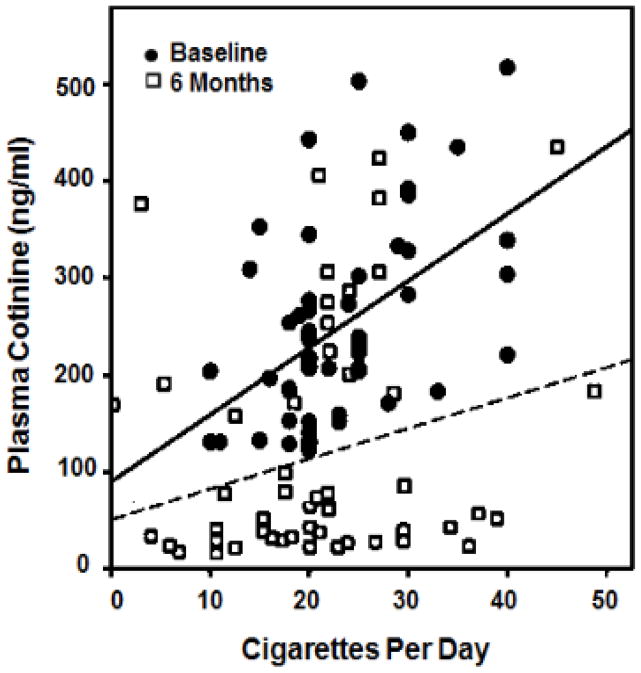
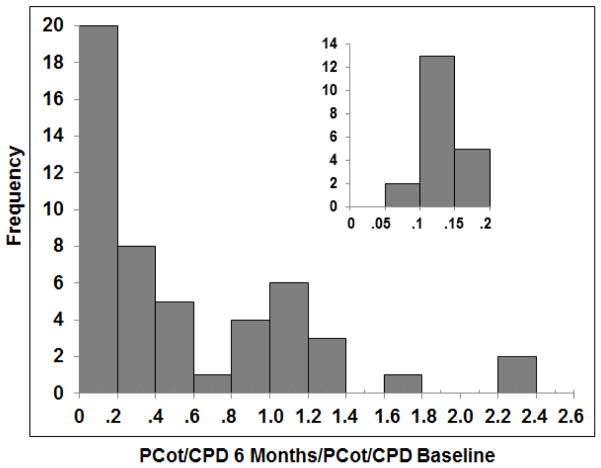
Figure 1 shows plasma cotinine concentrations vs. CPD for subjects at baseline and when smoking VLNC cigarettes. A substantial number of subjects while smoking VLNC had plasma cotinine levels similar to those seen smoking their usual brand conventional cigarette. Figure 2 shows the frequency distribution of ratios of plasma cotinine/CPD ratios in the VLNC and baseline conditions. 60% (30/50) or more of the subjects were non-compliant based on the within-subject plasma [cotinine/CPD RNC]/[plasma cotinine baseline] ratios of greater than 0.2. All subjects who reported non-compliance with research cigarettes were found to be non-compliant based on the cotinine/CPD ratio. Supplemental table 1 shows the ratio data for individual subjects.
Figure 1.
Observed plasma cotinine concentration vs cigarettes smoked per day in 50 subjects while smoking usual brand (circles) and VLNC cigarettes (boxes). Linear regression lines shown for different cigarette types (solid line = usual brand; dashed line = VLNC cigarettes). Data derived from Benowitz et al 2012 (14).
Figure 2.
Frequency distribution of within-subject ratios of the plasma cotinine/CPD ratio while smoking VLNC and plasma cotinine/CPD while smoking usual brand. A ratio of ratios greater than 0.2 suggests noncompliance with VLNC only smoking condition. The insert provides further details on those subjects with ratios ≤ 0.2. Data derived from Benowitz et al 2012. (14)
Theoretical Estimation of Cotinine Levels for Non-compliance
Nicotine pharmacokinetic data were used to compute a theoretical threshold ratio of cotinine/CPD for use in situations in which no baseline plasma cotinine data are available for comparison.
The relationship between plasma cotinine at steady state and the daily intake of nicotine depends on the percent conversion of nicotine to cotinine and on the total systemic clearance of cotinine, as expressed in the following equation:
| (13) | Equation (1) |
Dnic is the daily systemic dose of nicotine, PCot is plasma cotinine at steady state, fnic-cot is the fraction of nicotine that is converted to cotinine, and CLcot is the total systemic clearance of cotinine.
The daily dose of nicotine can also be determined in the following equation:
| Equation (2) |
where A is the nicotine content of the cigarette, F is the absolute bioavailability of nicotine and CPD is number of cigarettes smoked per day.
The ratio of fnic-cot/CLcot is a constant for an individual, which we call K.
| Equation (3) |
K is the factor that relates plasma cotinine level to daily systemic intake of nicotine:
| Equation (4) |
Rearranging the equation,
| Equation (5) |
Thus, the steady state plasma cotinine concentration per cigarette smoked each day is determined by the nicotine content of the cigarette, the absolute bioavailability of nicotine from smoking each cigarette, and K (which is a metabolic characteristic of the individual smoker). It should be noted that the use of urine total nicotine equivalents instead of cotinine would avoid the need to consider individual differences in nicotine and cotinine metabolism; however the total urine nicotine equivalents assay is much more complicated and costly, and validation data in relation to daily nicotine intake are not available for total nicotine equivalents.
On average, K is 0.083, with a coefficient of variation of 21% and range of 0.047 to 0.102. (13) For a smoker who takes in 1 mg per cigarette per day and has a typical 10% bioavailability, with K= 0.083 each cigarette results in a steady state plasma cotinine concentration of 12.0 ng/ml (range 9.8 to 21.2 ng/ml considering variability in K). Assume that a smoker has switched to a VLNC cigarette containing 0.5 mg nicotine. Without any change in bioavailability (i.e. assuming bioavailability is 10%, indicating no compensation), the steady state plasma cotinine generated per cigarette smoked per day would be (0.5 mg) (0.1)/0.083= 0.60 ng/ml per cigarette. Assuming extreme compensation of 40%, the maximum plasma cotinine per cigarette would be 2.4 ng/ml. As a sensitivity analysis, considering an extremely low value for K (K = 0.047), the maximal plasma cotinine could range up to 4.2 ng/ml per cigarette. Thus if a person smoked twenty cigarettes per day with 0.5 mg nicotine content per cigarette and 40% bioavailability, the maximum steady state plasma cotinine would be 48 ng/ml. For a person with both extreme compensation and an extremely low K value, plasma cotinine could range up to 85 ng/ml.
Using the data set described previously, we determined the number of subjects in the VLNC condition who exceeded the theoretical maximum of 2.4 ng/ml cotinine per cigarettes per day. As a sensitivity analysis for individual variation in K, we did the same analysis with a cut point of 4.2 ng/ml per cigarette, reflecting extreme compensatory smoking.
Figure 3 shows a frequency distribution of plasma cotinine/CPD at both baseline and 6 months. Based on a cotinine/CPD ratio of greater than 2.4 ng/ml, 62% of subjects were noncompliant. Based on an extremely conservative cut point of 4.2 ng/ml/CPD, 42 % were noncompliant. All subjects who reported non-compliance were found to be non-compliant by the absolute cotinine/CPD ratio criterion. Ratio data for individual subjects are shown in supplemental table 1.
Figure 3.
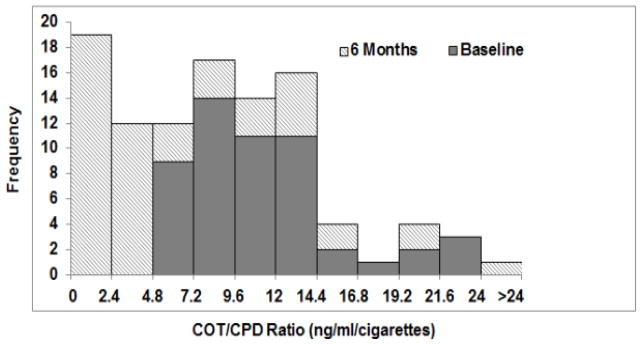
Frequency distribution of ratios of plasma cotinine/CPD while smoking usual brand (solid portion of the bar) and VLNC cigarettes (striped portion of the bars) while smoking usual cigarettes (baseline) and VLNC (6 months). A ratio value of greater than 2.4 ng/ml/cigarette suggests non-compliance with VLNC cigarettes. Data derived from Benowitz et al 2012. (14)
Concordance of methods for estimating non-compliance
There was a high degree of concordance between the two methods of estimating non-compliance. The correlation between the within subject ratio of [plasma cotinine/CPD VLNC]/[plasma cotinine/CPD baseline] and the absolute value of cotinine/CPD at 6 months was strong (r = 0.86, P < 0.001). Of the subjects determined to be non-compliant using the empirical analysis, 27/30 (90%) and 21/30 (70%) were also found to be non-compliant using an absolute cotinine/CPD values of 2.4 and 4.2 ng/ml/cigarette, respectively. Of the subjects determined to be non-compliant by the theoretical analysis using a threshold value of 2.4 or 4.2 ng/ml/cigarette, 87% and 100%, respectively, were determined to be non-compliant by the empirical analysis. Supplemental table 1 provides individual data and classifications as compliant or non-compliant by the various methods.
Discussion
To provide a science base for nicotine regulation it is important for clinical trial research to determine the effects of smoking RNCs. Essential to this determination is assessment of subject compliance with smoking only RNCs. This is especially challenging given the easy availability of conventional cigarettes on the market.
We present two approaches to using a biochemical measure (i.e. plasma cotinine) to estimate non-compliance of smoking VLNC cigarettes in a clinical trial. One approach is based within-subject comparisons of the ratio of plasma cotinine concentrations comparing the usual brand to VLNC, based on relative nicotine contents of the two cigarettes. The other approach uses absolute plasma cotinine/CPD values based on the nicotine content of VLNC cigarettes and the known pharmacokinetics of nicotine and cotinine. These measures showed concordance with an estimate of approximately 60% of subjects showing a high likelihood of non-compliance. Concordance between the two methods was approximately 90%. In a sensitivity analysis using extreme individual variability in metabolic factors, non-compliance is still 40%. These estimates contrast with the 21% self-reported non-compliance of subjects over the 6 months of the study. (14) Of note, in the latter study subjects were encouraged to report non-compliance with research cigarettes without penalty.
Because on average the absolute cotinine levels were 70% lower while smoking VLNC compared to baseline, our data suggest that most subjects were primarily smoking VLNC’s but were supplementing these cigarettes with some conventional cigarettes. The motivation for non-compliance is unclear, but may have to do with subjects’ desire to maintain some minimal daily intake of nicotine in order to avoid nicotine withdrawal symptoms, or perhaps to be able to smoke particularly rewarding cigarettes such as first thing in the morning or after a meal. It is worth noting that the impact of smoking a single conventional cigarette per day generating a cotinine level of 12.5 ng/ml will have a large impact on cotinine levels expected from a person smoking a VLNC cigarettes that are expected to result in 0.6 mg cotinine per cigarette. It is also possible that some subjects used nicotine medications or non-combustible forms of tobacco to deal with nicotine withdrawal symptoms without reported its use. Of note, non-compliance with low nicotine content cigarettes was reported by Finnegan et al in one the earliest studies to examine the effects of substituting such cigarettes for regular cigarettes. (15).
Our assumptions in generating these compliance estimations were conservative. We assumed that regular tobacco cigarettes contained 10 mg of nicotine, while many contain more than that. We also estimated a maximum bioavailability of 40% (a 4-fold increase), which is likely to be high. In a prior study in which we restricted cigarettes smoked per day we found that nicotine per cigarette increased by an average of 2.7-fold. (16) Thus some of the subjects who do not meet our criterion for non-compliance were nonetheless likely to have been non-compliant.
It is important to note that our analysis of clinical data is restricted to the number of subjects who remained in the trial up to the sixth month period. Some subjects in the RNC group dropped out of the trial before completing the taper, and most of whom reported disliking smoking the RNCs. (7) Had these subjects remained in the trial, they may have exhibited even greater degrees of non-compliance. Another limitation is that our analysis is most useful for estimating non-compliance with smoking VLNCs, where large differences in nicotine intake per cigarette compared to conventional cigarettes are expected. With only modest reductions in nicotine content and modest compensation there could be a large overlap in cotinine levels between conventional and RNC cigarette smokers, such that assessing noncompliance biochemically would be impossible.
Our analyses were based on plasma cotinine measurements. For the empirical analysis, comparing cotinine levels in two conditions within-subjects, either saliva or urine ratios could be used in exactly the same way as plasma levels. For the theoretical estimation of absolute cotinine per cigarette, the cotinine cut points can be multiplied by 1.2 and 4.5 when using saliva or urine, respectively. (17, 18) We propose our analysis as a tool for clinical researchers to use in assessing responses to switching from conventional cigarettes to VLNCs. By using this biochemical approach, assessing compliance is no longer limited only to self-report, which may be invalid due to self-presentation strategies as a research participant. Assessing smokers’ responses to switching to RNCs should include separate analyses for compliant and non-compliant smokers, and our paper proposes an approach to make that distinction.
Supplementary Material
Acknowledgments
Financial Support:
National Cancer Institute grants R01 CA78603 (NB), National Institute on Drug Abuse grant R01 DA12393 (NB) and National Institute on Drug Abuse and FDA Center for Tobacco Products grant U54 DA031659 (NB. NN, DH, ED).
We thank Drs. Katherine M. Dains, Sharon M. Hall, Susan Stewart, Delia Dempsey, Isabel Allen, and Peyton Jacob III and Ms. Margaret Wilson for their contributions to the design and conduct of the study, analysis of data and/or chemical analysis of biomarkers of exposure, and Scott Rostler for editorial assistance.
Footnotes
Conflict of interest:
Dr. Benowitz is a consultant to several pharmaceutical companies that market medications to aid smoking cessation and has served as a paid expert witness in litigation against tobacco companies. The other authors have no conflicts to declare.
References
- 1.HHS. A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. The Health Consequences of Smoking-50 Years of Progress. [PubMed] [Google Scholar]
- 2.Benowitz NL, Henningfield JE. Establishing a nicotine threshold for addiction. The implications for tobacco regulation. N Engl J Med. 1994;331(2):123–5. doi: 10.1056/NEJM199407143310212. [DOI] [PubMed] [Google Scholar]
- 3.Benowitz NL, Henningfield JE. Reducing the nicotine content to make cigarettes less addictive. Tobacco Control. 2013;22(suppl 1):i14–i7. doi: 10.1136/tobaccocontrol-2012-050860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henningfield JE, Benowitz NL, Slade J, Houston TP, Davis RM, Deitchman SD. Reducing the addictiveness of cigarettes. Council on Scientific Affairs, American Medical Association. Tob Control. 1998;7(3):281–93. doi: 10.1136/tc.7.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Warner KE. An endgame for tobacco? Tob Control. 2013;22(Suppl 1):i3–5. doi: 10.1136/tobaccocontrol-2013-050989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Husten CG, Deyton LR. Understanding the Tobacco Control Act: efforts by the US Food and Drug Administration to make tobacco-related morbidity and mortality part of the USA’s past, not its future. Lancet. 2013;381(9877):1570–80. doi: 10.1016/S0140-6736(13)60735-7. [DOI] [PubMed] [Google Scholar]
- 7.Benowitz NL, Dains KM, Hall SM, Stewart S, Peng M, Dempsey D, et al. Smoking Behavior and Exposure to Tobacco Toxicants During 6 months of Smoking Progressively Reduced Nicotine Content Cigarettes. Cancer Epidemiol Biomarkers Prev. 2012 doi: 10.1158/1055-9965.EPI-11-0644. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benowitz NL, Hall SM, Stewart S, Wilson M, Dempsey D, Jacob P., III Nicotine and Carcinogen Exposure with Smoking of Progressively Reduced Nicotine Content Cigarette. Cancer Epidemiol Biomarkers Prev. 2007 doi: 10.1158/1055-9965.EPI-07-0393. In Press. [DOI] [PubMed] [Google Scholar]
- 9.Hatsukami DK, Hertsgaard LA, Vogel RI, Jensen JA, Murphy SE, Hecht SS, et al. Reduced nicotine content cigarettes and nicotine patch. Cancer Epidemiol Biomarkers Prev. 2013;22(6):1015–24. doi: 10.1158/1055-9965.EPI-12-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hatsukami DK, Kotlyar M, Hertsgaard LA, Zhang Y, Carmella SG, Jensen JA, et al. Reduced nicotine content cigarettes: effects on toxicant exposure, dependence and cessation. Addiction. 2010;105(2):343–55. doi: 10.1111/j.1360-0443.2009.02780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hukkanen J, Jacob P, 3rd, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57(1):79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- 12.Benowitz NL, Jacob P, 3rd, Denaro C, Jenkins R. Stable isotope studies of nicotine kinetics and bioavailability. Clin Pharmacol Ther. 1991;49(3):270–7. doi: 10.1038/clpt.1991.28. [DOI] [PubMed] [Google Scholar]
- 13.Benowitz NL, Jacob P., 3rd Metabolism of nicotine to cotinine studied by a dual stable isotope method. Clin Pharmacol Ther. 1994;56(5):483–93. doi: 10.1038/clpt.1994.169. [DOI] [PubMed] [Google Scholar]
- 14.Benowitz NL, Dains KM, Hall SM, Stewart S, Wilson M, Dempsey D, et al. Smoking behavior and exposure to tobacco toxicants during 6 months of smoking progressively reduced nicotine content cigarettes. Cancer Epidemiol Biomarkers Prev. 2012;21(5):761–9. doi: 10.1158/1055-9965.EPI-11-0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finnegan JK, Larson PS, Haag HB. THE ROLE OF NICOTINE IN THE CIGARETTE HABIT. Science. 1945;102(2639):94–6. doi: 10.1126/science.102.2639.94. [DOI] [PubMed] [Google Scholar]
- 16.Benowitz NL, Jacob P, 3rd, Kozlowski LT, Yu L. Influence of smoking fewer cigarettes on exposure to tar, nicotine, and carbon monoxide. N Engl J Med. 1986;315(21):1310–3. doi: 10.1056/NEJM198611203152102. [DOI] [PubMed] [Google Scholar]
- 17.Bernert JT, Jr, McGuffey JE, Morrison MA, Pirkle JL. Comparison of serum and salivary cotinine measurements by a sensitive high-performance liquid chromatography-tandem mass spectrometry method as an indicator of exposure to tobacco smoke among smokers and nonsmokers. J Anal Toxicol. 2000;24(5):333–9. doi: 10.1093/jat/24.5.333. [DOI] [PubMed] [Google Scholar]
- 18.Benowitz NL, Dains KM, Dempsey D, Herrera B, Yu L, Jacob P., 3rd Urine nicotine metabolite concentrations in relation to plasma cotinine during low-level nicotine exposure. Nicotine Tob Res. 2009;11(8):954–60. doi: 10.1093/ntr/ntp092. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.