Abstract
Although natural killer (NK) cells are considered part of the innate immune system, recent studies have demonstrated the ability of antigen-experienced NK cells to become long-lived and contribute to potent recall responses similar to T and B cells. The precise signals that promote the generation of a long-lived NK cell response are largely undefined. Here, we demonstrate that NK cells require interleukin (IL)-18 signaling to generate a robust primary response during mouse cytomegalovirus (MCMV) infection, but do not require this signal for memory cell maintenance or recall responses. IL-12 signaling and STAT4 in activated NK cells increased the expression of the adaptor protein MyD88, which mediates signaling downstream of the IL-18 and IL-1 receptors. During MCMV infection, NK cells required MyD88 but not IL-1 receptor for optimal expansion. Thus, an IL-18-MyD88 signaling axis facilitates the prolific expansion of NK cells in response to primary viral infection, but not recall responses.
Introduction
Natural Killer (NK) cells play a significant role in the control of infected, stressed, or transformed cells that may be detrimental to the host. Recent studies in mice and humans have demonstrated that NK cells possess adaptive immune qualities (1). In mice infected with mouse cytomegalovirus (MCMV), Ly49H+ NK cells activated by the viral glycoprotein m157 undergo extensive proliferation, and contract resulting in the formation of a small pool of long-lived memory NK cells that can be recalled, and exhibit heightened effector function (1).
Pro-inflammatory cytokines strongly influence the NK cell response against MCMV infection (2). Although previous work has described the effect of pro-inflammatory cytokines on the general activation of NK cells during MCMV infection (2), their role in driving clonal-like expansion and memory in antigen-specific NK cells is largely unknown. We previously implicated IL-12, its signaling molecule STAT4, and the downstream transcription factor Zbtb32 as crucial signals in the generation of robust effector and memory NK cell responses against MCMV infection (3, 4). IL-18 has been suggested to “prime” resting NK cells for maximum IFN-γ production following ex vivo stimulation (5), and synergize with IL-12 during NK cell activation (6). Although IL-18 is produced early during MCMV infection (7), it is not known how IL-18 signals influence the virus-specific Ly49H+ NK cell response. Here, we investigate the direct effects of IL-18 signaling on primary and recall NK cell responses to MCMV infection.
Materials and methods
Mice and infections
All mice used in this study were bred and maintained at MSKCC in accordance with IACUC guidelines. Mixed bone marrow chimeric mice were generated, and adoptive transfer studies and viral infections were performed as previously described (8).
Flow cytometry and cell sorting
Fc receptors were blocked with 2.4G2 mAb before staining with the indicated surface or intracellular antibodies (BD, BioLegend, or eBioscience). Flow cytometry was performed on an LSR II (BD). Cell sorting was performed on an Aria II cytometer (BD). All data were analyzed with FlowJo software (TreeStar). NK cell enrichment and adoptive transfers were performed as previously described (3).
qRT-PCR and ChIP
qRT-PCR and chromatin immunoprecipitation (ChIP) were performed as previously described (4). The following qRT-PCR primers were used: Myd88, For: 5’-CACCTGTGTCTGGTCCATT-3’, Rev: 5’-AGGCTGAGTGCAAACTTG-3’; Actb, For: 5’-TGCGTGACATCAAAGAGAAG-3’, Rev: 5’-CGGATGTCAACGTCACACTT-3’. The following qPCR primers were used for ChIP studies: Myd88 promoter, For: 5’-AAGTAGGAAACTCCACAGGCGAGC-3’, Rev: 5’-TTCAAGAACAGCGATAGGCGGC-3’; Gene desert 50 kB upstream of Foxp3, For: 5’-TAGCCAGAAGCTGGAAAGAAGCCA-3’, Rev: 5’-TGATACCCTCCAGGTCCAACCATT-3’; Zpf42 promoter, For: 5’-AGAGGGCGGTGTGTACTGTGGTG-3’, Rev: 5’-CTTCTTCTTGCACCCGGCTTGAG-3’; Utf1 promoter, For: 5’-AGTCGTTGAATACCGCGTTGCTG-3’, Rev: 5’-CTGTTGAGATGTCGCCCAAGTGC-3’; Ifng promoter, For: 5’-GCTCTGTGGATGAGAAAT-3’, Rev: 5’-GCTCTGTGGATGAGAAAT-3’.
Ex vivo stimulation of NK cells
Purified NK cells were stimulated for 4 h (memory cells) or 18 h (for ChIP), as previously described (4). Negative and positive controls include NK cells incubated with media only, or with PMA (50 ng/mL) and Ionomycin (1 µg/mL), respectively.
Statistical methods
All graphs depict mean ± s.e.m. Two-tailed paired Student’s t-test was used to derive statistical differences. A p value < 0.05 was considered significant. Plots and statistical analyses were produced in GraphPad Prism.
Results and Discussion
Cell-Intrinsic IL-18 signaling required for the primary expansion of virus-specific NK cells
Although IL-18 has previously been shown to activate NK cells during viral infection, these studies involve directly infecting IL-18- and IL-18 receptor-deficient mice (7, 9). Therefore, to address whether IL-18 influences NK cell responses in a cell-intrinsic manner, we adoptively transferred equal numbers of WT and Il18r1−/− NK cells into Ly49h−/− mice, which harbor normal numbers of NK cells but are incapable of recognizing the MCMV-derived m157 protein (3, 8). Following infection with MCMV, WT NK cells preferentially expanded during the first week of infection and were higher in frequency than Il18r1−/− NK cells at day 7 post-infection (PI; Supp Figure 1A) and at later time points (Figure 1A). Consistent with the adoptive transfer experiment, we observed a similar expansion defect by Il18r1−/− Ly49H+ NK cells in WT:Il18r1−/− mixed bone marrow chimeric mice infected with MCMV (Figure 1B and Supp Figure 1B). Together, these studies confirm a cell-intrinsic requirement for IL-18 signaling in the antiviral NK cell response.
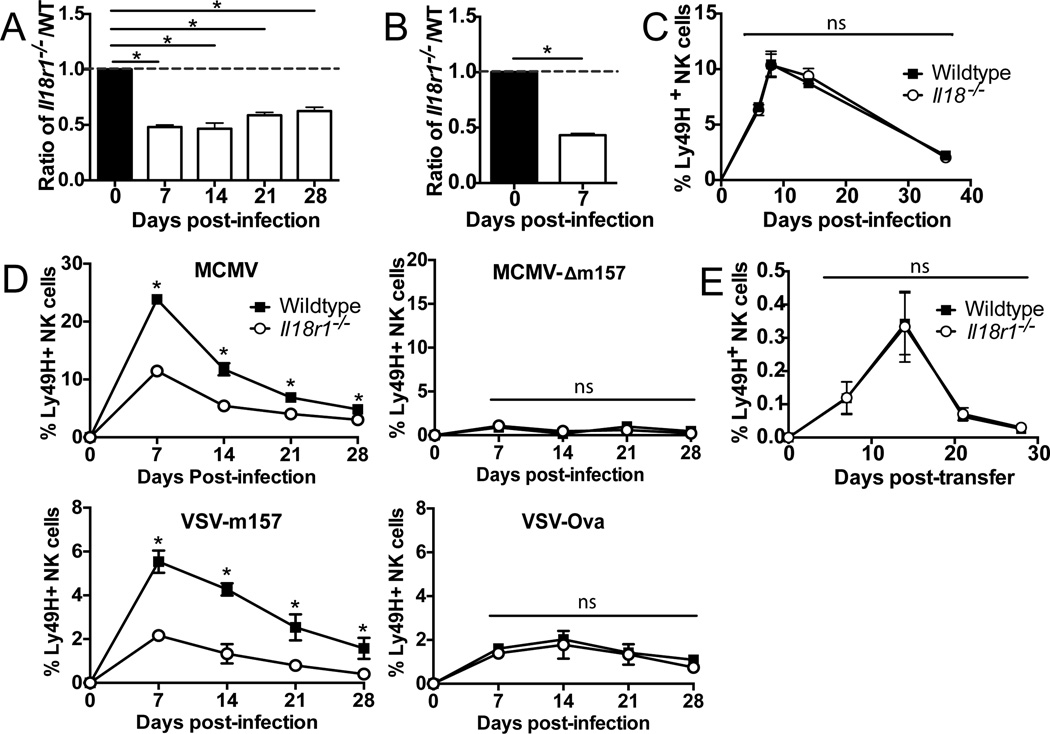
Figure 1. IL-18R-deficient NK cells mount a defective response to viral infection.
A. WT and Il18r1−/− NK cells were co-transferred into Ly49h−/− mice and infected with MCMV. The relative ratio of populations is shown for each time point compared to day 0. B. Mixed WT:Il18r1−/− chimeric mice were infected with MCMV and the relative ratio of Ly49H+ NK cells are shown for day 7 PI compared to uninfected. C. Percentages of co-transferred WT and Il18−/− Ly49H+ NK cells are shown during MCMV infection. D. Percentages of co-transferred WT and Il18r1−/− Ly49H+ NK cells are shown following infection with MCMV, MCMV-Δm157, VSV-m157, or VSV-Ova. E. WT and Il18r1−/− NK cells were co-transferred into Rag×Il2rg−/− mice and percentages of transferred Ly49H+ NK cells within the total cell population are shown. Data are mean ± s.e.m. representative of at least four independent experiments with at least n=3 biological replicates per condition. * p < 0.05 and ns, not significant, paired Student t-test.
IL-18 has been suggested enhance IL-12-induced effector functions of NK cells such as IFN-γ production (5, 6). To determine if IL-18 might also “prime” NK cells for MCMV-driven expansion, we isolated resting NK cells from Il18−/− mice and co-transferred them with equal numbers of WT NK cells into Ly49h−/− hosts. Following MCMV infection, WT and Il18−/− Ly49H+ NK cells exhibited comparable expansion and memory cell formation (Figure 1C), indicating that previous exposure to IL-18 during development or homeostasis was not required for normal expansion in response to viral challenge as long as IL-18 is present during infection.
To determine whether the effect of IL-18 signaling was limited to antigen-specific NK cell responses, we utilized MCMV and VSV viruses engineered to lack or express m157, respectively. Following adoptive transfer of WT and Il18r1−/− NK cells into Ly49h−/− hosts, we observed that in contrast to MCMV, infection with MCMV lacking m157 (MCMV-Δm157) elicited an equivalent (albeit modest) expansion of both WT and Il18r1−/− NK cell populations (Figure 1D), indicating that IL-18 signaling is not required for the low-level proliferation of “bystander” NK cells responding to viral infection. Consistent with this observation, infection with VSV-m157, but not with VSV-Ova, elicited a preferential antigen-specific expansion of WT NK cells over Il18r1−/− NK cells (Figure 1D). We next investigated whether IL-18 was required for NK cells to undergo homeostatic proliferation. We found that WT and Il18r1−/−NK cells proliferated similarly when transferred into Rag2−/−×Il2rg−/− mice, generating comparable long-lived populations more than 4 weeks later (Figure 1E), demonstrating IL-18 signaling is not required for NK cells to proliferate in response to common gamma chain cytokines. Altogether, these data suggest that IL-18 signaling directly regulates antigen-driven expansion of NK cells during viral infection, but not during bystander or homeostatic expansion.
IL-18 signaling is necessary for optimal IFN-γ production and maturation of NK cells during infection
Because IL-18 is required for optimal proliferation of NK cells during viral infection, we investigated additional NK cell effector functions that may be compromised in the absence of IL-18 signaling. At day 1.5 PI we observed that Il18r1−/− NK cells exhibited a defect in IFN-γ production, but not Granzyme B (Figure 2A), consistent with a previous report (7). Although both NK cell populations became similarly activated at days 1.5 and 7 PI (Supp Figure 1C and 1D), WT NK cells were found to be more mature, as measured by CD27 and CD11b staining, compared to Il18r1−/− cells (Figure 2B), suggesting IL-18 may have a role in promoting the maturation of activated NK cells.
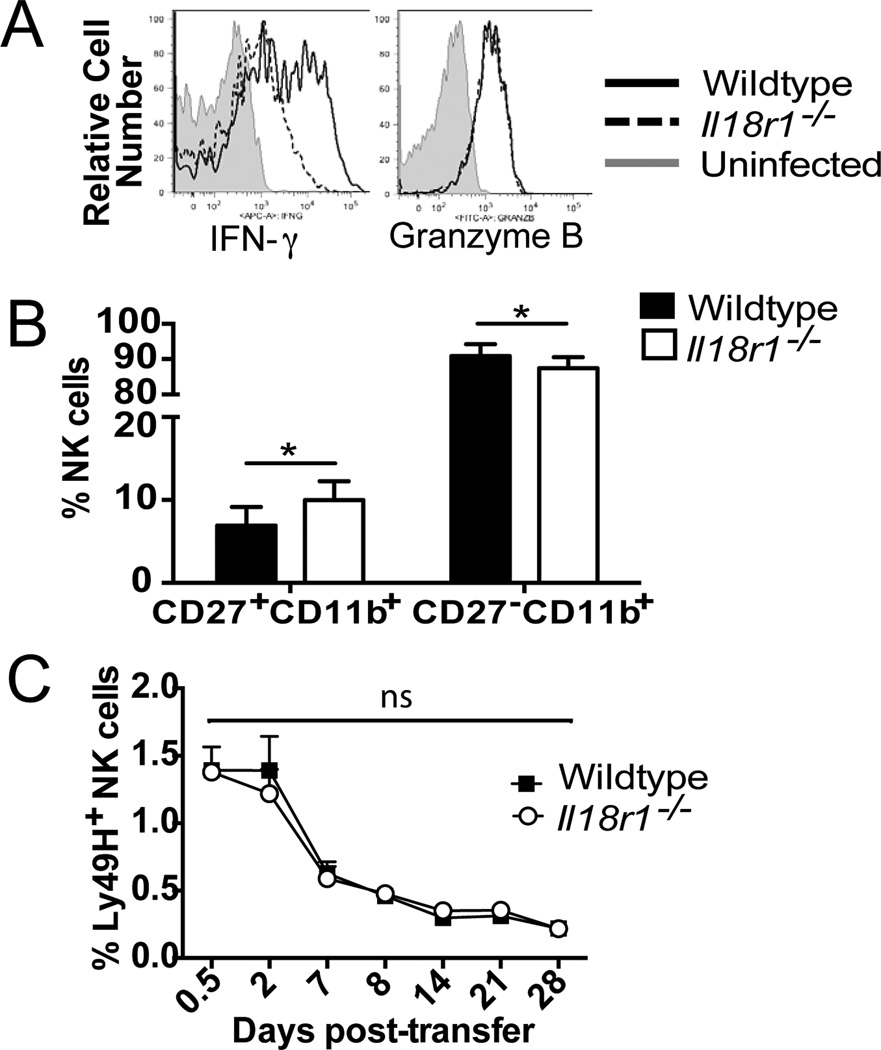
Figure 2. IL-18 is necessary for optimal NK cell maturation and IFN-γ production following MCMV infection.
A. Mixed WT:Il18r1−/− chimeric mice were infected with MCMV and amount of IFN-γ and granzyme B in NK cells at day 1.5 PI are shown. B. WT and Il18r1−/− NK cells were co-transferred into Ly49h−/− mice and infected with MCMV. CD27 and CD11b staining is shown for WT and Il18r1−/− Ly49H+ NK cells at day 7 PI. C. Equal numbers of purified effector WT and Il18r1−/− NK cells (at day 7 PI) were co-transferred into a naïve Ly49h−/−host. Percentages of Ly49H+ NK cells are shown. Data are mean ± s.e.m. representative of at least 3 independent experiments with at least n=3 biological replicates per condition. * p < 0.05 and ns, not significant, paired Student t-test.
Adoptive co-transfer and bone marrow chimeric studies revealed an expansion defect in the Il18r1−/− NK cell response to MCMV infection, which could be a consequence of decreased proliferation or increased apoptosis of Il18r1−/− NK cells relative to WT NK cells. We were not able to detect differences in BrdU incorporation, Ki67 staining, or CFSE dilution by WT and Il18r1−/− NK cells in MCMV-infected bone marrow chimeric mice (data not shown), consistent with a prior study (10). In addition, FLICA incorporation studies did not reveal any differences in pan-caspase activity between Il18r1−/− and WT NK cells on day 7 PI (Supp Figure 1E), suggesting that Il18r1−/− NK cells were not more susceptible to apoptosis. Lastly, when WT and Il18r1−/− effector NK cells were sorted from MCMV-infected hosts on day 7 PI, and equal numbers co-transferred into naïve recipients, the two populations contracted at similar rates (Figure 2C), indicating that Il18r1−/− NK cells are not undergoing increased cell death at later time points, and that IL-18 signaling does not regulate the contraction phase or maintenance of memory NK cells.
Antiviral NK cell response depends on MyD88, but not the IL-1 receptor
Because the IL-18 receptor requires the adapter molecule MyD88 for downstream signaling (11), we investigated the contribution of MyD88 to the NK cell response to MCMV infection. WT:Myd88−/− mixed bone marrow chimeric mice were generated and both NK cell populations reconstituted similarly (data not shown). Following MCMV infection, Myd88−/− Ly49H+ NK cells exhibited a cell-intrinsic expansion defect compared to WT NK cells (Figure 3A), similar to Il18r1−/− NK cells (Figure 1C). In addition, fewer Myd88−/− NK cells produced IFN-γ after MCMV infection despite comparable upregulation of CD69 (Figure 3B). Similar to Il18r1−/− NK cells, Myd88−/− NK cells failed to mature as efficiently as WT NK cells at day 7 PI (Figure 3C). When equal numbers of WT and Myd88−/− NK cells were transferred into Ly49h−/− hosts followed by infection with MCMV, we observed preferential expansion and memory cell formation of the WT NK cells compared to Myd88−/− NK cells (Figure 3D). The IL-1 receptor, which is expressed by NK cells, is also known to use MyD88 for signaling (11); however, unlike Il18r1−/− or Myd88−/− NK cells, Il1r−/− NK cells expanded comparably to WT NK cells at day 7 PI (Figure 3E) and showed similar effector function and phenotype as WT NK cells at days 1.5 and 7 PI (Supp Figures 2A–C). These findings support a mechanism for IL-18 signaling on NK cells through a MyD88-dependent signaling axis.
Figure 3. MyD88-deficient NK cells exhibit defective proliferation during MCMV infection.
A. WT: Myd88−/− chimeric mice were infected with MCMV and percentages of splenic NK cells are shown for uninfected and day 7 PI. B. CD69 and IFN-γ are shown for wildtype and Myd88−/− NK cells (compared to uninfected mice) at day 1.5 PI. C. CD27 and CD11b staining is shown for WT and Il18r1−/− Ly49H+ NK cells at day 7 PI. D. Percentages of co-transferred WT (CD45.1) and MyD88−/− (CD45.2) Ly49H+ NK cells are shown following MCMV infection. E. Percentages of co-transferred WT and Il1r−/− Ly49H+ NK cells are shown during MCMV infection. Data are mean ± s.e.m. representative of three independent experiments with at least n=3 biological replicates per condition. * p < 0.05 and ns, not significant, paired Student t-test.
IL-12 signaling and STAT4 increase expression of MyD88 in activated NK cells
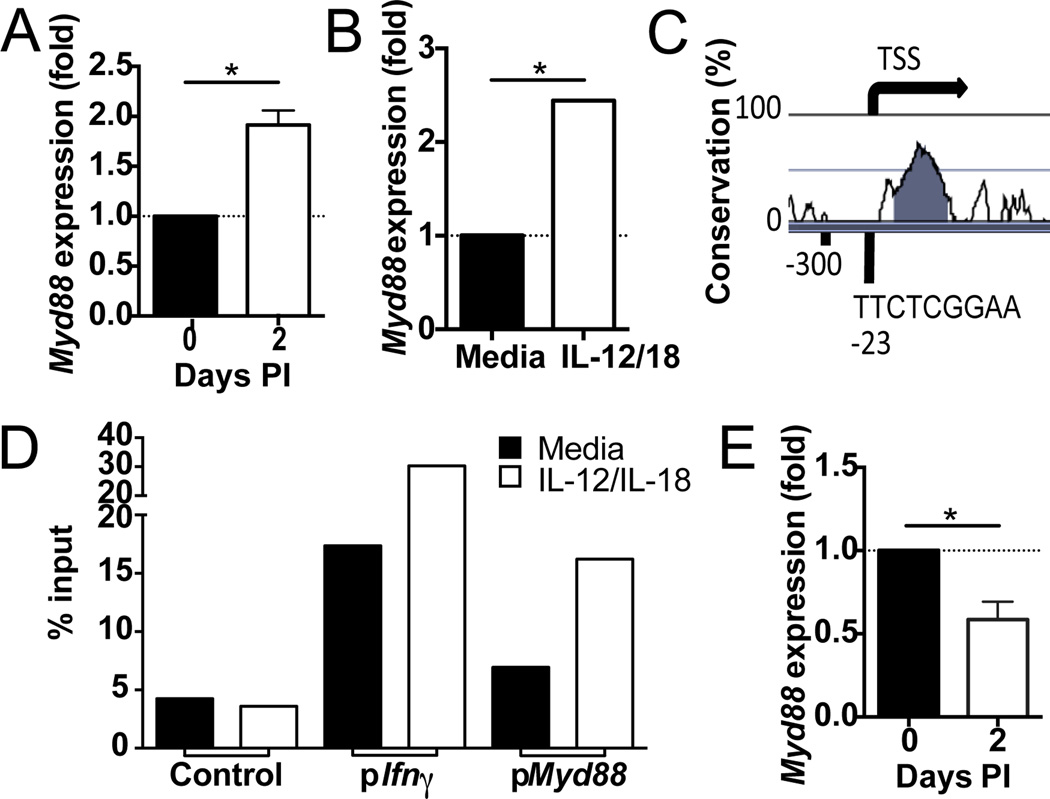
During MCMV infection, we observed an increase in Myd88 expression in sorted NK cells by microarray and qRT-PCR (Figure 4A, data not shown). Given the important early role of IL-12 in activating NK cells, and a report that proinflammatory cytokines induce IL-18 signaling components in human NK cells (12), we investigated whether IL-12 directly influences the expression of the IL-18 signaling machinery. Overnight stimulation of sorted NK cells with IL-12 and IL-18 also showed an increase in the expression of Myd88 by qRT-PCR (Figure 4B). STAT4 is a transcription factor that mediates signals downstream from the IL-12 receptor (13), and analysis of the Myd88 promoter revealed one putative STAT4 binding site upstream of the transcriptional start site (Figure 4C). STAT4 ChIP followed by qRT-PCR identified a significant enrichment of STAT4 binding directly upstream of the Myd88 transcriptional start site (Figure 4D). This increase in STAT4 binding was dependent on IL-12 stimulation, suggesting Myd88 is a target gene of IL-12 signaling. Finally, we compared the expression of Myd88 in WT and Stat4−/− NK cells during MCMV infection. Activated Stat4−/− NK cells exhibited lower levels of Myd88 expression compared to WT NK cells. Altogether, these data indicate that IL-12 signaling acts through STAT4 to increase the expression of Myd88, identifying a novel role for IL-12 in potentiating IL-18 signaling early during MCMV infection.
Figure 4. IL-12 signaling induces expression of Myd88 in NK cells.
A. qRT-PCR analysis of Myd88 mRNA abundance in NK cells sorted from the spleen of WT mice following infection with MCMV (n=4 biological replicates per condition). Fold expression is shown relative to naïve mice. B. qRT-PCR analysis of Myd88 mRNA abundance in WT NK cells stimulated with IL-12 and IL-18 for 18 h (n=3 biological replicates per condition). Fold expression is shown relative to medium only. C. Vista browser image of mouse Myd88 promoter showing predicted STAT4 binding site. D. STAT4 binding at Myd88, Ifng, and control promoters as assessed through ChIP followed by qPCR in sorted WT NK cells stimulated with IL-12 and IL-18 for 18 h. STAT4 occupancy as percent of input is shown for target (Myd88) and control DNA (negative control: average of gene desert 50 kb upstream of Foxp3, Zfp42, and Utf1 promoters; positive control: Ifng promoter). Data were confirmed in two independent experiments. E. qRT-PCR analysis of Myd88 mRNA abundance in Stat4−/− NK cells relative to WT NK cells from mice infected with MCMV (n=4 biological replicates per condition). Data are mean ± s.e.m. representative of three independent experiments. * p < 0.05, paired Student t-test.
IL-18 signaling is dispensable for recall responses by memory NK cells
To assess whether IL-18 signaling regulates the functional capabilities of memory NK cells, we stimulated WT and Il18r1−/− memory NK cells with Ly49D, Ly49H, or IL-12 and IL-18 and measured their ability to degranulate and to make IFN-γ. Similar to resting NK cells, Il18r1−/− memory NK cells produced less IFN-γ than their WT counterparts only when stimulated with pro-inflammatory cytokines, but not with Ly49D, Ly49H or PMA and ionomycin (Figure 5A and data not shown). Il18r1−/− memory NK cells degranulated similarly to WT (Figure 5B). Thus, like resting NK cells (5), memory NK cells continue to depend on IL-18 for IL-12-mediated IFN-γ production.
Figure 5. IL-18 signaling is dispensable for recall response of NK cells.
Memory WT and Il18r1−/− NK cells were stimulated with Ly49D, IL-12 and IL18, or PMA and Ionomycin, and percent IFN-γ (A) or CD107a (B) expression shown. C. Percentage of recalled WT and Il18r1−/− NK cells (at day 28 PI) are shown following secondary challenge with MCMV. Data are mean ± s.e.m. representative of five independent experiments with at least n=4 biological replicates per condition. * p < 0.05 or ns, not significant, paired Student t-test.
Given the importance of IL-18 signaling on the optimal expansion of naïve NK cells during MCMV infection, we investigated its role during a recall response. Equal numbers of effector WT and Il18r1−/− NK cells were co-transferred and “parked” in a naïve host for three weeks, followed by infection with MCMV. Surprisingly, Il18r1−/− memory NK cells expanded similarly to their WT counterparts during secondary challenge by MCMV (Figure 5C), demonstrating a stage-specific requirement for IL-18 signals for proliferation. Following the peak of expansion, both cell populations contributed to equal frequencies of secondary memory NK cells. Thus, IL-18 signaling is specifically required for the primary expansion of virus-specific NK cells, but is dispensable in the subsequent recall response.
NK cells possess adaptive immune qualities in response to cytokine treatment (14) or exposure to pathogen and non-pathogen antigens (15). It is poorly understood what the cytokine signal requirements are in resting and memory NK cells. Here, we find a stage-specific requirement for IL-18, where IL-18 promotes the antigen-specific primary expansion of resting NK cells, but not the recall response of memory NK cells. Furthermore, we have identified a previously unknown role for IL-12 in promoting the expression of Myd88 to enhance the IL-18 signaling cascade, and possibly sensitize NK cells to lower levels of IL-18 cytokine.
Our current findings suggest that as naïve NK cells differentiate into antigen-experienced memory cells, they become “specialized” and may rely more on antigen-specific signals and less on pro-inflammatory cytokine signals for clonal-like expansion. This mechanism would allow previously antigen experienced NK cells to respond more robustly during subsequent pathogen encounter. Because memory and recall NK cell responses have also been documented in humans following viral infection (1), determining whether a homologous role for IL-18 in the recall response of human NK cells may impact the use of this cytokine in clinical settings.
Supplementary Material
Acknowledgments
S.M. was supported by MSTP (T32GM07739), NIH (T32AI007621), and the Cancer Research Institute (CRI) grants. J.C.S. was supported by the Searle Scholars Program, the CRI, and NIH grant AI100874.
Footnotes
The authors declare no financial conflicts of interest.
References
- 1.Sun JC, Lanier LL. NK cell development, homeostasis and function: parallels with CD8(+) T cells. Nat Rev Immunol. 2011 doi: 10.1038/nri3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- 3.Sun JC, Madera S, Bezman NA, Beilke JN, Kaplan MH, Lanier LL. Proinflammatory cytokine signaling required for the generation of natural killer cell memory. J Exp Med. 2012;209:947–954. doi: 10.1084/jem.20111760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beaulieu AM, Zawislak CL, Nakayama T, Sun JC. The transcription factor Zbtb32 controls the proliferative burst of virus-specific natural killer cells responding to infection. Nat Immunol. 2014;15:546–553. doi: 10.1038/ni.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaix J, Tessmer MS, Hoebe K, Fuseri N, Ryffel B, Dalod M, Alexopoulou L, Beutler B, Brossay L, Vivier E, Walzer T. Cutting edge: Priming of NK cells by IL-18. J Immunol. 2008;181:1627–1631. doi: 10.4049/jimmunol.181.3.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takeda K, Tsutsui H, Yoshimoto T, Adachi O, Yoshida N, Kishimoto T, Okamura H, Nakanishi K, Akira S. Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity. 1998;8:383–390. doi: 10.1016/s1074-7613(00)80543-9. [DOI] [PubMed] [Google Scholar]
- 7.Pien GC, Satoskar AR, Takeda K, Akira S, Biron CA. Cutting edge: selective IL-18 requirements for induction of compartmental IFN-gamma responses during viral infection. J Immunol. 2000;165:4787–4791. doi: 10.4049/jimmunol.165.9.4787. [DOI] [PubMed] [Google Scholar]
- 8.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457:557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andrews DM, Scalzo AA, Yokoyama WM, Smyth MJ, Degli-Esposti MA. Functional interactions between dendritic cells and NK cells during viral infection. Nat Immunol. 2003;4:175–181. doi: 10.1038/ni880. [DOI] [PubMed] [Google Scholar]
- 10.French AR, Sjolin H, Kim S, Koka R, Yang L, Young DA, Cerboni C, Tomasello E, Ma A, Vivier E, Karre K, Yokoyama WM. DAP12 signaling directly augments proproliferative cytokine stimulation of NK cells during viral infections. J Immunol. 2006;177:4981–4990. doi: 10.4049/jimmunol.177.8.4981. [DOI] [PubMed] [Google Scholar]
- 11.Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 12.Sareneva T, Julkunen I, Matikainen S. IFN-alpha and IL-12 induce IL-18 receptor gene expression in human NK and T cells. J Immunol. 2000;165:1933–1938. doi: 10.4049/jimmunol.165.4.1933. [DOI] [PubMed] [Google Scholar]
- 13.Thierfelder WE, van Deursen JM, Yamamoto K, Tripp RA, Sarawar SR, Carson RT, Sangster MY, Vignali DA, Doherty PC, Grosveld GC, Ihle JN. Requirement for Stat4 in interleukin-12-mediated responses of natural killer and T cells. Nature. 1996;382:171–174. doi: 10.1038/382171a0. [DOI] [PubMed] [Google Scholar]
- 14.Cooper MA, Elliott JM, Keyel PA, Yang L, Carrero JA, Yokoyama WM. Cytokine-induced memory-like natural killer cells. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0813192106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paust S, von Andrian UH. Natural killer cell memory. Nat Immunol. 2011;131:500–508. doi: 10.1038/ni.2032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.