Abstract
Three human cases of H10N8 viruses were reported in China in late 2013 and early 2014, two of which were fatal. This was the first time the H10N8 subtype has been detected in humans and no vaccine candidates or antibody therapy has been developed for these viruses so far. We developed an H10N8 vaccine candidate virus based on A/Jiangxi-Donghu/346/13 that can also be used in a murine challenge model for vaccine and monoclonal antibody research. The vaccine virus is a 6:2 re-assortant virus expressing the surface glycoproteins of A/Jiangxi-Donghu/346/13 on an A/Puerto Rico/8/34 backbone. Vaccination with inactivated challenge virus or recombinant hemagglutinin or neuraminidase derived from this strain protected mice from viral challenge.
Introduction
During the 2013/2014 winter season China reported three human infections with H10N8 virus in the Jiangxi province, two of which were fatal [1–4]. Subsequent studies showed the presence of H10N8 in poultry and live bird markets in this province [3, 5, 6]. Serological evidence for the virus was also found in poultry workers in the neighboring Guangdong province and in feral dogs that frequent live bird markets in the same province [3, 7]. Although it is likely that these cases are isolated episodes, it is possible that transmission events will re-occur in future winter seasons as with H7N9 infections. Regular transmission of the virus from avian species to humans increases the risk of adaptive mutations and/or re-assortment events with human influenza A strains which could result in a strain with high pandemic potential. It is therefore warranted to design, test and optimize pre-pandemic H10N8 vaccines and therapeutic antibodies. To facilitate this process, we generated an H10N8 vaccine strain based on a re-assorted virus that possesses the hemagglutinin (HA) and neuraminidase (NA) genomic segments from the human isolate A/Jiangxi-Donghu/346/13 (JD13) and the safe A/Puerto Rico/8/34 (PR8) backbone [8–11]. This virus can also be used in challenge experiments necessary for evaluation of vaccines and therapeutics. We assessed the growth properties of the generated virus in embryonated eggs and in Madin-Darby canine kidney (MDCK) cells and tested vaccine efficacy of an inactivated whole virus preparation and recombinant H10 HA and N8 NA protein vaccines against a lethal H10N8 challenge.
Materials and Methods
The re-assortant H10N8 virus was rescued using the 6 internal genomic segments of PR8 in combination with HA and NA segments from A/Jiangxi-Donghu/346/13 (JD13, synthesized by Genewiz Inc., NJ) as described before [12, 13]. The H10N8 6:2 re-assortant virus and PR8 were grown in 10 day old embryonated chicken eggs. Growth curves were generated in embryonated chicken eggs (initial inoculum 100 PFU) or on MDCK cells (MOI of 0.001). Virus in allantoic fluid or culture supernatant was quantified via plaque assay as described before [13]. HA assays were performed using chicken or turkey red blood cells as described before [12, 13]. The ectodomains of the H10 HA (rH10) and N8 NA (rN8) were expressed and purified as described previously [14]. ELISA, ELLA and HI assays were performed using established protocols [12, 13].
For determining the murine lethal dose 50 (mLD50) 6–8 week old BALB/c mice (4 per group) were intranasally infected with 5×100, 5×101, 5×102, 5×103, 5×104 or 5×105 plaque forming units (PFU) of recombinant H10N8 virus (under anesthesia, 0.15 mg/kg ketamine and 0.03 mg/kg xylazine intraperitoneally; virus was diluted in 50 ul of PBS). Weight was recorded daily and animals that lost more than 20% of their initial body weight were scored dead and euthanized according to institutional guidelines. For the vaccine studies, animals (4–5 per group) were vaccinated intramuscularly with inactivated H10N8 virus (1 ug/mouse) or recombinant H10 protein, N8 protein or bovine serum albumin (BSA) as negative control (5 ug/mouse, adjuvanted with 5 ug polyI:C per dose). Three weeks later, post prime animals received a boost (same formulations, same amounts) and were then challenged 4 weeks post boost intranasally under anesthesia with 10 LD50 of H10N8 virus in 50 ul PBS. All animal experiments were performed in accordance with the Icahn School of Medicine at Mount Sinai Institutional Animal Care and Use Committee.
Results
Rescue and characterization of the JD13 H10N8 re-assortant virus
The JD13 H10N8 re-assortant virus was successfully rescued and plaque purified virus was grown up in embryonated eggs resulting in a virus stock with a titer of 1.7×107PFU/ml. Virus identity was confirmed by Sanger sequencing and plaque staining with a monoclonal antibody (mAb) that recognizes PR8 H1 (mAb PY102, in house produced) or H10 (mAb 9H10 [15], in house produced) HA. Plaque sizes of the JD13 virus were considerably smaller than that of a PR8 (Figure 1). To better characterize the growth properties of the virus, we assessed its growth kinetics in MDCK cells and embryonated eggs. In eggs, JD13 reached peak titers in the range of 107 PFU/ml at 48 hours post infection, 2 logs lower than that of PR8. An experiment with a low multiplicity of infection (MOI) in MDCK cells showed similar results with JD13 titers in the 106 PFU/ml range at 72 hours post infection, approximately 1.5 logs lower than PR8 (Figure 1). This was also reflected by HA titers measured at 72 hours post infection in allantoic fluid or MDCK supernatants, which reached 256 and 64 hemagglutination units (HAU) respectively when turkey red blood cells were used (Figure 1). Titers with chicken red blood cells were slightly lower with a mean of 171 HAU for allantoic fluid 72 hours post infection. Next, we wanted to assess mLD50 of JD13 in the mouse model. For this we used BALB/c mice, a popular Mx−/− mouse breed used for influenza virus vaccine studies. Mice were intranasally infected with escalating doses of virus. Groups infected with 5 or 50 PFU did not show significant weight loss or any other signs of disease. Mice infected with 500 PFU showed moderate transient weight loss (maximum 14% on day 7 post infection) but regained weight to near-initial levels by the end of the 14 day observation period (Figure 2). Mice that received higher doses - 5×103, 5×104 and 5×105 PFU - lost more than 20% of their initial weight, showed clinical signs of infection (ruffled fur, lethargy, shivering) and were euthanized according to institutional guidelines. Based on these results, we calculated an mLD50 of 1.6×103 PFU for JD13 virus which was not mouse-adapted in vitro or in vivo after rescue.
Figure 1. Growth characteristics of the A/Jiangxi-Donghu/346/13 6:2 re-assortant (JD13 H10N8) as compared to A/PR/8/34 (PR8, H1N1).
Growth of both viruses in A embryonated eggs (initial inoculum 100 PFU) and B MDCK cells (MOI 0.001). HA units in allantoic fluid C or embryonated eggs D over time as measured with turkey red blood cells. E Chicken red blood cells gave a lower readout of the same allantoic fluid. Plaque size of JD13 H10N8 and PR8 virus are shown in F and G respectively. The plaque phenotype of JD13 H10N8 is considerably smaller than that of PR8. Plaques were immunostained with mAb PY102 (PR8) or mAbs 9H10 (JD13) [15]. Data in A–E is shown as mean with standard deviation.
Figure 2. mLD50 determination of the A/Jiangxi-Donghu/346/13 6:2 re-assortant in BALB/c mice.
A Weight loss curves after intranasal inoculation with virus doses ranging from 5 to 5×105 PFU. B Survival of animals is shown in A. The mLD50 of the virus was calculated to be of 1.6×103 PFU (using the Reed and Munch method).
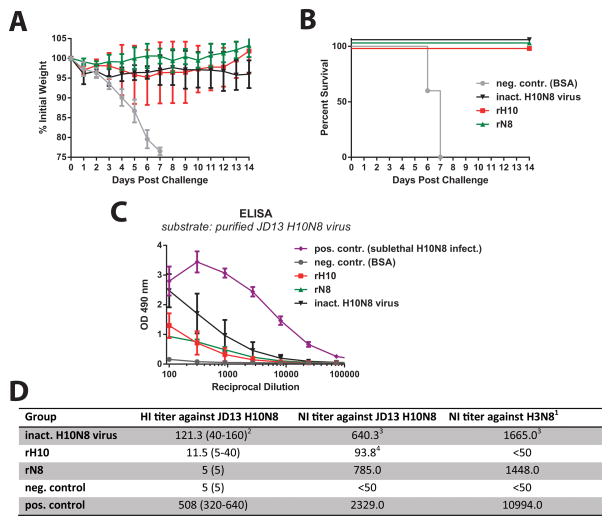
Vaccination with inactivated virus, rH10 or rN8 protects mice from challenge
To test if vaccine constructs could protect from H10N8 challenge we vaccinated mice with formalin-inactivated whole virus H10N8 vaccine, rH10 or rN8. Animals were then challenged intranasally with 10 mLD50 of JD13 virus and monitored for weight loss for 14 days. All three vaccine constructs were able to protect from viral challenge but to different degrees. Control animals succumbed to infection by day 7 (Figure 3). While protection in the rN8 and inactivated vaccine groups was relatively homogenous, one animal in the rH10 group lost more weight than the others but finally recovered. Surprisingly, rN8 seemed to induce the most robust protection from weight loss. It is also of note that mice which received the whole virus vaccine did not recover as well as rH10 or rN8 vaccinated mice. This difference was statistically significant for whole virus vaccine and rN8 groups on day 14 (p=0.0074). To further investigate the immune response to the vaccine constructs, we performed an ELISA with purified JD13 virus as substrate. Reactivity was best for the animals vaccinated with inactivated vaccine followed by rH10 and rN8 vaccinated groups. A positive control sera from animals that were sublethaly infected with JD13 reacted strongly to the virus preparation while sera from negative control animals showed no reactivity (Figure 3). In addition we measured HI and NI titers against homologous and heterologous strains. Animals that experienced a sublethal JD13 infection exhibited the highest HI titers followed by animals that were vaccinated with inactivated virus vaccine and animals that received rH10 HA. Negative control and rN8 vaccinated animals were HI negative to JD13. Animals vaccinated with inactivated virus or rN8 as well as positive control sera from re-convalescent animals had strong NI activity against JD13 H10N8 but also against a heterologous North American H3N8 virus while NI activity was absent from rH10 and BSA vaccinated control mice.
Figure 3. Vaccination with inactivated H10N8 virus, rH10 and rN8 protects mice from viral challenge.
Weight loss A and survival B of animals that received an intramuscular prime-boost regimen with inactivated H10N8, rH10 or rN8 vaccines. C ELISA titers of vaccinated animals against purified JD13 H10N8 virus. Sera from rH10 or rN8 can only react to one component of the virus while sera from animals vaccinated with inactivated virus or exposed to a sublethal infection react to both surface glyoproteins and to the internal proteins of damage virions on the ELISA plate as well. D shows HI and NI titers against JD13 H10N8 virus as well as NI titers against a heterologous H3N8 isolate. 1The virus strain used for this assay was A/Northern shoveler/Alaska/7MP1708/07 (H3N8). 2Geometric mean plus range in parenthesis, negative wells were assigned a value of 5 for calculation purpose. 3Values are expressed as the reciprocal serum dilution that was able to inhibit NA activity by 50%. 4Anti-HA antibodies can cause low titers of NI activity by steric hindrance [20].
Discussion
H10N8 infection was detected in three human individuals in China in the 2013/14 winter season - two ended fatally [1, 3, 5, 6]. Although these cases are likely to be isolated events, it is worth noting that viruses of the H10 subtype have infected humans - with non-lethal outcomes - before [16]. An H10N7 outbreak in seals is currently ongoing on the Danish, Swedish and German coast (http://www.waddensea-secretariat.org/news-and-service/news/14-28-10increased-seal-mortality-in-denmark-and-schleswig-holstein). It is therefore warranted to investigate if vaccines or therapeutic mAbs [15, 17–19] would be effective countermeasures against this subtype if it were to ever emerge as a pandemic strain. To facilitate this, we generated an H10N8 vaccine strain based on a virus that expresses the H10 HA and N8 NA of JD13 and the remaining proteins of PR8. The PR8 backbone is safe for humans (and poultry), does not allow for efficient transmission in mammals, and has an excellent safety track record for re-assortant vaccine strains [8–11]. In accordance to the WHO guidelines for H7N9 vaccine strains (‘Update of WHO biosafety risk assessment and guidelines for the production and quality control of human influenza vaccines against avian influenza A(H7N9) virus’, May 10th 2013; no specific guidelines for H10N8 are currently available) we sequenced the rescued virus to confirm the absence of a polybasic cleavage site in the HA and embryocidal activity in ovo. However, safety testing in ferrets and stability studies over multiple passages have to commence before the re-assortant strain is fit for industrial vaccine production. The rescued virus showed acceptable growth in embryonated eggs - although significantly lower peak titers than the parental PR8 virus. Furthermore, we determined that the virus shows better hemagglutination ability with turkey red blood cells than with chicken red blood cells. Vaccination with inactivated virus induced higher HI titers than those typically seen with similar viruses of avian origin. Vaccination with recombinant H10 HA induced low HI titers but could nonetheless protect animals from virus challenge - an important finding since recombinant HA vaccines have recently been licensed for use in the US and could be produced rapidly in a pandemic scenario. Surprisingly, recombinant N8 NA also offered solid protection from challenge by inducing high titers of NI activity. In summary we established a mouse challenge model for novel H10N8 viruses that will be useful for future development of H10N8 pre-pandemic vaccines and antibody therapies.
Acknowledgments
We thank Dr. Jonathan Runstadler (Massachusetts Institute of Technology) for the A/Northern shoveler/Alaska/7MP1708/07 (H3N8) virus. This study was partially supported by the NIH Centers for Excellence in Influenza Research and Surveillance (CEIRS) contract HHSN272201400008C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chen H, Yuan H, Gao R, Zhang J, Wang D, Xiong Y, et al. Clinical and epidemiological characteristics of a fatal case of avian influenza A H10N8 virus infection: a descriptive study. Lancet. 2014;383:714–21. doi: 10.1016/S0140-6736(14)60111-2. [DOI] [PubMed] [Google Scholar]
- 2.http://www.wpro.who.int/china/mediacentre/factsheets/h10n8/en/.
- 3.Su S, Qi W, Zhou P, Xiao C, Yan Z, Cui J, et al. First Evidence of H10N8 Avian Influenza Virus Infections among Feral Dogs in Live Poultry Markets in Guangdong Province, China. Clin Infect Dis. 2014 doi: 10.1093/cid/ciu345. [DOI] [PubMed] [Google Scholar]
- 4.García-Sastre A, Schmolke M. Avian influenza A H10N8--a virus on the verge? Lancet. 2014;383:676–7. doi: 10.1016/S0140-6736(14)60163-X. [DOI] [PubMed] [Google Scholar]
- 5.Qi W, Zhou X, Shi W, Huang L, Xia W, Liu D, et al. Genesis of the novel human-infecting influenza A(H10N8) virus and potential genetic diversity of the virus in poultry, China. Euro Surveill. 2014:19. doi: 10.2807/1560-7917.es2014.19.25.20841. [DOI] [PubMed] [Google Scholar]
- 6.Ni X, He F, Hu M, Zhou X, Wang B, Feng C, et al. Investigation of avian influenza virus in poultry and wild birds due to novel avian-origin influenza A(H10N8) in Nanchang City, China. Microbes Infect. 2014 doi: 10.1016/j.micinf.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 7.Qi W, Su S, Xiao C, Zhou P, Li H, Ke C, et al. Antibodies against H10N8 avian influenza virus among animal workers in Guangdong Province before November 30, 2013, when the first human H10N8 case was recognized. BMC Med. 2014;12:205. doi: 10.1186/s12916-014-0205-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beare AS, Schild GC, Craig JW. Trials in man with live recombinants made from A/PR/8/34 (H0 N1) and wild H3 N2 influenza viruses. Lancet. 1975;2:729–32. doi: 10.1016/s0140-6736(75)90720-5. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez A, Pérez-González A, Hossain MJ, Chen LM, Rolling T, Pérez-Breña P, et al. Attenuated strains of influenza A viruses do not induce degradation of RNA polymerase II. J Virol. 2009;83:11166–74. doi: 10.1128/JVI.01439-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsuoka Y, Chen H, Cox N, Subbarao K, Beck J, Swayne D. Safety evaluation in chickens of candidate human vaccines against potential pandemic strains of influenza. Avian Dis. 2003;47:926–30. doi: 10.1637/0005-2086-47.s3.926. [DOI] [PubMed] [Google Scholar]
- 11.Campbell PJ, Danzy S, Kyriakis CS, Deymier MJ, Lowen AC, Steel J. The M segment of the 2009 pandemic influenza virus confers increased neuraminidase activity, filamentous morphology, and efficient contact transmissibility to A/Puerto Rico/8/1934-based reassortant viruses. J Virol. 2014;88:3802–14. doi: 10.1128/JVI.03607-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krammer F, Albrecht RA, Tan GS, Margine I, Hai R, Schmolke M, et al. Divergent H7 immunogens offer protection from H7N9 virus challenge. J Virol. 2014;88:3976–85. doi: 10.1128/JVI.03095-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klausberger M, Wilde M, Palmberger D, Hai R, Albrecht RA, Margine I, et al. One-shot vaccination with an insect cell-derived low-dose influenza A H7 virus-like particle preparation protects mice against H7N9 challenge. Vaccine. 2014;32:355–62. doi: 10.1016/j.vaccine.2013.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Margine I, Palese P, Krammer F. Expression of Functional Recombinant Hemagglutinin and Neuraminidase Proteins from the Novel H7N9 Influenza Virus Using the Baculovirus Expression System. J Vis Exp. 2013 doi: 10.3791/51112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan GS, Lee PS, Hoffman RM, Mazel-Sanchez B, Krammer F, Leon PE, et al. Characterization of a broadly neutralizing monoclonal antibody that targets the fusion domain of group 2 influenza a virus hemagglutinin. J Virol. 2014;88:13580–92. doi: 10.1128/JVI.02289-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arzey GG, Kirkland PD, Arzey KE, Frost M, Maywood P, Conaty S, et al. Influenza virus A (H10N7) in chickens and poultry abattoir workers, Australia. Emerg Infect Dis. 2012;18:814–6. doi: 10.3201/eid1805.111852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friesen RH, Lee PS, Stoop EJ, Hoffman RM, Ekiert DC, Bhabha G, et al. A common solution to group 2 influenza virus neutralization. Proc Natl Acad Sci U S A. 2014;111:445–50. doi: 10.1073/pnas.1319058110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ekiert DC, Friesen RH, Bhabha G, Kwaks T, Jongeneelen M, Yu W, et al. A highly conserved neutralizing epitope on group 2 influenza A viruses. Science. 2011;333:843–50. doi: 10.1126/science.1204839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dreyfus C, Laursen NS, Kwaks T, Zuijdgeest D, Khayat R, Ekiert DC, et al. Highly conserved protective epitopes on influenza B viruses. Science. 2012;337:1343–8. doi: 10.1126/science.1222908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Couzens L, Gao J, Westgeest K, Sandbulte M, Lugovtsev V, Fouchier R, et al. An optimized enzyme-linked lectin assay to measure influenza A virus neuraminidase inhibition antibody titers in human sera. J Virol Methods. 2014;210C:7–14. doi: 10.1016/j.jviromet.2014.09.003. [DOI] [PubMed] [Google Scholar]